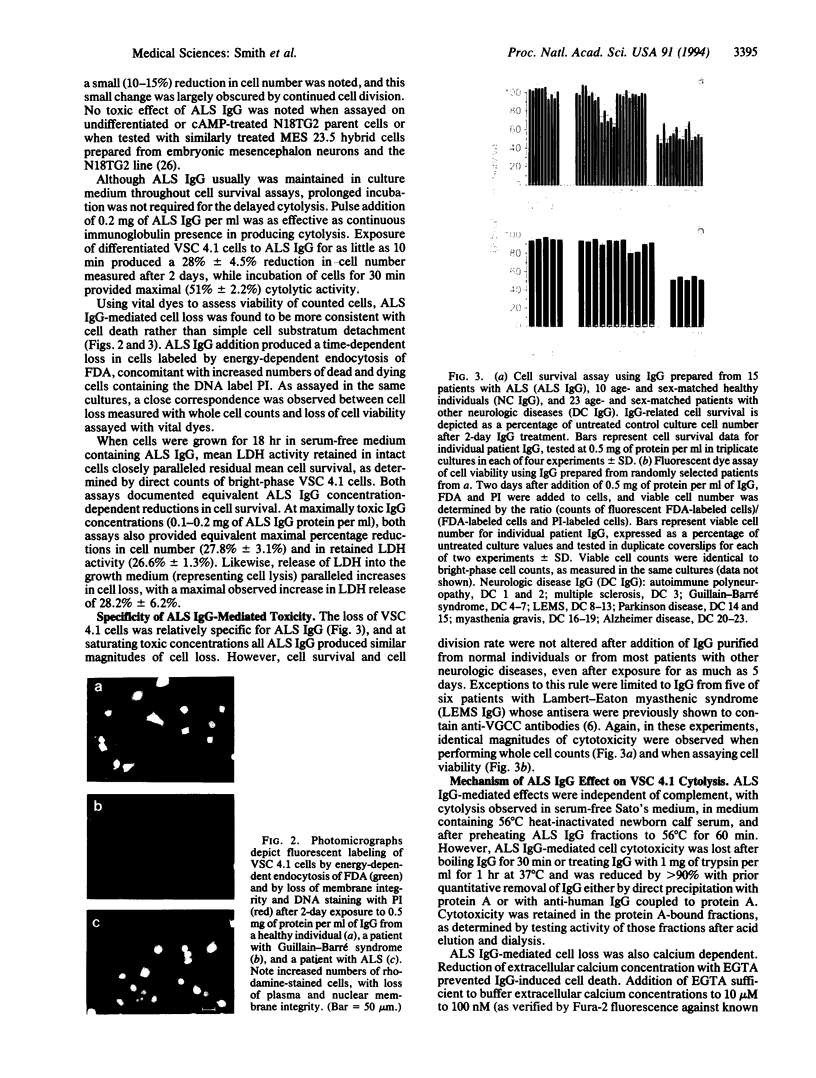
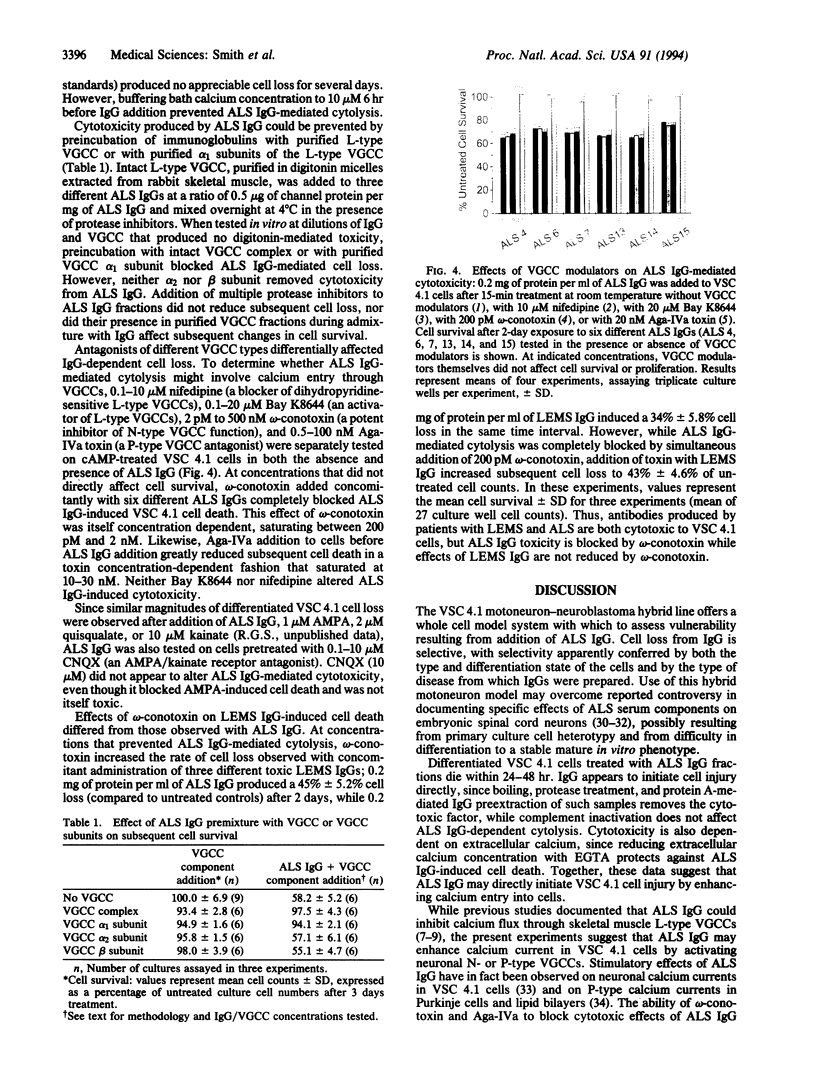
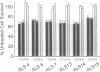Abstract
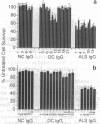
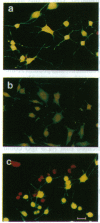
Patients with amyotrophic lateral sclerosis possess antibodies (ALS IgGs) that bind to L-type skeletal muscle voltage-gated calcium channels (VGCCs) and inhibit L-type calcium current. To determine whether interaction of ALS IgGs with neuronal VGCCs might influence motoneuron survival, we used a motoneuron-neuroblastoma hybrid (VSC 4.1) cell line expressing binding sites for inhibitors of L-, N-, and P-type VGCCs. Using direct viable cell counts, quantitation of propidium iodide- and fluorescein diacetate-labeled cells, and lactate dehydrogenase release to assess cell survival, we document that ALS IgG kills 40-70% of cAMP-differentiated VSC 4.1 cells within 2 days. ALS IgG-mediated cytotoxicity is dependent on extracellular calcium and is prevented by peptide antagonists of N- or P-type VGCCs but not by dihydropyridine modulators of L-type VGCCs. Preincubating IgG with purified intact L-type VGCC or with isolated VGCC alpha 1 subunit also blocks ALS IgG-mediated cytotoxicity. These results suggest that ALS IgG may directly lead to motoneuron cell death by a mechanism requiring extracellular calcium and mediated by neuronal-type calcium channels.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appel S. H., Engelhardt J. I., García J., Stefani E. Immunoglobulins from animal models of motor neuron disease and from human amyotrophic lateral sclerosis patients passively transfer physiological abnormalities to the neuromuscular junction. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):647–651. doi: 10.1073/pnas.88.2.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appel S. H. Excitotoxic neuronal cell death in amyotrophic lateral sclerosis. Trends Neurosci. 1993 Jan;16(1):3–5. doi: 10.1016/0166-2236(93)90039-o. [DOI] [PubMed] [Google Scholar]
- Cashman N. R., Durham H. D., Blusztajn J. K., Oda K., Tabira T., Shaw I. T., Dahrouge S., Antel J. P. Neuroblastoma x spinal cord (NSC) hybrid cell lines resemble developing motor neurons. Dev Dyn. 1992 Jul;194(3):209–221. doi: 10.1002/aja.1001940306. [DOI] [PubMed] [Google Scholar]
- Crawford G. D., Jr, Le W. D., Smith R. G., Xie W. J., Stefani E., Appel S. H. A novel N18TG2 x mesencephalon cell hybrid expresses properties that suggest a dopaminergic cell line of substantia nigra origin. J Neurosci. 1992 Sep;12(9):3392–3398. doi: 10.1523/JNEUROSCI.12-09-03392.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deakin C. D., Fagan E. A., Williams R. Cytoprotective effects of calcium channel blockers. Mechanisms and potential applications in hepatocellular injury. J Hepatol. 1991 Mar;12(2):251–255. doi: 10.1016/0168-8278(91)90947-a. [DOI] [PubMed] [Google Scholar]
- Delbono O., García J., Appel S. H., Stefani E. Calcium current and charge movement of mammalian muscle: action of amyotrophic lateral sclerosis immunoglobulins. J Physiol. 1991 Dec;444:723–742. doi: 10.1113/jphysiol.1991.sp018903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbono O., Magnelli V., Sawada T., Smith R. G., Appel S. H., Stefani E. Fab fragments from amyotrophic lateral sclerosis IgG affect calcium channels of skeletal muscle. Am J Physiol. 1993 Mar;264(3 Pt 1):C537–C543. doi: 10.1152/ajpcell.1993.264.3.C537. [DOI] [PubMed] [Google Scholar]
- Dubinsky J. M. Intracellular calcium levels during the period of delayed excitotoxicity. J Neurosci. 1993 Feb;13(2):623–631. doi: 10.1523/JNEUROSCI.13-02-00623.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen M. R. Ca(2+)-dependent changes in the mitochondrial energetics in single dissociated mouse sensory neurons. Biochem J. 1992 Apr 1;283(Pt 1):41–50. doi: 10.1042/bj2830041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin J. L., Johnson E. M., Jr Suppression of programmed neuronal death by sustained elevation of cytoplasmic calcium. Trends Neurosci. 1992 Dec;15(12):501–508. doi: 10.1016/0166-2236(92)90103-f. [DOI] [PubMed] [Google Scholar]
- Hamilton B. R., Smith D. O. Calcium currents in rat motor nerve terminals. Brain Res. 1992 Jul 3;584(1-2):123–131. doi: 10.1016/0006-8993(92)90885-d. [DOI] [PubMed] [Google Scholar]
- Hong S. J., Tsuji K., Chang C. C. Inhibition by neosurugatoxin and omega-conotoxin of acetylcholine release and muscle and neuronal nicotinic receptors in mouse neuromuscular junction. Neuroscience. 1992;48(3):727–735. doi: 10.1016/0306-4522(92)90416-y. [DOI] [PubMed] [Google Scholar]
- Horwich M. S., Engel W. K., Chauvin P. B. Amyotrophic lateral sclerosis sera applied to cultured motor neurons. Arch Neurol. 1974 Apr;30(4):332–333. doi: 10.1001/archneur.1974.00490340060015. [DOI] [PubMed] [Google Scholar]
- Juntti-Berggren L., Larsson O., Rorsman P., Ammälä C., Bokvist K., Wåhlander K., Nicotera P., Dypbukt J., Orrenius S., Hallberg A. Increased activity of L-type Ca2+ channels exposed to serum from patients with type I diabetes. Science. 1993 Jul 2;261(5117):86–90. doi: 10.1126/science.7686306. [DOI] [PubMed] [Google Scholar]
- Kim Y. I., Neher E. IgG from patients with Lambert-Eaton syndrome blocks voltage-dependent calcium channels. Science. 1988 Jan 22;239(4838):405–408. doi: 10.1126/science.2447652. [DOI] [PubMed] [Google Scholar]
- Kimura F., Smith R. G., Delbono O., Nyormoi O., Schneider T., Nastainczyk W., Hofmann F., Stefani E., Appel S. H. Amyotrophic lateral sclerosis patient antibodies label Ca2+ channel alpha 1 subunit. Ann Neurol. 1994 Feb;35(2):164–171. doi: 10.1002/ana.410350207. [DOI] [PubMed] [Google Scholar]
- Koh J. Y., Choi D. W. Quantitative determination of glutamate mediated cortical neuronal injury in cell culture by lactate dehydrogenase efflux assay. J Neurosci Methods. 1987 May;20(1):83–90. doi: 10.1016/0165-0270(87)90041-0. [DOI] [PubMed] [Google Scholar]
- Koh J. Y., Cotman C. W. Programmed cell death: its possible contribution to neurotoxicity mediated by calcium channel antagonists. Brain Res. 1992 Aug 7;587(2):233–240. doi: 10.1016/0006-8993(92)91002-v. [DOI] [PubMed] [Google Scholar]
- Lang B., Newsom-Davis J., Peers C., Prior C., Wray D. W. The effect of myasthenic syndrome antibody on presynaptic calcium channels in the mouse. J Physiol. 1987 Sep;390:257–270. doi: 10.1113/jphysiol.1987.sp016698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larmet Y., Dolphin A. C., Davies A. M. Intracellular calcium regulates the survival of early sensory neurons before they become dependent on neurotrophic factors. Neuron. 1992 Sep;9(3):563–574. doi: 10.1016/0896-6273(92)90193-h. [DOI] [PubMed] [Google Scholar]
- Legendre P., Rosenmund C., Westbrook G. L. Inactivation of NMDA channels in cultured hippocampal neurons by intracellular calcium. J Neurosci. 1993 Feb;13(2):674–684. doi: 10.1523/JNEUROSCI.13-02-00674.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Sugimori M., Cherksey B. D., Smith R. G., Delbono O., Stefani E., Appel S. IgG from amyotrophic lateral sclerosis patients increases current through P-type calcium channels in mammalian cerebellar Purkinje cells and in isolated channel protein in lipid bilayer. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11743–11747. doi: 10.1073/pnas.90.24.11743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoPresti P., Poluha W., Poluha D. K., Drinkwater E., Ross A. H. Neuronal differentiation triggered by blocking cell proliferation. Cell Growth Differ. 1992 Sep;3(9):627–635. [PubMed] [Google Scholar]
- Magnelli V., Sawada T., Delbono O., Smith R. G., Appel S. H., Stefani E. The action of amyotrophic lateral sclerosis immunoglobulins on mammalian single skeletal muscle Ca2+ channels. J Physiol. 1993 Feb;461:103–118. doi: 10.1113/jphysiol.1993.sp019504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nastainczyk W., Ludwig A., Hofmann F. The dihydropyridine-sensitive calcium channel of the skeletal muscle: biochemistry and structure. Gen Physiol Biophys. 1990 Aug;9(4):321–329. [PubMed] [Google Scholar]
- Plaitakis A., Constantakakis E., Smith J. The neuroexcitotoxic amino acids glutamate and aspartate are altered in the spinal cord and brain in amyotrophic lateral sclerosis. Ann Neurol. 1988 Sep;24(3):446–449. doi: 10.1002/ana.410240314. [DOI] [PubMed] [Google Scholar]
- Protti D. A., Szczupak L., Scornik F. S., Uchitel O. D. Effect of omega-conotoxin GVIA on neurotransmitter release at the mouse neuromuscular junction. Brain Res. 1991 Aug 23;557(1-2):336–339. doi: 10.1016/0006-8993(91)90156-p. [DOI] [PubMed] [Google Scholar]
- Rosen D. R., Siddique T., Patterson D., Figlewicz D. A., Sapp P., Hentati A., Donaldson D., Goto J., O'Regan J. P., Deng H. X. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993 Mar 4;362(6415):59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. R., Wong E., Dalmau J., Manley G., Posner J. B., Sher E., Furneaux H. M. Cloning and characterization of a Lambert-Eaton myasthenic syndrome antigen. Ann Neurol. 1993 Jan;33(1):113–120. doi: 10.1002/ana.410330126. [DOI] [PubMed] [Google Scholar]
- Rothstein J. D., Martin L. J., Kuncl R. W. Decreased glutamate transport by the brain and spinal cord in amyotrophic lateral sclerosis. N Engl J Med. 1992 May 28;326(22):1464–1468. doi: 10.1056/NEJM199205283262204. [DOI] [PubMed] [Google Scholar]
- Roveri A., Coassin M., Maiorino M., Zamburlini A., van Amsterdam F. T., Ratti E., Ursini F. Effect of hydrogen peroxide on calcium homeostasis in smooth muscle cells. Arch Biochem Biophys. 1992 Sep;297(2):265–270. doi: 10.1016/0003-9861(92)90671-i. [DOI] [PubMed] [Google Scholar]
- Salazar-Grueso E. F., Kim S., Kim H. Embryonic mouse spinal cord motor neuron hybrid cells. Neuroreport. 1991 Sep;2(9):505–508. doi: 10.1097/00001756-199109000-00002. [DOI] [PubMed] [Google Scholar]
- Smith R. G., Appel S. H. Extracts of skeletal muscle increase neurite outgrowth and cholinergic activity of fetal rat spinal motor neurons. Science. 1983 Mar 4;219(4588):1079–1081. doi: 10.1126/science.6823568. [DOI] [PubMed] [Google Scholar]
- Smith R. G., Engelhardt J. I., Tajti J., Appel S. H. Experimental immune-mediated motor neuron diseases: models for human ALS. Brain Res Bull. 1993;30(3-4):373–380. doi: 10.1016/0361-9230(93)90268-g. [DOI] [PubMed] [Google Scholar]
- Smith R. G., Hamilton S., Hofmann F., Schneider T., Nastainczyk W., Birnbaumer L., Stefani E., Appel S. H. Serum antibodies to L-type calcium channels in patients with amyotrophic lateral sclerosis. N Engl J Med. 1992 Dec 10;327(24):1721–1728. doi: 10.1056/NEJM199212103272405. [DOI] [PubMed] [Google Scholar]
- Touzeau G., Kato A. C. ALS serum has no effect on three enzymatic activities in cultured human spinal cord neurons. Neurology. 1986 Apr;36(4):573–576. doi: 10.1212/wnl.36.4.573. [DOI] [PubMed] [Google Scholar]
- Uchitel O. D., Protti D. A., Sanchez V., Cherksey B. D., Sugimori M., Llinás R. P-type voltage-dependent calcium channel mediates presynaptic calcium influx and transmitter release in mammalian synapses. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3330–3333. doi: 10.1073/pnas.89.8.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent A., Lang B., Newsom-Davis J. Autoimmunity to the voltage-gated calcium channel underlies the Lambert-Eaton myasthenic syndrome, a paraneoplastic disorder. Trends Neurosci. 1989 Dec;12(12):496–502. doi: 10.1016/0166-2236(89)90109-4. [DOI] [PubMed] [Google Scholar]
- Wolfgram F., Myers L. Amyotrophic lateral sclerosis: effect of serum on anterior horn cells in tissue culture. Science. 1973 Feb 9;179(4073):579–580. doi: 10.1126/science.179.4073.579. [DOI] [PubMed] [Google Scholar]
- Zhang J. H., Morita Y., Hironaka T., Emson P. C., Tohyama M. Ontological study of calbindin-D28k-like and parvalbumin-like immunoreactivities in rat spinal cord and dorsal root ganglia. J Comp Neurol. 1990 Dec 22;302(4):715–728. doi: 10.1002/cne.903020404. [DOI] [PubMed] [Google Scholar]