Abstract
Uncontrolled hemorrhage has been considered as one of the most important factors for causing death on the battlefront. If given timely and efficient hemostatic medicines in pre-hospital setting, patients will obtain more time and chance to wait for medical treatment so as to save their lives. However, there is not a certain answer about which kind of hemostatic drugs can achieve efficacious effect to hemostasis in the battle. This review aims to summarize effective hemostatic medicines applied in battlefield from 41 articles. After analyzing and comparing the efficacy and complications of those products, we conclude that Fibrin Sealant Dressing, Celox and Woundstat are prior to other materials to stanch life-threatening extremity hemorrhage on the battlefield based on present research in the related area. Therefore, in the prevalence of some inevitable battlefield throughout the world, especially in the Middle Eastern countries, our findings suggest for the first time that the effective hemostatic device is not only a key point to link pre-hospital and hospital care but also an essential way to increase the survival rate of battlefront in the foreseeable future.
Keywords: Battlefield, hemostatic, celox, woundstat, farin sealant dressing
Introduction
Uncontrolled hemorrhage in the war field has been recognized as the greatest threat to soldiers’ life through history [1]. It was estimated that injuries in major arteries with hemorrhage probably accounted for 50% and 31% of the total death in the war and civilian setting, respectively [2-5]. Army surgeons in 1993 also identified a significant high mortality due to bleeding among soldiers in Somalia [1,6]. It was well known that huge loss of blood reduced the blood volume for circulation, leading to obstruction of human body microcirculation and hypoxia in brain and other organs. If the situation continued to develop without effective intervention, uncontrolled hemorrhagic shock or other severe attacks might happen. Furthermore, the complications of hemorrhage-hypothermia and metabolic acidosis which disturbed the blood coagulation system made hemostasis more challenging [7]. Moreover, large amount of blood transfusion due to hemorrhage increased the risk of multiple organ failure and made the follow-up treatment more difficult [8,9]. Especially in the battle front, accompanying complex geographical conditions and urgency of warfare, soldiers suffering severe bleeding could not always get helped. Bellay’s land mark essays reported that about 9% of the death in Vietnam War caused by extremity hemorrhage could be prevented by effective hemostasis method in 1984 [1]. Controlling hemorrhage in pre-hospital treatment could reduce morbidity, which was also evidenced by United States military personnel [1,10,11]. Therefore, effective pre-hospital hemostatic care should be given as soon as possible in battlefield.
Although wars in modern times are fewer than before, the destructiveness of new weapons applied at the battle is more powerful. Under this circumstance, it is more urgent to apply effective hemostatic agents in war to control bleeding. The hemostatic materials Hemcon and QickClot are widely used in the battle and proved to be the most effective ways to stop bleeding. However, as new agents get to emerge and the side effects of those well-known efficient devices have been exposed gradually, people start to doubt the efficacy of them. Although plenty of papers were published to demonstrate the best hemostasis method in war field, no final conclusion has been reached. To find out a definite answer to that issue, a review to explore and evaluate effective hemostasis medicines is presented and we find that Fibrin Sealant dressing, Celox and Woundstat are more effective to stanch hemorrhage than others.
Methods
Approaches searching for literatures
This review collected relevant articles from Science Director, Ovid Medline and PubMed up to April, 2014. There was no limitation on language in our collection. The search terminology are: (((((((hemorrhage [MeSH Terms]) OR hemorrhage [All Fields]) OR blood loss [All Fields]) OR ((blood [All Fields]) AND loss [All Fields]))) AND (((((((injuries [All Fields]) OR trauma [All Fields])) OR ((wounds [All Fields]) AND injuries [All Fields])) OR (((wounds and injuries [All Fields])) OR (wounds and injuries [MeSH Terms])))) AND ((war [All Fields]) OR battle [All Fields])))) AND (((haemostasis [All Fields]) OR hemostasis [MeSH Terms]) OR hemostasis [All Fields]).
Inclusion standards
Those articles which reported effective results or reached a conclusion benefiting controlling hemorrhage were accepted, while any individual or personal cases werenot included even though they were somewhat meaningful. Articles could be about experiments on animals, demonstrating the efficacy of a method or retrospective surveys of soldiers in battle field with definite outcomes. Figure 1 showed the detailed results of searching. The results were addressed separately by two partners through applying the abovecriteria and no difference was found between the two.
Figure 1.
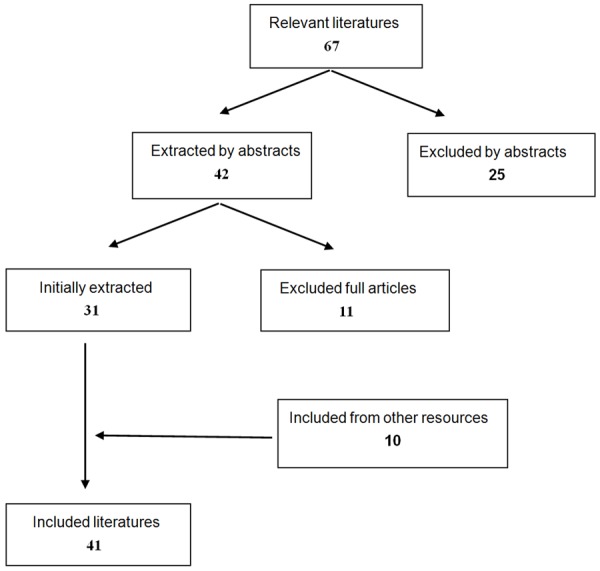
Searching results.
Information abstracted and integrated
Data collected about the number of subjects, intervention and final results were the main objects of the study. Besides, some information about the weight of animals or age of patients were gathered.
Results
Analysis on the included articles
The significant ones of 41 studies were recorded in the review (Tables 1 and 2). Among them, 3 were descriptive and 38 were comparative essays. The majority of the studies were suitable for application in emergency conditions because of convenience and efficacy. Additionally, the methods described above were all employed externally, which made hemostasis in wounds easier for caregivers.
Table 1.
The basic information of the human survey included in this review
Table 2.
The basic information of the animal study included in this review
| Ref. | Type of article | Intervention | Number | Weight (kg) |
|---|---|---|---|---|
| [13] | Comparative | ND, SD, (SD+QC), (SD+RDH), (SD+TDEX) | 30 swine | 42~55 kg |
| [14] | Comparative | ND, SD, (SD+1% ZH), (SD+4% ZH), (SD+1% ZH 2oz), (SD+8% ZH), (SD+HC), othersa | 72 swine | 37±0.8 kg |
| [15] | Comparative | QC, gauze sponge | 16 swine | 38.8~39.7 kg |
| [16] | Comparative | QC, biological agents | 8 swine | 75~100 kg |
| [19] | Comparative | QCG, AFB | 30 swine | NS |
| [20] | Comparative | QCG, QCX, CX, HCG, CTG | 50 pigs | 34~45 kg |
| [21] | Comparative | HCG, CG | 14 swine | 35~45 kg |
| [27] | Comparative | HC Bandage, SMC | 9 sheep | 56~70 kg |
| [28] | Comparative | QC, Actcel, HC, CX combination with tourniquet | 50 swine | NS |
| [29] | Comparative | WS, HC, CX, SQR | 46 swine | 37 kg |
| [30] | Comparative | CX, ACS+, HC, WS | 30 swine | NS |
| [31] | Comparative | CX, HC, QC, SD | 46 swine | 35.5±1.1 kg |
| [32] | Comparative | ACS+, CX, IC, WS, AB, BLS, XS, CHI, HC, HP-21, SD | 88 swine | 25-35 kg |
| [33] | Comparative | ACS+, CX, IC, WS, BLS, XS, CHI, AB, HC, FP-21, SD | 88 swine | 25-35 kg |
| [34] | Comparative | FSD bandage, HC, AFD | 19 pigs | 37.2±3.3 kg |
| [35] | Comparative | FSD, SD | 15 pigs | 38.6±3.1 kg |
| [36] | Comparative | FSD powder, HC Bandage, QC, AFB | 60 swine | 37.7±2.5 kg |
| [37] | Comparative | FSD, HC, suture, othersb | 59 pigs | 41.9±0.4 kg |
| [38] | Comparative | Bandage containing fibrinogen and thrombin, plain gauze | 6 swine | NS |
| [39] | Comparative | Gauze bandage, DFSD, IgG placebo sealant dressing, ND | 21 swine | 40±3.0 kg |
| [40] | Comparative | DFSD, gauze bandage, IgG placebo sealant dressing | 19 swine | 39.7 kg |
| [41] | Comparative | Chitosan dressing, gauze sponges | 15 swine | NS |
| [42] | Comparative | WS, HC bandage, ACS, QCG, AFB | 25 swine | 42±3 kg |
| [43] | Comparative | WSc, QCG | 14 swine | 35~44 kg |
| [44] | Comparative | WS, AFB, CHI | 24 swine | NS |
| [45] | Comparative | WS, SD | 21 swine | NS |
| [46] | Comparative | WS, CA, QCG, CHI | 80 swine | 43.0±7.7 kg |
QC, QuikClot agent; HC, HemCon dressing; ND, no dressing; SD, standard dressing; RDH, Rapid Deployment Hemostat; TDEX, TraumaDEX; 1% ZH, 3.5 ozQuikClot with 1% residual moisture; NS, not stated; 4% ZH, 3.5 ozQuikClot with 4% residual moisturz; alimbs; 1% ZH 2oz, 2 oz ZH with 1% residual moisture; 8% ZH, 3.5 ozQuikClot with 8% residual moisture;
SD+nonzeolite mineral hemostat (NZH), SD+bovine clotting factor hemostat, Fast Act (FA), SD+TraumaDex 30 g (TDEX);
QCG, QuikClot Combat Gauze; AFB, Army Field Bandage; QCX, QuikClot CombatGauze XL; HCG, ChitoGauze; CTG, CeloxTrauma Gauze; CG, Combat Gauze; SMC, standard manual compression; WS, WoundStat; CX: Celox; SQR, super quick relief; ACS+, QuikClot zeolite Advance Clotting Sponge; IC, Instaclot; AB: Alpha Bandage; BLS, Bloodstop; XS, X-Sponge; CHI, Chitoflex; FP-21, Polymem FP-21; FSD, fibrin sealant dressing;
two gauze (Army Field Dressing, Ellwyn, Inc., Ellwyn, PA) and six dressings are proprietary formulations;
DFSD, Dry Fibrin Sealant dressing;
modified WoundStat;
CA, Celox-A.
QuikClot
QuikClot (QC), which was approved by Food and Drug Administration (FDA), promoted hemostasis by absorbed water to concentrate blood clotting. It has become a necessary commodity for emergency treatment in United States Marine Corps. The product got used widely in Iraq War. The experts of America once said that QC changed totally the 130 years history of failure of hemostasis. Additionally, the low price allows it a wider market. However, the burn injury caused by it needs the second generation to address. QuikClot Advanced Clotting Sponge (ACS+), still based on a zeolite material, is designed to reduce exothermic reaction when reacting with liquid [12] and filled in a loose bag to facilitate the convenience of application and removal. The third generation including QuikClot Combat Gauze (QCG) and QuikClot Combat Gauze XL (QCX) consists of a material called kaolin, which promotes clotting formation in a wound.
Alam et al. compared QC with other hemostatic agents to test the hemostatic efficacy, only QC reaching 0% mortality (X2 test, P<0.05) [13]. They also demonstrated QC group had the lowest blood loss (4.4±1.4 ml/kg) comparing with other products. But the study didn’t mention the tissue injury following bleeding controlling. The next year, Alam et al. conducted another experiment to explore the heat caused by exothermic reaction and demonstrated the effective hemostasis effect again [14]. They tested heat effect of QC with different residual moisture. The results were that decreasing or increasing RM did not lower significantly the temperature of the wound. Instead, it affected the efficacy of hemostasis. The application of QC (3.5 ozQuikClot with 4% residual moisture) decreased blood loss and reduced mortality to 0% (X2 test, P<0.01) comparing with others in lethal groin artery hemorrhage. The authors also suggested heat sensitive tissue should not be applied with QC. Pusateri et al. described that QC shortened hemostatic time (Wilcoxon test, P<0.05), reduced resuscitation volume (Wilcoxon test, P<0.05) and posttreatment blood loss (Wilcoxon test, P<0.01) comparing with standard gauze in severe liver injury pig models [15]. But the summit temperature at the tissue interface was increased with QC (93.3±0.5) when comparing with standard gauze (37.5±6.5) (P<0.01). Wright JK et al. demonstrated a superior hemostatic effect with QC compared to biological agent (two mean test, P<0.05) [16]. At the same time, the clinical assessment of wounds and historical examination showed the use of QC could cause huge thermal injury resulting in necrosis of tissue. Peter et al. described a convey of 103 patients, 83 with external use and 20 intracorporeal use in war field [17]. They found the survival rate was up to 97% by the use of QC, wiith only 8 failure cases because of coagulopathic problem probably. There were three cases suffering burn injures, one of whom needed grafting. Ran Y et al. described a report about battle injuries, which found 79 percent (11/14) of patients applied with QCG succeed to stop bleeding [18]. There was no complication happening among the soldiers in the study. Johnson D et al. also proved the amount of bleeding in QCG group was less than that in the control group (36±112 ml versus 340±297 ml, non-parametric test, P<0.002) [19]. It was not difficult to see that the complication of QC damaged the normal issue seriously. Rall et al. conducted a study, in which they demonstrated the experimental data in HemCon ChitoGauze (HCG) is superior than in QCG [20]. Among those data, the survival rate (60%) and initial hemostasis (30%) in QCG were lower than those in HCG group (70% and 60%). Schwartz et al. also found that the hemostasis time, blood loss and survival rate in ChitoGauze group presented favorable trends compared with those with QCG in swine model [21] Although QCG overcame the bad effects of QC, more researches are needed to demonstrate its efficacy.
HemCon dressing
HemCon dressing (HC) received the approval of FDA in 2003 owing to its efficacy in stopping hemorrhage. The major component is chitosan, which is a kind of biodegradable polysaccharide amine derived from shellfish. The positive polysaccharide amine could attract negative red blood cells to help with blood clotting. Besides, once contacting with the blood, it will adhere to the wounds. The antibacterial character of chitosan gives it more advantages than others [22-24]. The dressing was applied on human in Iraq and Afghanistan War firstly. But a relatively stiff backing limits its use in small wounds and leads to the emerging of more flexible one-ChitoFlex (CHI). The new version, which is first produced in 2006, is a double sided chitosan roll that is conform and easy to use. ChitoGauze (HCG) is another kind of dressing covered with chitosan, manufactured by HemCon Medical Technologies Inc. The new product performs better effect in stopping bleeding and is used as the test agent by The NMRU study. Celox Gauze is also an available material for the treatment of hemorrhage. The main ingredients are a various of polymer chitosan compounds, which are accessible both on a bandage and in granular form. Celox received FDA approval in June of 2006.
Wedmore et al. demonstrated the high benefit of pre-hospital HC application in a retrospective and observational survey in Iraq [25]. They found 97% of cases could achieve to stop bleeding and 66% of patients were reached 100% success of hemostasis after the failure with standard gauze. There was not an adverse effect to be found in their study. Cox ED et al. reported that 95% of 1,691 patients survived by the use of hemostatic dressing including HC and QC [26]. Whereas the former presented more benefits than the latter. Pawanrat et al. demonstrated better efficacy of HC over standard manual compression (SMC) for hemostasis in animal models [27]. In their study, they proved the hemostasis time in HC group (6.9±3.9 min) was less compared to SMC group (10.8±2.8 min, Student’s T-test, P<0.019). Hematoma occurrence rate in HC group was 2/9 against 8/9 in SMC group and no complication happened in HC group. Maclntyre et al. conducted an experiment in swine models, demonstrating tourniquet use combined with Hemcon reduced tourniquet releasing time to 3 minutes and realized 100% hemostasis [28], which presented more effect than other three groups. Kheirabadi et al. conducted a research, which proved Celox was superior to both HC and ACS+ [29]. Hemostasis success was 6 in Celox, but 1 in HC after 2 minutes’ pressure, and the survival time of animal applied with Celox was longer than that of HC treated animals (Fisher’s test, P<0.05). The tissue damage caused by Celox was unknown. But WoundStat was more effective than it (100% vs 60%). Clay JG et al. also demonstrated that 83% of swine applied with Celox achieved 2 hours’ survival time, which suggested more advantages of Celox than HC and ACS+ (67% and 50%) [30]. In Kozen BG’s severe femoral bleeding experiment, Celox improved greatly hemorrhage control and achieved 100% survival compared with 50% with standard gauze, which did not show difference from HC and QC [31]. Two studies comparing 10 hemostatic dressings demonstrated the hemostatic effect of Celox was more obvious in improving hemostasis and survival time than HC and CHI in severe vascular damage [32,33] (Logrank test, P<0.05). HC improved hemostasis greatly, being deployed widely in military hospital. However, a new generation Celox showed more benefits than that.
The fibrin sealant dressing
The compositions of the Fibrin Sealant Dressing (FSD), which is purified from human clotting protein, are fibrinogen, thrombin, blood coagulation factors and calcium. FSD was exploited by United States Military researchers and American Red Cross. When the clotting protein of the dressing contacts with the blood, the enzymatic system will be invoked and fibrin layer is produced to adhere to the injured tissue and absorbed totally. Because of its biological property from human blood, it is still in clinical experiment to go a further step for the sake of safety.
Kheirabadi et al. compared the hemostatic efficacy of HC, FSD and standard gauze army field dressing. In the experiment, they proved HC and FSD stopped initial hemorrhage which could not be controlled by standard gauze [34]. Those pigs receiving FSD all could begin normal activities and even lived up to 96 hours, whereas the survival time of HC group was less (the log-rank test, P<0.004). But the injury site applied with FSD formed pseudoaneurysm, which might be in a risk of rebleeding. Two years later, Kheirabadi et al. finished another study, which proved more evidently that FSD stopped arterial hemorrhage effectively [35]. The initial hemorrhage was rated 93% (14/15) after 4 minutes’ pressure and prevented 80% of rebleeding for at least 7 days. The pseudoaneurysm caused by FSD at the aortotomy site disappeared after 2 to 3 weeks by CT, necropsy and histology examination. Acheson et al. compared the hemostatic effectiveness of QC, HC, FSD and AFB [36]. They also demonstrated that FSD increased the survival time compared with other agents (the Logrank test, P<0.001). Blood loss with FSD was less than in HC group (Kruskal-Wallis test, P<0.05), and blood loss with HC was still less than that in other two groups. Sondeen et al. described ten hemostatic materials of hemorrhage control [37]. The study proved that those receiving Fibrin Sealant dressing and suture repair survived 1 hour, while other groups almost no more than 10 minutes (Dunnett’s test, P<0.001). Larson MJ et al. showed the blood loss in FSD group was less than the plain dressing group in lethal artery lacerations model (Kruskal-Wallis test, P=0.0022) [38]. What’s more, FSD reduced the amount of hemorrhage and improved the survival in the model of Grade V liver injury [39-41]. It seemed that FSD performed better than others in the war because of fewer complications and more effective hemostasis effect.
WoundStat
The new product is composed of chitosan within silica and polyacrylic acid. The former with powerful absorption ability derives from hydrated alumino silicates. Another absorbs water 200 more times as heavy as its weight. The combination of components in it ensures superior hemostatic performance. Although FDA approved WoundStat (WS) for treating external hemorrhage, it could only be applied in animal trials to test for further benefits.
Kevin R et al. described the efficacy of hemostatic dressing in WS, HC, QCG, AFB and ACS [42]. They reported the amount of survival animals treated with WS was larger than that in other groups (Fisher’s exact test, P<0.05). Five in WS group all survived for 3 hours, whereas no one survived in others, except only one animal treated with HC succeed (the Logrank test, P<0.001). The resuscitation liquid for WS was less compared with other four groups (Kruskal-Wallis test, P<0.006). Joseph W. Carraway et al. compared the hemostatic agent of modified WS (containing only silica) with QCG in a lethal vascular injury model [43]. They demonstrated 100% animals in modified WS group survived for 2 hours, comparing with 0% of those treated with QCG after 120 minutes (the Logrank test, P=0.005). The total Post-treatment blood (Post-TBL) and resuscitation liquid needed for WS groups were reduced significantly compared to QCG group. Chitra. N. Sambasivan et al. demonstrated WS was able to realize hemostasis more effectively compared to CHI and standard gauze with 30 seconds compression [44]. In their study, Post-TBL and lactated Ringer’s solution for resuscitation were less than those in other two groups (Mann-Whitney U test P<0.05). Kheirabadi et al. conducted a research, in which they demonstrated the hemostatic success rate was 100% for WS, showing an obvious advantage over other groups (Fisher’s exact test, P<0.05) [29]. The side effect of WS was unknown. Gerlach T et al. demonstrated that WS succeed in stopping fatal artery bleeding in swine [45]. None animal died and no apparent complication was found. However, histopathologic evaluation in WS group revealed that its application associating with vessel inflammatory response led to neurovascular injury. The longer time of l hour application time probably also effected the happening of the complication or WS did produce damage to normal tissue. Littlejohn LF et al. found there was no significant advantage for WS compared with Celox-A, CHI, and combat gauze in treating uncontrolled hemorrhage in smaller track wounds [46]. WS could perform obvious hemostasis effect in life-threatening artery hemorrhage in animal models. But the unwelcomed effect on local tissue in some situations still calls for our attention.
Discussion
The common hemostatic medicines can be grouped into three types: factor concentrators, pro-coagulant activators and mucoadhesive agents. Almost all developed and marketed hemostatic drugs bear trade names and they are more familiar to care providers. Therefore we adopt trade names in this review.
Factor concentrators
QuikClot performs better effect in stopping bleeding. However, the tissue damage caused by exothermic reaction leads to serious tissue injury [13,15-17]. Although the second generation QuikClot ACS+ overcome the above complication, the hemostatic results are unsatisfactory [29,30,47].
Procoagulant supplementor
The invention of QuikClot Combat Gauze improves the hemostatic efficacy and causes few complication [18,19], but the hemostatic effects are not so hopeful in other studies [20,21,46,48]. The Fibrin Sealant Dressing, which has been proved to be with outstanding hemostatic effect in arterial hemorrhage models, stays ahead of HemCon and/or QuikClot in comparative studies [34,36,37]. While the potential price is high, the unusual origin from human blood can be replaced by the blood plasma from Atlantic salmon fish [34]. This new discovery brings hope to the application of the Fibrin Sealant Dressing.
Mucoadhesive agents
HemCon is effective in controlling artery hemorrhage [25,26]. The improved version has more advantages in hemorrhage control [27]. However, Celox, the new product originates from the same material, performs more excellent hemostatic effect than HemCon bandage [29,30] and the cheap price makes it easier to get (£15-20). Moreover, it not only arrests bleeding with clotting dysfunctions [49], but is also effective in wound healing [50-52]. Animal experiments for RDH show that it is ineffective to stop bleeding [53-55] and the modified RDH (mRDH bandage) does not express out obvious hemostatic effects [56]. WoundStat has been proved with almost 100% success of hemostasis in experimental literatures, which excels HemCon, Celox and QuikClot ACS+ in stopping severe artery bleeding [29,42-44] and its market price is £23. But it is required to take into consideration its limitation to big-size wounds and potential complication when applying [46].
Pusateri et al [57] cited that the ideal hemostatic agent used in military should meet the following seven criteria: the first and the most important characteristic is that it is effective to stop arterial bleeding in 2 minutes; then it is convenient for medics or anyone to use and has few complication. Besides, it is easy for soldiers to carry and has long shelf life (such as 2~3 years). The material applied on the injury location is reabsorbable by the body, which may make it safer. Finally, a cheap price gives it a more wide application. However, there is no such a product meeting all standards above. Currently, the hemostatic efficacy is considered as the critical principle in choosing a hemostatic product. From the collected datas, three hemostatic materials are outperformed HemCon and QuikClot. The Fibrin Sealant Dressing is able to control aggressive, fatal bleeding in animal models and the complication caused by it is few [34,36,37]. Another is Celox, performing effectively and safely in arresting artery bleeding [29-31]. The last is Woundstat, which achieves 100% hemostasis, reduces hemorrhage time and improves survival rate [29,42,43] and it is also suitable for medics or the unprofessionals. As to the arterial or venous hemorrhage, it is well known that the two kinds of hemorrhage present different ways and colors. However, due to the seriousness and complexity of injuries in the battle, it is hard to recognize which kind it belongs to. Hughes in a study noted that soldiers had difficulty in making a distinction between arterial and venous hemorrhage [58]. So there is no necessary to distinguish whether the product is for artery or vein hemostasis. The product which presents excellent hemostasis effect in arterial bleeding will function well in other ways.
In summary, Fibrin Sealant Dressing, Celox and Woundstat show more advantages over others proved by the data we collected. J. Granville-Chapman also concluded Celox and WoundStat were effective to stop bleeding caused by pre-hospital trauma in battlefield [59].
When choosing a hemostatic agent, the advantages should be balanced with the disadvantages. Therefore, we need more researches to gradually validate their efficacy and safety in future.
Limitations
There are only a few descriptive cases included in the review talking about hemostatic medicine due to the harsh environment in battle field. Data from some articles collected are not intact or detailed because of the difficulty in recording.
Acknowledgements
This work is supported by the Foundation of Science and Technology of Shaaxi Province (2013JM4061). No benefits in any form have been or will be received from a commercial organization directly or indirectly.
Disclosure of conflict of interest
None.
References
- 1.Bellamy RF. The causes of death in conventional land warfare: implications for combat casualty care research. Mil Med. 1984;149:55–62. [PubMed] [Google Scholar]
- 2.Alam HB, Burris D, DaCorta JA, Rhee P. Hemorrhage control in the battlefield: role of new hemostatic agents. Mil Med. 2005;170:63–69. doi: 10.7205/milmed.170.1.63. [DOI] [PubMed] [Google Scholar]
- 3.Simon D, Taylor MJ, Elrifai AM, Shih SR, Bailes JE, Davis D, Kluger Y, Diamond DL, Maroon JC. Hypothermic blood substitution enables resuscitation after hemorrhagic shock and 2 hours of cardiac arrest. Asaio J. 1995;41:M297–M300. doi: 10.1097/00002480-199507000-00017. [DOI] [PubMed] [Google Scholar]
- 4.Starnes BW, Beekley AC, Sebesta JA, Andersen CA, Rush RJ. Extremity vascular injuries on the battlefield: tips for surgeons deploying to war. J Trauma. 2006;60:432–442. doi: 10.1097/01.ta.0000197628.55757.de. [DOI] [PubMed] [Google Scholar]
- 5.Walters TJ, Mabry RL. Issues related to the use of tourniquets on the battlefield. Mil Med. 2005;170:770–775. doi: 10.7205/milmed.170.9.770. [DOI] [PubMed] [Google Scholar]
- 6.Holcomb JB. Fluid resuscitation in modern combat casualty care: lessons learned from Somalia. J Trauma. 2003;54:S46–S51. doi: 10.1097/01.TA.0000051936.91915.A2. [DOI] [PubMed] [Google Scholar]
- 7.Mabry RL, Holcomb JB, Baker AM, Cloonan CC, Uhorchak JM, Perkins DE, Canfield AJ, Hagmann JH. United States Army Rangers in Somalia: an analysis of combat casualties on an urban battlefield. J Trauma. 2000;49:515–528. 528–529. doi: 10.1097/00005373-200009000-00021. [DOI] [PubMed] [Google Scholar]
- 8.Cosgriff N, Moore EE, Sauaia A, Kenny-Moynihan M, Burch JM, Galloway B. Predicting life-threatening coagulopathy in the massively transfused trauma patient: hypothermia and acidoses revisited. J Trauma. 1997;42:857–861. 861–862. doi: 10.1097/00005373-199705000-00016. [DOI] [PubMed] [Google Scholar]
- 9.Sauaia A, Moore FA, Moore EE, Haenel JB, Read RA, Lezotte DC. Early predictors of postinjury multiple organ failure. Arch Surg. 1994;129:39–45. doi: 10.1001/archsurg.1994.01420250051006. [DOI] [PubMed] [Google Scholar]
- 10.Holcomb J, MacPhee M, Hetz S, Harris R, Pusateri A, Hess J. Efficacy of a dry fibrin sealant dressing for hemorrhage control after ballistic injury. Arch Surg. 1998;133:32–35. doi: 10.1001/archsurg.133.1.32. [DOI] [PubMed] [Google Scholar]
- 11.Calkins D, Snow C, Costello M, Bentley TB. Evaluation of possible battlefield tourniquet systems for the far-forward setting. Mil Med. 2000;165:379–384. [PubMed] [Google Scholar]
- 12.Arnaud F, Tomori T, Carr W, McKeague A, Teranishi K, Prusaczyk K, McCarron R. Exothermic reaction in zeolite hemostatic dressings: QuikClot ACS and ACS+ Ann Biomed Eng. 2008;36:1708–1713. doi: 10.1007/s10439-008-9543-7. [DOI] [PubMed] [Google Scholar]
- 13.Alam HB, Uy GB, Miller D, Koustova E, Hancock T, Inocencio R, Anderson D, Llorente O, Rhee P. Comparative analysis of hemostatic agents in a swine model of lethal groin injury. J Trauma. 2003;54:1077–1082. doi: 10.1097/01.TA.0000068258.99048.70. [DOI] [PubMed] [Google Scholar]
- 14.Alam HB, Chen Z, Jaskille A, Querol RI, Koustova E, Inocencio R, Conran R, Seufert A, Ariaban N, Toruno K, Rhee P. Application of a zeolite hemostatic agent achieves 100% survival in a lethal model of complex groin injury in Swine. J Trauma. 2004;56:974–983. doi: 10.1097/01.ta.0000127763.90890.31. [DOI] [PubMed] [Google Scholar]
- 15.Pusateri AE, Delgado AV, Dick EJ, Martinez RS, Holcomb JB, Ryan KL. Application of a granular mineral-based hemostatic agent (QuikClot) to reduce blood loss after grade V liver injury in swine. J Trauma. 2004;57:555–562. doi: 10.1097/01.ta.0000136155.97758.cd. discussion 562. [DOI] [PubMed] [Google Scholar]
- 16.Wright JK, Kalns J, Wolf EA, Traweek F, Schwarz S, Loeffler CK, Snyder W, Yantis LJ, Eggers J. Thermal injury resulting from application of a granular mineral hemostatic agent. J Trauma. 2004;57:224–230. doi: 10.1097/01.ta.0000105916.30158.06. [DOI] [PubMed] [Google Scholar]
- 17.Rhee P, Brown C, Martin M, Salim A, Plurad D, Green D, Chambers L, Demetriades D, Velmahos G, Alam H. QuikClot use in trauma for hemorrhage control: case series of 103 documented uses. J Trauma. 2008;64:1093–1099. doi: 10.1097/TA.0b013e31812f6dbc. [DOI] [PubMed] [Google Scholar]
- 18.Ran Y, Hadad E, Daher S, Ganor O, Kohn J, Yegorov Y, Bartal C, Ash N, Hirschhorn G. QuikClot Combat Gauze use for hemorrhage control in military trauma: January 2009 Israel Defense Force experience in the Gaza Strip--a preliminary report of 14 cases. Prehosp Disaster Med. 2010;25:584–588. doi: 10.1017/s1049023x00008797. [DOI] [PubMed] [Google Scholar]
- 19.Johnson D, Agee S, Reed A, Gegel B, Burgert J, Gasko J, Loughren M. The effects of QuikClot Combat Gauze on hemorrhage control in the presence of hemodilution. US Army Med Dep J. 2012:36–39. [PubMed] [Google Scholar]
- 20.Rall JM, Cox JM, Songer AG, Cestero RF, Ross JD. Comparison of novel hemostatic dressings with QuikClot combat gauze in a standardized swine model of uncontrolled hemorrhage. J Trauma Acute Care Surg. 2013;75:S150–S156. doi: 10.1097/TA.0b013e318299d909. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz RB, Reynolds BZ, Shiver SA, Lerner EB, Greenfield EM, Solis RA, Kimpel NA, Coule PL, McManus JG. Comparison of two packable hemostatic Gauze dressings in a porcine hemorrhage model. Prehosp Emerg Care. 2011;15:477–482. doi: 10.3109/10903127.2011.598615. [DOI] [PubMed] [Google Scholar]
- 22.Burkatovskaya M, Castano AP, Demidova-Rice TN, Tegos GP, Hamblin MR. Effect of chitosan acetate bandage on wound healing in infected and noninfected wounds in mice. Wound Repair Regen. 2008;16:425–431. doi: 10.1111/j.1524-475X.2008.00382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burkatovskaya M, Tegos GP, Swietlik E, Demidova TN, P Castano A, Hamblin MR. Use of chitosan bandage to prevent fatal infections developing from highly contaminated wounds in mice. Biomaterials. 2006;27:4157–4164. doi: 10.1016/j.biomaterials.2006.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dai T, Tegos GP, Burkatovskaya M, Castano AP, Hamblin MR. Chitosan acetate bandage as a topical antimicrobial dressing for infected burns. Antimicrob Agents Chemother. 2009;53:393–400. doi: 10.1128/AAC.00760-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wedmore I, McManus JG, Pusateri AE, Holcomb JB. A special report on the chitosan-based hemostatic dressing: experience in current combat operations. J Trauma. 2006;60:655–658. doi: 10.1097/01.ta.0000199392.91772.44. [DOI] [PubMed] [Google Scholar]
- 26.Cox ED, Schreiber MA, McManus J, Wade CE, Holcomb JB. New hemostatic agents in the combat setting. Transfusion. 2009;49(Suppl 5):248S–255S. doi: 10.1111/j.1537-2995.2008.01988.x. [DOI] [PubMed] [Google Scholar]
- 27.Kranokpiraksa P, Pavcnik D, Kakizawa H, Uchida BT, Jeromel M, Keller FS, Rosch J. Hemostatic efficacy of chitosan-based bandage for closure of percutaneous arterial access sites: An experimental study in heparinized sheep model. Radiol Oncol. 2010;44:86–91. doi: 10.2478/v10019-010-0021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson D, Agee S, Reed A, Gegel B, Burgert J, Gasko J, Loughren M. The effects of QuikClot Combat Gauze on hemorrhage control in the presence of hemodilution. US Army Med Dep J. 2012:36–39. [PubMed] [Google Scholar]
- 29.Kheirabadi BS, Edens JW, Terrazas IB, Estep JS, Klemcke HG, Dubick MA, Holcomb JB. Comparison of new hemostatic granules/powders with currently deployed hemostatic products in a lethal model of extremity arterial hemorrhage in swine. J Trauma. 2009;66:316–326. doi: 10.1097/TA.0b013e31819634a1. discussion 327-328. [DOI] [PubMed] [Google Scholar]
- 30.Clay JG, Grayson JK, Zierold D. Comparative testing of new hemostatic agents in a swine model of extremity arterial and venous hemorrhage. Mil Med. 2010;175:280–284. doi: 10.7205/milmed-d-09-00185. [DOI] [PubMed] [Google Scholar]
- 31.Kozen BG, Kircher SJ, Henao J, Godinez FS, Johnson AS. An alternative hemostatic dressing: comparison of CELOX, HemCon, and QuikClot. Acad Emerg Med. 2008;15:74–81. doi: 10.1111/j.1553-2712.2007.00009.x. [DOI] [PubMed] [Google Scholar]
- 32.Arnaud F, Parreno-Sadalan D, Tomori T, Delima MG, Teranishi K, Carr W, McNamee G, McKeague A, Govindaraj K, Beadling C, Lutz C, Sharp T, Mog S, Burris D, McCarron R. Comparison of 10 hemostatic dressings in a groin transection model in swine. J Trauma. 2009;67:848–855. doi: 10.1097/TA.0b013e3181b2897f. [DOI] [PubMed] [Google Scholar]
- 33.Arnaud F, Teranishi K, Tomori T, Carr W, McCarron R. Comparison of 10 hemostatic dressings in a groin puncture model in swine. J Vasc Surg. 2009;50:632–639. doi: 10.1016/j.jvs.2009.06.010. discussion 631-639. [DOI] [PubMed] [Google Scholar]
- 34.Kheirabadi BS, Acheson EM, Deguzman R, Sondeen JL, Ryan KL, Delgado A, Dick EJ, Holcomb JB. Hemostatic efficacy of two advanced dressings in an aortic hemorrhage model in Swine. J Trauma. 2005;59:25–34. doi: 10.1097/01.ta.0000171458.72037.ee. discussion 34-35. [DOI] [PubMed] [Google Scholar]
- 35.Kheirabadi BS, Acheson EM, Deguzman R, Crissey JM, Delgado AV, Estep SJ, Holcomb JB. The potential utility of fibrin sealant dressing in repair of vascular injury in swine. J Trauma. 2007;62:94–103. doi: 10.1097/01.ta.0000251595.45451.d0. [DOI] [PubMed] [Google Scholar]
- 36.Acheson EM, Kheirabadi BS, Deguzman R, Dick EJ, Holcomb JB. Comparison of hemorrhage control agents applied to lethal extremity arterial hemorrhages in swine. J Trauma. 2005;59:865–874. doi: 10.1097/01.ta.0000187655.63698.9f. discussion 874-875. [DOI] [PubMed] [Google Scholar]
- 37.Sondeen JL, Pusateri AE, Coppes VG, Gaddy CE, Holcomb JB. Comparison of 10 different hemostatic dressings in an aortic injury. J Trauma. 2003;54:280–285. doi: 10.1097/01.TA.0000037431.19185.B4. [DOI] [PubMed] [Google Scholar]
- 38.Larson MJ, Bowersox JC, Lim RJ, Hess JR. Efficacy of a fibrin hemostatic bandage in controlling hemorrhage from experimental arterial injuries. Arch Surg. 1995;130:420–422. doi: 10.1001/archsurg.1995.01430040082018. [DOI] [PubMed] [Google Scholar]
- 39.Holcomb JB, Pusateri AE, Harris RA, Charles NC, Gomez RR, Cole JP, Beall LD, Bayer V, MacPhee MJ, Hess JR. Effect of dry fibrin sealant dressings versus gauze packing on blood loss in grade V liver injuries in resuscitated swine. J Trauma. 1999;46:49–57. doi: 10.1097/00005373-199901000-00009. [DOI] [PubMed] [Google Scholar]
- 40.Holcomb JB, Pusateri AE, Harris RA, Reid TJ, Beall LD, Hess JR, MacPhee MJ. Dry fibrin sealant dressings reduce blood loss, resuscitation volume, and improve survival in hypothermic coagulopathic swine with grade V liver injuries. J Trauma. 1999;47:233–240. 240–242. doi: 10.1097/00005373-199908000-00002. [DOI] [PubMed] [Google Scholar]
- 41.Pusateri AE, McCarthy SJ, Gregory KW, Harris RA, Cardenas L, McManus AT, Goodwin CJ. Effect of a chitosan-based hemostatic dressing on blood loss and survival in a model of severe venous hemorrhage and hepatic injury in swine. J Trauma. 2003;54:177–182. doi: 10.1097/00005373-200301000-00023. [DOI] [PubMed] [Google Scholar]
- 42.Ward KR, Tiba MH, Holbert WH, Blocher CR, Draucker GT, Proffitt EK, Bowlin GL, Ivatury RR, Diegelmann RF. Comparison of a new hemostatic agent to current combat hemostatic agents in a Swine model of lethal extremity arterial hemorrhage. J Trauma. 2007;63:276–283. 283–284. doi: 10.1097/TA.0b013e3180eea8a5. [DOI] [PubMed] [Google Scholar]
- 43.Carraway JW, Kent D, Young K, Cole A, Friedman R, Ward KR. Comparison of a new mineral based hemostatic agent to a commercially available granular zeolite agent for hemostasis in a swine model of lethal extremity arterial hemorrhage. Resuscitation. 2008;78:230–235. doi: 10.1016/j.resuscitation.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 44.Sambasivan CN, Cho SD, Zink KA, Differding JA, Schreiber MA. A highly porous silica and chitosan-based hemostatic dressing is superior in controlling hemorrhage in a severe groin injury model in swine. Am J Surg. 2009;197:576–580. doi: 10.1016/j.amjsurg.2008.12.011. discussion 580. [DOI] [PubMed] [Google Scholar]
- 45.Gerlach T, Grayson JK, Pichakron KO, Sena MJ, DeMartini SD, Clark BZ, Estep JS, Zierold D. Preliminary study of the effects of smectite granules (WoundStat) on vascular repair and wound healing in a swine survival model. J Trauma. 2010;69:1203–1209. doi: 10.1097/TA.0b013e3181c452b5. [DOI] [PubMed] [Google Scholar]
- 46.Littlejohn LF, Devlin JJ, Kircher SS, Lueken R, Melia MR, Johnson AS. Comparison of Celox-A, ChitoFlex, WoundStat, and combat gauze hemostatic agents versus standard gauze dressing in control of hemorrhage in a swine model of penetrating trauma. Acad Emerg Med. 2011;18:340–350. doi: 10.1111/j.1553-2712.2011.01036.x. [DOI] [PubMed] [Google Scholar]
- 47.Gustafson SB, Fulkerson P, Bildfell R, Aguilera L, Hazzard TM. Chitosan dressing provides hemostasis in swine femoral arterial injury model. Prehosp Emerg Care. 2007;11:172–178. doi: 10.1080/10903120701205893. [DOI] [PubMed] [Google Scholar]
- 48.Kheirabadi BS, Mace JE, Terrazas IB, Fedyk CG, Estep JS, Dubick MA, Blackbourne LH. Safety evaluation of new hemostatic agents, smectite granules, and kaolin-coated gauze in a vascular injury wound model in swine. J Trauma. 2010;68:269–278. doi: 10.1097/TA.0b013e3181c97ef1. [DOI] [PubMed] [Google Scholar]
- 49.Millner R, Lockhart AS, Marr R. Chitosan arrests bleeding in major hepatic injuries with clotting dysfunction: an in vivo experimental study in a model of hepatic injury in the presence of moderate systemic heparinisation. Ann R Coll Surg Engl. 2010;92:559–561. doi: 10.1308/003588410X12699663903593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burkatovskaya M, Castano AP, Demidova-Rice TN, Tegos GP, Hamblin MR. Effect of chitosan acetate bandage on wound healing in infected and noninfected wounds in mice. Wound Repair Regen. 2008;16:425–431. doi: 10.1111/j.1524-475X.2008.00382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burkatovskaya M, Tegos GP, Swietlik E, Demidova TN, P Castano A, Hamblin MR. Use of chitosan bandage to prevent fatal infections developing from highly contaminated wounds in mice. Biomaterials. 2006;27:4157–4164. doi: 10.1016/j.biomaterials.2006.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dai T, Tegos GP, Burkatovskaya M, Castano AP, Hamblin MR. Chitosan acetate bandage as a topical antimicrobial dressing for infected burns. Antimicrob Agents Chemother. 2009;53:393–400. doi: 10.1128/AAC.00760-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alam HB, Uy GB, Miller D, Koustova E, Hancock T, Inocencio R, Anderson D, Llorente O, Rhee P. Comparative analysis of hemostatic agents in a swine model of lethal groin injury. J Trauma. 2003;54:1077–1082. doi: 10.1097/01.TA.0000068258.99048.70. [DOI] [PubMed] [Google Scholar]
- 54.Pusateri AE, McCarthy SJ, Gregory KW, Harris RA, Cardenas L, McManus AT, Goodwin CJ. Effect of a chitosan-based hemostatic dressing on blood loss and survival in a model of severe venous hemorrhage and hepatic injury in swine. J Trauma. 2003;54:177–182. doi: 10.1097/00005373-200301000-00023. [DOI] [PubMed] [Google Scholar]
- 55.Sondeen JL, Pusateri AE, Coppes VG, Gaddy CE, Holcomb JB. Comparison of 10 different hemostatic dressings in an aortic injury. J Trauma. 2003;54:280–285. doi: 10.1097/01.TA.0000037431.19185.B4. [DOI] [PubMed] [Google Scholar]
- 56.King DR. Thirty consecutive uses of a hemostatic bandage at a US Army combat support hospital and forward surgical team in Operation Iraqi Freedom. J Trauma. 2011;71:1775–1778. doi: 10.1097/TA.0b013e3182231615. [DOI] [PubMed] [Google Scholar]
- 57.Pusateri AE, McCarthy SJ, Gregory KW, Harris RA, Cardenas L, McManus AT, Goodwin CJ. Effect of a chitosan-based hemostatic dressing on blood loss and survival in a model of severe venous hemorrhage and hepatic injury in swine. J Trauma. 2003;54:177–182. doi: 10.1097/00005373-200301000-00023. [DOI] [PubMed] [Google Scholar]
- 58.Butler FJ. Tactical medicine training for SEAL mission commanders. Mil Med. 2001;166:625–631. [PubMed] [Google Scholar]
- 59.Granville-Chapman J, Jacobs N, Midwinter MJ. Pre-hospital haemostatic dressings: a systematic review. Injury. 2011;42:447–459. doi: 10.1016/j.injury.2010.09.037. [DOI] [PubMed] [Google Scholar]


