Abstract
OBJECTIVES
The objective of the present study was to evaluate short- and mid-term outcomes of the left subclavian artery (LSA) chimney stent implantation (LSACSI) during thoracic endovascular aortic repair (TEVAR), and to summarize our experience with this technique.
METHODS
From June 2010 to September 2012, 59 patients (49 men; mean age of 57.4 ± 13.3 years, range from 26 to 83 years) who underwent TEVAR and LSACSI were enrolled. Patients suffered from Stanford type B aortic dissection (n = 27), penetrating aortic ulcer (n = 18), aortic arch aneurysm (n = 9), pseudoaneurysm of the aortic arch (n = 4) and proximal type I endoleak after TEVAR of aortic dissection (n = 1). Elective settings were performed in 72% and emergent in 38% of all patients. Follow-up was performed at postoperative 3 months, 6 months and yearly thereafter.
RESULTS
The technical success rate was 98.3% (58/59), and 69 thoracic stent grafts were used. Sixty-two chimney stents, including 55 uncovered and 7 covered stents, were implanted in 59 LSAs. The overall immediate endoleak rate was 15.3% (9/59); type I endoleak was observed in 5 patients and type II in 4 patients. The difference in the immediate endoleak rate related to the anatomy between the outer and the inner curvature was statistically significant (35 vs 4%, P = 0.018). Chimney stent compression was observed in 3 patients and another stent was deployed inside the first one. Perioperative complications included stroke (3.4%, 2/59) and left upper limb ischaemia (1.7%, 1/59). The median follow-up period was 16.5 (range 1–39 months). The mortality rate during follow-up was 5.4% (3/56). Complications during follow-up included endoleak [overall, n = 8 (14.3%, 8/56); type I, n = 5; type II, n = 3], retrograde type A aortic dissection (n = 1), collapse (n = 3, 5.4%) or occlusion (n = 2, 3.6%) of the chimney stent.
CONCLUSIONS
Short- and mid-term results showed that it is feasible to preserve the patency of the LSA in TEVAR with the chimney technique for thoracic aortic pathologies close to the LSA. However, TEVAR combined with LSACSI was not advocated for lesions located at the outer curve of the aortic arch due to a high possibility of endoleak.
Keywords: Endovascular, Aortic arch, Left subclavian artery, Chimney technique
INTRODUCTION
Thoracic endovascular aortic repair (TEVAR) has demonstrated good short- and mid-term results for a variety of thoracic aortic pathologies. For thoracic aortic pathologies with inadequate proximal sealing zones, TEVAR necessitates coverage of the left subclavian artery (LSA) and even the left common carotid artery (LCCA), in order to obtain good fixation of stent grafts. However, covering the origins of these arteries may induce some complications, such as left upper limb ischaemia, posterior circulation ischaemia or cerebral infarction [1, 2]. These considerations make the reconstruction of supra-arch branch vessels necessary for favourable outcomes. TEVAR combined with graft bypass (hybrid procedure) has gained a widespread implementation and extended endovascular treatment options [3]. This technique does not require extracorporeal circulation and hypothermic arrest; morbidity and mortality outcomes can be improved, especially in high-risk patients. However, the long-term durability of hybrid procedures remains to be defined. Endovascular supra-arch branch preservation was successfully attempted using fenestrated, scalloped or branched stent grafts [4, 5], all of which have to be customized before implantation, limiting their applicability in emergent settings. Use of a chimney stent makes it possible to use standard off-the-shelf stent grafts to instantly treat lesions with inadequate sealing zones, providing an alternative in emergent cases [6, 7]. However, reports regarding the application of the chimney technique to revascularize aortic arch branches during TEVAR have been limited to case reports or small series. We retrospectively reviewed our experience and evaluated the effectiveness of TEVAR combined with LSACSI for thoracic aortic pathologies close to the LSA.
METHODS
The study was approved by the Institutional Review Board of Anzhen Hospital affiliated to Capital Medical University (Beijing, China), and informed signed consents were obtained from each patient involved in this study.
From June 2010 to December 2012, a total of 59 patients (49 males; mean 57 ± 13 years, range 26–83) with thoracic aortic pathologies close to the LSA underwent TEVAR and LSACSI at Anzhen Hospital (Beijing, China). Preoperative computed tomography angiography (CTA) measured the length (8.5 ± 2.2 mm) and diameter (30.2 ± 3.2 mm) of the landing zone. Clinical entities included 27 Stanford type B aortic dissections, 18 penetrating aortic ulcers, 9 aortic arch aneurysms, 4 pseudoaneurysms of the aortic arch and 1 proximal endoleak after TEVAR. Of these subjects, 44 had concomitant hypertension, 12 had coronary heart disease, 5 had diabetes mellitus and 5 had cerebrovascular disease. Patients' characteristics are listed in Table 1.
Table 1:
Characteristics of 59 patients
| N or mean | % | |
|---|---|---|
| Age (years) | 57.4 ± 13.3 | |
| Male gender | 49 | 83.1 |
| Main disease | ||
| Aortic dissection | 27 | 45.8 |
| Aortic arch aneurysm | 9 | 15.3 |
| Pseudoaneurysm of the aortic arch | 4 | 6.8 |
| Aortic ulcer | 18 | 30.5 |
| Proximal endoleak following TEVAR | 1 | 1.7 |
| Comorbidities | ||
| Hypertension | 44 | 74.6 |
| Coronary heart disease | 12 | 20.3 |
| Diabetes mellitus | 5 | 8.5 |
| Cerebrovascular disease | 5 | 8.5 |
| Length of the proximal landing zone (mm) | 8.5 ± 2.2 | |
| Diameter of the proximal landing zone (mm) | 30.2 ± 3.2 | |
TEVAR: thoracic endovascular aortic repair.
All patients received local and potentiated anaesthesia. Thoracic aortic stent grafts were implanted through the femoral artery using a surgical (n = 53) or totally percutaneous method (n = 6). Elective settings were performed in 72% and emergent in 38% of all patients. The aortic stent grafts were 10–15% oversized. For 10 patients with long thoracic pathology, implantation of two overlapped thoracic aortic stent grafts was necessary. Types of endografts included Relay (Bolton, Spain; n = 5), Zenith TX2 (Cook, Denmark; n = 9), Valiant (Medtronic, USA; n = 12), Captivia (Medtronic, USA; n = 7), Hercules (MicroPort, China; n = 7), E-vita (JOTEC, Germany; n = 13) and Grikin (GRIKIN, China; n = 10).
In the current study, only one LSA was implanted with a balloon-expandable chimney stent, and the others were implanted with self-expandable chimney stents. Chimney stents were deployed into the LSA through a 6-F arterial sheath after puncturing the left radial artery for the bare stent delivery systems [Sinus (OptiMed, Germany; n = 49), Precise (Cordis, USA; n = 2), Astron (BIOTRONIK, Germany; n = 1) and Hippocampus (Invatec, Italy; n = 1)], or a 9-F sheath by surgically exposing the left brachial artery for the covered stent delivery system [Fluency Plus (Bard, USA; n = 7)]. At the initial stage, we preferred to choose a self-expandable covered chimney stent. However, it needed a large delivery system and was delivered through surgical exposure, greatly affecting the blood supply of the left upper limb and leading to a gap with the aortic stent graft. Instead, the bare stent has both good long-term patency in peripheral vessels and a thinner delivering system, which can be delivered totally percutaneously. Therefore, at the late stage of this study, only one case complicated with an LSA pseudoaneurysm was implanted with the covered chimney stent and all the others were implanted with the bare stent in the LSA.
Concerning the LSACSI, after the wire access was obtained in the LSA, a pigtail catheter was sent into the LSA, through which a super-stiff guidewire was sent into the ascending aorta. Then the pigtail catheter was removed and the chimney stent was delivered into the LSA along the guidewire and placed at the LSA take-off. After the endo-aortic device was deployed, the chimney stent was deployed rapidly parallel to the main aortic stent graft, with 1 cm overlapping and 1 cm protruding proximally. Completion ascending aortic angiogram was performed to evaluate the immediate results (Fig. 1). Technical success was defined as the instant postoperative aortogram demonstrating successful proximal fixation of the aortic stent graft and preserved LSA. The lesion was excluded successfully and the LSA had favourable antegrade blood flow. No severe complications occurred, such as large amount of endoleak, stroke or death. All patients received a hypodermic injection of 5000 U low-molecular-weight heparin once a day for 2–3 days, and then took 100 mg/day aspirin orally for life. Table 2 gives the characteristics of all procedures.
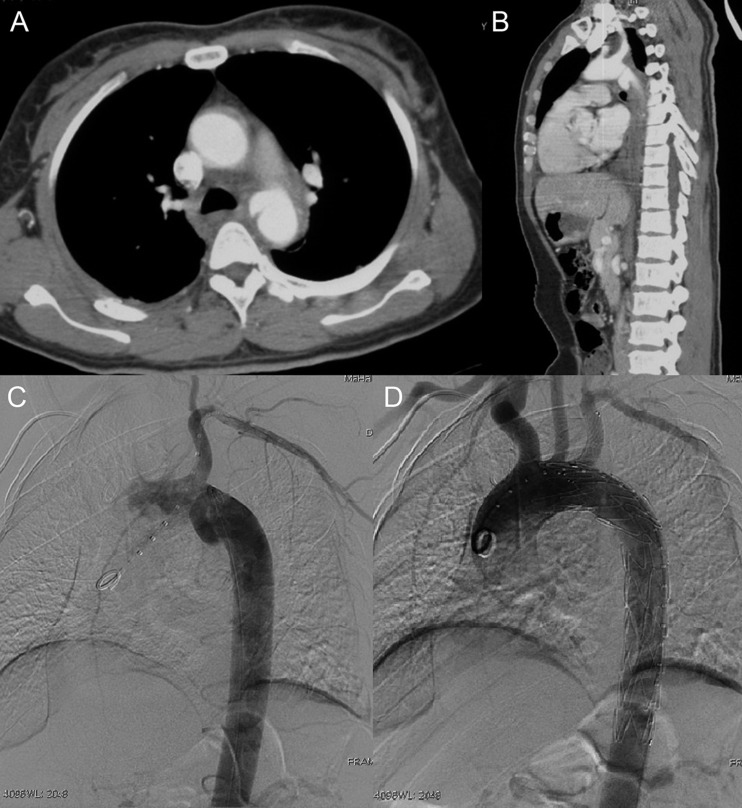
Figure 1:
Emergent left subclavian artery (LSA) chimney stenting in TEVAR for a traumatic aortic pseudoaneurysm. (A and B) Preoperative CTA showed pseudoaneurysm of the aortic arch. (C and D) digital subtraction angiography (DSA) demonstrated total exclusion of the pseudoaneurysm and preserved LSA blood flow after TEVAR plus LSA chimney stent implantation.
Table 2:
TEVAR characteristics
| N or mean | % | |
|---|---|---|
| LSA diameter (mm) | 8.3 ± 2.4 | |
| Distance between LSA and LVA (mm) | 43.1 ± 3.2 | |
| Diameter of chimney stents (mm) | 9.2 ± 1.3 (7–12) | |
| Length of chimney stents (mm) | 41.8 ± 6.5 (24–60) | |
| Chimney stent implantation access | ||
| Excision | 8 | 12.9 |
| Percutaneous | 54 | 87.1 |
| Two chimney stents | 3 | 5.1 |
| Two aortic stent grafts | 10 | 16.9 |
| Diameter of landing zones (mm) | 29.5 ± 2.6 | |
| Diameter of aortic stent grafts (mm) | 32.8 ± 3.2 (24–42) | |
| Length of aortic stent grafts (mm) | 172.9 ± 29.0 (80–230) | |
| Stent graft implantation access | ||
| Excision | 62 | 89.9 |
| Percutaneous | 7 | 10.1 |
| Postoperative immediate endoleak | 9 | 15.3 |
| Stroke | 2 | 3.4 |
| Death during follow-up | 3 | 5.1 |
LSA: left subclavian artery; LVA: left vertebral artery.
Follow-up examinations included chest X-ray and CTA, which were performed at discharge, at postoperative 3, 6 and 12 months, and then yearly thereafter. Follow-up examinations including x-ray and CTA could be finished in any hospitals, but the images were always read by the physicians involved in this study.
SPSS 17.0 (SPSS, Inc., Chicago, IL, USA) was used for statistical analysis. Continuous variables are expressed as mean ± standard deviation. For comparison of the endoleak rate, the continuity corrected chi-square test in a 2 × 2 table was used. A P-value of <0.05 was considered statistically significant.
RESULTS
The technical success rate was 98.3% (58/59). A total of 69 aortic stent grafts were used. The diameter of aortic stent grafts was 32.8 ± 3.2 mm (range 24–42 mm), and the length was 172.9 ± 29.0 mm (range 80–230 mm). Chimney stent compression was observed in 3 patients and another stent was deployed inside the first one. A total of 62 chimney stents, including 55 bare and 7 covered stents, were deployed in 59 LSAs. The diameter of all chimney stents was 9.2 ± 1.3 mm (range 7–12 mm), and the length was 41.8 ± 6.5 mm (range 24–60 mm).
Immediate postoperative endoleak was detected in 9 cases (9/59, 15.3%) on postoperative aortogram, including 5 type I and 4 type II, in most (88.9%, 8/9) of which the lesion was located at the outer curve of the aortic arch (versus lesions located at the inner curve, P = 0.018). Perioperative complications included stroke (n = 2), distal left radial arterial occlusion (n = 1), and sharp chest and back pain possibly arising from acute spinal cord ischaemia (SCI; n = 1). The incidence rate of stroke was 3.4% (2/59). Characteristics of the population detected on immediate postoperative endoleak are listed in Table 3.
Table 3:
Characteristics of the population detected with immediate postoperative endoleak
| Patient | Sex/age | Aortic pathology | Location of lesion | Procedural status | Endograft (type and size) | Chimney stent (type and size) | Endoleak |
||
|---|---|---|---|---|---|---|---|---|---|
| Type | Volume | Follow-up result | |||||||
| 1 | M/56 | AD (chronic) | Outer curvature | Elective | E-vita 36 × 170 | Covered/Fluency 8 × 40 | Ia | Medium | Resolved |
| 2 | M/59 | AD (acute) | Outer curvature | Elective | Relay 28 × 145 | Covered/Fluency 10 × 40 | Ia | Slight | Diminished |
| 3 | M/79 | PAU | Outer curvature | Elective | E-vita 33 × 170 | Covered/Fluency 8 × 40 | Ia | Slight | Resolved |
| 4 | M/60 | TAA, PAU | Outer curvature | Elective | E-vita 36 × 230, Hercules 34 × 80 |
Bare/Sinus 10 × 40 | II | Slight | Resolved |
| 5 | M/53 | AD (subacute) | Outer curvature | Elective | Valiant 30 × 200 | Bare/Sinus 10 × 40 | Ia | Medium | Diminished |
| 6 | M/51 | AD (chronic) | Inner curvature | Elective | Grikin 30 × 180 | Bare/Sinus 8 × 40 | Ia | Slight | No change |
| 7 | M/62 | TAA | Outer curvature | Elective | E-vita 33 × 230 | Bare/Sinus 8 × 40 | II | Slight | No change |
| 8 | M/45 | PAU, IMH (acute) | Outer curvature | Elective | E-vita 33 × 230 | Bare/Sinus 10 × 40 | II | Slight | Increased |
| 9 | M/41 | AD (subacute) | Outer curvature | Elective | Captivia 30 × 150 | Bare/Sinus 10 × 40 | II | Medium | Diminished, but converted to retrograde type A AD |
AD: aortic dissection; TAA: thoracic aortic aneurysm; PAU: penetrating aortic ulcer; IMH: intramural haematoma.
Among the 59 subjects, 56 (91.5%) patients were successfully followed up with a median period of 16.5 months. During follow-up, 3 patients died. One died of abrupt haemoptosis of unclear reason. One died of a ruptured pseudoaneurysm secondary to surgical replacement of the thoraco-abdominal aorta for dissected aortic aneurysm 19 months after TEVAR. The other died of cerebral infarction 19 months after TEVAR. Five patients suffered from chimney stent collapse or occlusion, but no reintervention was needed. All the other patients showed good LSA patency (Fig. 2). Among the 9 cases with immediate endoleak, 3 resolved spontaneously, 3 diminished, 1 increased and the other 2 were stable without clinical consequences during follow-up. However, there were three newly generated endoleaks. The incidence rate of endoleak during follow-up was 14.3% (8/56).
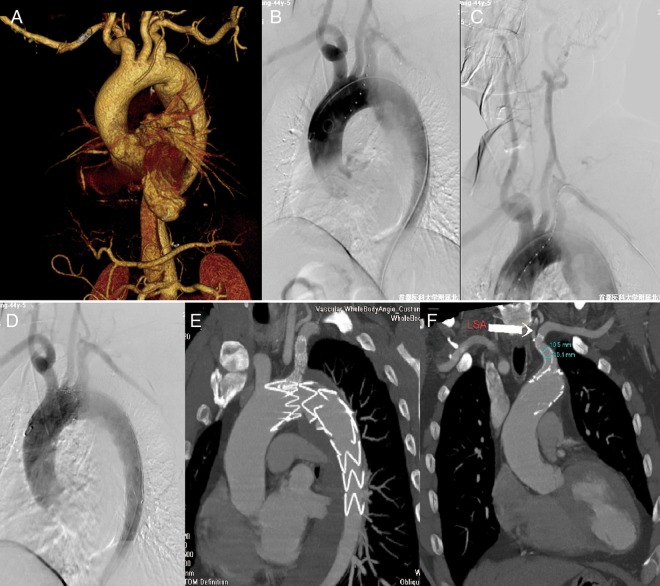
Figure 2:
Left subclavian artery (LSA) chimney stenting in TEVAR for type B aortic dissection. (A) Preoperative CTA showed type B aortic dissection with the tear close to the LSA. (B and C) Intraoperative DSA demonstrated type B aortic dissection with the tear close to the LSA and the left dominant vertebral artery. (D) Postoperative DSA showed total exclusion of the tear and preserved LSA blood flow. (E and F) Follow-up CTA showed thrombosis of the false lumen around the aortic stent graft and a patent chimney stent in the LSA.
DISCUSSION
In 2002, Criado et al. [8] used stent implantation to reconstruct covered aortic branch vessels. In 2003, Greenberg et al. [9] used this same method to treat abdominal aortic aneurysm with an insufficient proximal landing zone. To maintain blood flow of the renal artery, they implanted another renal stent parallel to the aortic stent graft. In 2007, Criado [10] systemically introduced this technique for aortic endovascular repair. Considering the parallel relationship of the stent in the branch vessel with the proximal stent graft in the aorta, the stent in the branch vessel was defined as the chimney stent, and the technique was named the chimney technique [11]. In the past few years, this technique has been increasingly reported to expand endograft repair in the aortic arch. However, reports about the chimney technique in TEVAR have been limited to small series with a short follow-up [7, 12]. This study may be the largest reported series about the application of the chimney technique in TEVAR at a single medical centre so far including mid-term results. We evaluated the available data regarding LSACSI in TEVAR and summarized our experience with it.
As we know, the LSA provides extensive circulation to the left upper limb, spinal cord and brain. Consequently, intentional coverage of this great vessel might not be physiologically tolerated. However, LSA coverage was necessary to achieve good proximal fixation for thoracic aortic pathologies with an insufficient proximal landing zone. To keep antegrade blood supply to the LSA, both surgical and endovascular methods were attempted. While the result of surgical bypass was satisfactory, it was more invasive and had associated complications, which was not recommended for high-risk patients (e.g. comorbidities, advanced age or low cardiac function). The introduction of fenestrated and branched endografts has made it possible to preserve blood flow of the LSA with a totally percutaneous method. However, these stent grafts had to be custom-made based on the patients' individual anatomy and were thus time-consuming, limiting their application in urgent situations. The chimney technique may be taken as a less invasive alternative not only in emergent cases, but also as a routine technique in TEVAR.
Concerning the indication of LSACSI in TEVAR, it can be applied in all cases requiring the LSA opening to be preserved after coverage of it in TEVAR, especially for those with dominant left vertebral artery and severely stenotic or occlusive right vertebral artery or congenital right vertebral artery dysplasia [13], and for those with left upper extremity dialysis access and pedicled left internal mammary grafts [14]. One patient with thoracic aortic aneurysm presented with dizziness, nausea and blood pressure elevation after the LSA was completely covered by the aortic stent graft. The chimney stent was instantly implanted into the LSA, and then symptomatic and antihypertensive treatments were provided. Although blood pressure returned to normal after a short time, the patient suddenly became unconscious despite 100% blood oxygen saturation, equally responsive pupils and normal blood gas results. Then left radial artery pulse diminution was detected. Urgent angiogram of the aortic arch and brachiocephalic arteries detected stenosis of the right internal carotid artery (70%) and the right vertebral artery (70%), and diminished antegrade LSA blood flow and LSA stealing, but no intracranial haemorrhage. After local anaesthesia was converted to general anaesthesia, the left brachial artery was exposed and punctured. The guidewire was inserted into the ascending aorta through the LSA. A Cook balloon catheter was deployed into the chimney stent to dilate it and another chimney stent was implanted. Completion ascending aortogram showed favourable LSA antegrade blood flow. However, postoperative brain CT scan showed bilateral cerebellar infarction of this patient. This further demonstrated the necessity of LSA revascularization during TEVAR for some patients with cerebrovascular disease.
SCI is a devastating complication after TEVAR [15]. Risk factors for SCI after TEVAR have been previously reported to include length of aortic coverage [16], prior abdominal aortic aneurysm repair, hypotension, iliac artery injury, renal failure and LSA coverage. For patients with both long aortic coverage and LSA coverage, LSA revascularization was necessary to maintain perfusion of the spinal cord. In this group, 13 cases had aortic coverage more than 230 mm in length and simultaneous LSA coverage. LSACSI was performed instantly to restore the blood supply of the LSA. There was no paraplegia observed in this group, which was consistent with the results of other studies [17]. Only one patient developed sharp back pain arising from suspected acute spinal cord ischaemia after the deployment of the aortic stent graft and LSA coverage, and the symptom resolved after LSACSI. We suggest that patients associated with risk factors for SCI as mentioned above should be considered for LSACSI in TEVAR.
Stroke is another major complication of intentional LSA coverage in TEVAR [18]. In this group, 2 patients developed abrupt loss of consciousness after deployment of the aortic stent graft and instantly LSACSI was performed. Postoperative CT scan of the brain demonstrated hemispheric cerebral infarction in both patients. The stroke rate in this group was 3.4% (2/59), which was unrelated with chimney stent implantation (CSI). The reported stroke rate in a review about the chimney-graft technique for preserving supra-aortic branches was 4% [17].
The chimney technique is expected to prevent an endoleak by prolonging the proximal landing zone. However, it was reported that the deployment of the chimney stent may potentially displace the aortic stent graft and even induce an endoleak [19]. Furthermore, the inadequacy of the proximal sealing zone has been reported to be a main cause of failure to exclude the entry tear or aneurysmal sac, especially when the tear or aneurysmal sac is located at the outer curvature of the aortic arch [20], potentially resulting in a type Ia endoleak. We observed 9 (9/59, 15.3%) immediate endoleaks, including 5 type I and 4 type II. The difference in the immediate endoleak rate related to the anatomy between the outer and the inner curvature was statistically significant (35 vs 4%, P = 0.018). Therefore, the chimney technique should not be recommended for aortic dissection with the tear or false lumen located at the greater curvature [21], or for a true aneurysm with fusiform intumescence [22]. In this case, endoleak management was very difficult. For endovascular abdominal aortic repair (EVAR), type I endoleak after the procedure can be treated by expanding the aortic stent graft and the chimney stent with a kissing balloon technique [23]. However, the safety of this approach in TEVAR needs further study.
It is reported that retrograde type A aortic dissection is a rare but lethal complication after TEVAR. The incidence rate of retrograde type A aortic dissection after TEVAR was ∼2.5% [24]. One patient with acute type B aortic dissection in this group developed a type II endoleak after TEVAR, which can increase the shear stress on the aortic arch, strengthening interaction among the aortic stent graft, the chimney stent and the fragile aortic wall and causing retrograde type A aortic dissection 3.5 months after TEVAR. He was converted to open surgery and underwent total arch replacement combined with stented elephant trunk implantation.
It is crucial to select and deploy the aortic stent graft to achieve technical success. The radial supporting power of the aortic stent graft is positively associated with the long-term patency of the chimney stent. Reinforced stent grafts with no proximal bare components are more beneficial for the deployment of the chimney stent. Considering long-term patency, we do not recommend that the aortic stent graft go too far from the LSA opening. The LSA chimney technique is more suitable if the proximal landing zone can be obtained just at the region where the aortic stent graft covers the LSA opening [25]. Otherwise, technologies other than the chimney technique should be recommended. In this study, among 5 cases with chimney stent collapse or occlusion, the aortic stent was found to be close to the posterior edge of the LCCA in 4 cases. We inferred that the patency of the chimney stent decreased as the distance increased between the proximal end of the aortic stent graft and the LSA opening.
Both covered and uncovered stents can be used in the chimney technique. However, consensus has not been reached about which is more suitable. The covered stent may be more helpful for establishing a blood channel and to decrease the risk of endoleak around the chimney stent [11, 25]. However, there is still a gap between the chimney stent and the aortic stent graft, and the risk of endoleak still exists. On the other hand, the bare stent has both good long-term patency in peripheral vessels and a thinner delivery system on the basis of the same chimney efficacy. Zhu et al. [25] compared the two types of stents as chimney stents to treat aortic dissection and concluded that the bare stent seemed to be related with a higher probability of the immediate proximal type I endoleak. How to select the approach to deploy the chimney stent is also very important. Owing to the large delivery system, the covered stent has to be implanted by surgical left brachial artery access, whereas the bare stent can be implanted by percutaneous left radial artery access. It is best to first place the chimney stent at the ostium of the LSA, and then deploy the aortic stent graft, followed by deployment of the chimney stent. The proximal end of the chimney stent should properly exceed the proximal aortic stent graft (1 cm is better) to prevent the opening from being covered by the aortic stent graft, and to prevent a too long anterior free segment to reduce thrombosis formation [7]. The chimney stent should also have a proper landing zone in the LSA.
The primary limitation of this study was that comparisons between the CSI group and supra-arch branch bypass combined with endograft implantation (hybrid procedure) group were not made. Though the preliminary result of the LSA chimney technique in TEVAR was satisfactory, it is necessary to evaluate whether this technique for aortic pathologies close to the LSA can improve the long-term outcome.
CONCLUSION
Short- and mid-term results showed that it is feasible to preserve the patency of the LSA in TEVAR with the chimney technique for thoracic aortic pathologies close to the LSA, especially for lesions in the lesser curvature of the aortic arch. However, we cannot come to any conclusion on which kind of chimney stent should be the first choice between covered and bare ones, and also between self-expandable and balloon-expandable ones. Further control studies are necessary to identify which kind of chimney stent should be the first choice.
Funding
This study was supported by The National Natural Science Foundation (no. 81270388). Funding to pay the Open Access publication charges for this article was provided by the National Natural Science Foundation.
Conflict of interest: none declared.
ACKNOWLEDGEMENTS
The authors thank Weiguo Ma, Yu Li and Ruidong Qi for their advice with regard to the revision of this article. The authors thank The National Natural Science Foundation for their funding support to this study.
REFERENCES
- 1.Chung J, Kasirajan K, Veeraswamy RK, Dodson TF, Salam AA, Chaikof EL, et al. Left subclavian artery coverage during thoracic endovascular aortic repair and risk of perioperative stroke or death. J Vasc Surg. 2011;54:979–84. doi: 10.1016/j.jvs.2011.03.270. [DOI] [PubMed] [Google Scholar]
- 2.Manninen H, Tulla H, Vanninen R, Ronkainen A. Endangered cerebral blood supply after closure of left subclavian artery: postmortem and clinical imaging studies. Ann Thorac Surg. 2008;85:120–5. doi: 10.1016/j.athoracsur.2007.08.035. [DOI] [PubMed] [Google Scholar]
- 3.Schoder M, Lammer J, Czerny M. Endovascular aortic arch repair: hopes and certainties. Eur J Vasc Endovasc Surg. 2009;38:255–61. doi: 10.1016/j.ejvs.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Hongo N, Miyamoto S, Shuto R, Wada T, Matsumoto S, Kiyosue H, et al. Endovascular aortic arch reconstruction using in situ stent-graft fenestration in the brachiocephalic artery. J Vasc Intervent Radiol. 2011;22:1144–8. doi: 10.1016/j.jvir.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Shahverdyan R, Gawenda M, Brunkwall J. Triple-barrel graft as a novel strategy to preserve supra-aortic branches in arch-TEVAR procedures: clinical study and systematic review. Eur J Vasc Endovasc Surg. 2013;45:28–35. doi: 10.1016/j.ejvs.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 6.Yang J, Xiong J, Liu X, Jia X, Zhu Y, Guo W. Endovascular chimney technique of aortic arch pathologies: a systematic review. Ann Vasc Surg. 2012;26:1014–21. doi: 10.1016/j.avsg.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 7.Gehringhoff B, Torsello G, Pitoulias GA, Austermann M, Donas KP. Use of chimney grafts in aortic arch pathologies involving the supra-aortic branches. J Endovasc Ther. 2011;18:650–5. doi: 10.1583/11-3504.1. [DOI] [PubMed] [Google Scholar]
- 8.Criado FJ, Barnatan MF, Rizk Y, Clark NS, Wang CF. Technical strategies to expand stent-graft applicability in the aortic arch and proximal descending thoracic aorta. J Endovasc Ther. 2002;9(Suppl 2):II32–8. [PubMed] [Google Scholar]
- 9.Greenberg RK, Clair D, Srivastava S, Bhandari G, Turc A, Hampton J, et al. Should patients with challenging anatomy be offered endovascular aneurysm repair? J Vasc Surg. 2003;38:990–6. doi: 10.1016/s0741-5214(03)00896-6. [DOI] [PubMed] [Google Scholar]
- 10.Criado FJ. A percutaneous technique for preservation of arch branch patency during thoracic endovascular aortic repair (TEVAR): retrograde catheterization and stenting. J Endovasc Ther. 2007;14:54–8. doi: 10.1583/06-2010.1. [DOI] [PubMed] [Google Scholar]
- 11.Criado FJ. Chimney grafts and bare stents: aortic branch preservation revisited. J Endovasc Ther. 2007;14:823–4. doi: 10.1583/07-2247.1. [DOI] [PubMed] [Google Scholar]
- 12.Shu C, Luo MY, Li QM, Li M, Wang T, He H. Early results of left carotid chimney technique in endovascular repair of acute non-a-non-B aortic dissections. J Endovasc Ther. 2011;18:477–84. doi: 10.1583/11-3401.1. [DOI] [PubMed] [Google Scholar]
- 13.Feezor RJ, Lee WA. Management of the left subclavian artery during TEVAR. Semin Vasc Surg. 2009;22:159–64. doi: 10.1053/j.semvascsurg.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 14.Weigang E, Parker JA, Czerny M, Lonn L, Bonser RS, Carrel TP, et al. Should intentional endovascular stent-graft coverage of the left subclavian artery be preceded by prophylactic revascularisation? Eur J Cardiothorac Surg. 2011;40:858–68. doi: 10.1016/j.ejcts.2011.01.046. [DOI] [PubMed] [Google Scholar]
- 15.Zipfel B, Buz S, Redlin M, Hullmeine D, Hammerschmidt R, Hetzer R. Spinal cord ischemia after thoracic stent-grafting: causes apart from intercostal artery coverage. Ann Thorac Surg. 2013;96:31–8. doi: 10.1016/j.athoracsur.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 16.Feezor RJ, Martin TD, Hess PJ, Jr, Daniels MJ, Beaver TM, Klodell CT, et al. Extent of aortic coverage and incidence of spinal cord ischemia after thoracic endovascular aneurysm repair. Ann Thorac Surg. 2008;86:1809–14. doi: 10.1016/j.athoracsur.2008.09.022. discussion 14. [DOI] [PubMed] [Google Scholar]
- 17.Moulakakis KG, Mylonas SN, Dalainas I, Sfyroeras GS, Markatis F, Kotsis T, et al. The chimney-graft technique for preserving supra-aortic branches: a review. Ann Cardiothorac Surg. 2013;2:339–46. doi: 10.3978/j.issn.2225-319X.2013.05.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cooper DG, Walsh SR, Sadat U, Noorani A, Hayes PD, Boyle JR. Neurological complications after left subclavian artery coverage during thoracic endovascular aortic repair: a systematic review and meta-analysis. J Vasc Surg. 2009;49:1594–601. doi: 10.1016/j.jvs.2008.12.075. [DOI] [PubMed] [Google Scholar]
- 19.Ohrlander T, Sonesson B, Ivancev K, Resch T, Dias N, Malina M. The chimney graft: a technique for preserving or rescuing aortic branch vessels in stent-graft sealing zones. J Endovasc Ther. 2008;15:427–32. doi: 10.1583/07-2315.1. [DOI] [PubMed] [Google Scholar]
- 20.Pamler RS, Kotsis T, Gorich J, Kapfer X, Orend KH, Sunder-Plassmann L. Complications after endovascular repair of type B aortic dissection. J Endovasc Ther. 2002;9:822–8. doi: 10.1177/152660280200900616. [DOI] [PubMed] [Google Scholar]
- 21.Sze DY, van den Bosch MA, Dake MD, Miller DC, Hofmann LV, Varghese R, et al. Factors portending endoleak formation after thoracic aortic stent-graft repair of complicated aortic dissection. Circ Cardiovasc Interv. 2009;2:105–12. doi: 10.1161/CIRCINTERVENTIONS.108.819722. [DOI] [PubMed] [Google Scholar]
- 22.Piffaretti G, Mariscalco G, Lomazzi C, Rivolta N, Riva F, Tozzi M, et al. Predictive factors for endoleaks after thoracic aortic aneurysm endograft repair. J Thorac Cardiovasc Surg. 2009;138:880–5. doi: 10.1016/j.jtcvs.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 23.Bin Jabr A, Sonesson B, Lindblad B, Dias N, Resch T, Malina M. Chimney grafts preserve visceral flow and allow safe stenting of juxtarenal aortic occlusion. J Vasc Surg. 2013;57:399–405. doi: 10.1016/j.jvs.2012.08.108. [DOI] [PubMed] [Google Scholar]
- 24.Dong ZH, Fu WG, Wang YQ, Guo da Q, Xu X, Ji Y, et al. Retrograde type A aortic dissection after endovascular stent graft placement for treatment of type B dissection. Circulation. 2009;119:735–41. doi: 10.1161/CIRCULATIONAHA.107.759076. [DOI] [PubMed] [Google Scholar]
- 25.Zhu Y, Guo W, Liu X, Jia X, Xiong J, Wang L. The single-centre experience of the supra-arch chimney technique in endovascular repair of type B aortic dissections. Eur J Vasc Endovasc Surg. 2013;45:633–8. doi: 10.1016/j.ejvs.2013.02.016. [DOI] [PubMed] [Google Scholar]