Abstract
The aim is to investigate the molecular mechanisms underlying the PM2.5-induced autophagy in human lung cancer epithelial cells (A549). The effects of the PM2.5 on morphological and biochemical markers of autophagy in A549 were analyzed by electron microscopy, GFP-LC3 puncta was observed by confocal fluorescence microscope. The effects of phosphorylation of AMPK, mTOR, AKT, ERK, JNK, and p53 on LC3II in A549 were observed following PM2.5 exposure; the role of autophagy in PM2.5-induced apoptosis was examined using 3-methyladenine and rapamycin. PM2.5 induced morphological and biochemical markers of autophagy in A549. Phosphorylation of AMPK and dephosphorylation of mTOR were observed following PM2.5 treatment, and AMPK inhibitor blocked LC3B-II expression. In addition, we demonstrated that PM2.5-induced autophagy confers a pro-survival role in host defense.
Keywords: PM2.5, autophagy, oxidative stress, A549, COPD
Introduction
Particulate matter (PM2.5) air pollutants have been shown to exacerbate a variety of pulmonary disorders, including chronic obstructive pulmonary disease (COPD) [1,2]. Strong evidence indicates that daily variation in exposure to outdoor air pollution correlates with acute exacerbation of COPD [3,4]. Recently, autophagy was shown to influence the pathogenesis of COPD, although controversy exists as to whether it promotes the development of COPD or serves as a defense mechanism [5,6]. Autophagy is a catabolic process that maintains cellular homeostasis in response to a wide spectrum of cellular stresses. Furthermore, PM2.5 was shown to induce autophagy in A549 cells, a widely used type II pulmonary epithelial cell model [7]. Thus, we sought to identify the molecular mechanisms of PM2.5-induced autophagy using A549 cells as a relevant system for assessing the mechanisms of PM2.5-induced autophagy.
The regulation of autophagy is complex and context-dependent. Certain kinases, such as mTOR, phosphatidylinositol 3-kinase and AMP-dependent protein kinase (AMPK, phosphorylation sites at Thr-172), directly regulate components of the autophagic machinery; other kinases, such as the mitogen-activated protein kinases (MAPKs), regulate autophagy indirectly via the modulation of the levels/function of autophagy related proteins [8-12]. AMPK induces autophagy under low energy conditions by dual regulation of mTORC1 and ULK1 [13]. Inhibition of mTORC1 activity leads to the activation of a set of evolutionarily conserved autophagy-regulating proteins (Atg proteins) and the formation of autophagosomes, which fuse with lysosomes to form autolysosomes [14]. Autophagy may also be influenced by MAPKs, which are upstream regulators of mTOR, a serine/threonine kinase that mediates the responses to various extracellular stimuli. The four categories of MAPKs, extracellular signal-regulated kinase (ERK), p38, c-Jun N-terminal kinase (JNK)/stress-activated protein kinase (SAPK) and big MAP kinase (BMK), all have been reported to regulate autophagy, although it is unclear whether they promote or inhibit autophagy [11,15]. The AMPK and MAPK/mTOR pathways converge on the dual regulation of p53, which also has a reported role in the regulation of autophagy [16]. Consistently, our early studies demonstrated that p53 mediates alveolar epithelial cell mitochondria-regulated apoptosis induced by particulate matter [17]. Finally, reactive oxygen species (ROS) is generally accepted as an intracellular inducer of autophagy, and autophagy, in turn, serves to reduce oxidative damage [18]. Recent studies indicate that PM2.5 induces ROS generation and damage in COPD patients [19]. Thus, we examined the potential role for a variety of signaling pathways in PM2.5-induced autophagy in cell level. Recently, we confirmed that AKT/mTOR and c-Jun N-terminal Kinase (JNK) signaling pathways are involved for chrysotile asbestos-induced autophagy in human lung epithelial cells (A549) [20]. In this study, we report that PM2.5-induced autophagy in A549 cells is mediated by AMPK activation, while the MAPKs p38 and ERK have inhibitory roles on autophagy and JNK and p53 have no apparent role. Inhibition of autophagy by 3-MA increases cell death significantly, whereas the induction of autophagy by rapamycin decreases cell death. In conclusion, PM2.5-induced autophagy in A549 cells confers a pro-survival phenotype, the activation of which is mediated through the AMPK signaling pathway. Our study provides a better understanding of the complex signaling events that characterize PM2.5-induced alveolar autophagy and cell death and may shed a new light on the development of novel therapies for COPD.
Materials and methods
Reagents and antibodies
DMEM culture media, penicillin, streptomycin and fetal bovine serum were purchased from Invitrogen-Life Technologies (Carlsbad, CA). Acridine orange, monodansylcadaverine (MDC), trypsin-EDTA, 3-MA and rapamycin were obtained from Sigma-Aldrich (St. Louis, MO). U0126, SB203580, SP600125, pifithrin-α (PFT-α) and AMPK inhibitor dorsomorphin (DM) were purchased from Calbiochem (Merck KGaA, Darmstadt, Germany). All antibodies were obtained from Cell Signaling Technology (Beverly, MA). Lipofectamine 2000 and TRIzol reagent were from Invitrogen Life Technologies (Carlsbad, CA). pBABE-EGFP-mCherry-LC3 was purchased from Biovector Science Lab, Inc (China).
PM2.5 sampling and composition
We obtained PM2.5 from the Zhanjiang, a China sea-side City located on the southern coast of the South China Sea. The PM2.5 we used in the present work provided from the QJS-100 multi-level flow particulate matter cutter, maintaining a constant aspiration flow rate (100 L/min) for a period of 48 h. Place the sample containing the PM2.5 fiber filters to ultrapure water, ultrasonic oscillations 15 min to elude the particulate matter, the sample was then vacuum-freeze drying for 24 h, weighing, formulated into a stock solution by adding a certain amount of PBS and autoclave, PM2.5 suspensions were vortexed and put 4°C stored. PM2.5 filter samples were analyzed to determine the main composition using gas-mass spectrometry [21] and X-Ray fluorescence spectroscopy [22] as shown in the paper [36].
Cell culture and treatment
Human lung epithelial carcinoma A549 cells were purchased from American Tissue Culture Collection (ATCC) (Rockville, MD, USA). A549 Cells were grown in RPMI-1640 medium with 10% fetal bovine serum (FBS) (Gibco/BRL, MD), supplemented with 100 U/ml penicillin G and 100 ng/ml streptomycin (Sigma-Aldrich Corp., St. Louis, MO). Cells were maintained at 37°C in a humidified 5% CO2 incubator.
For assessment of PM2.5 effects, cells were treated with PM2.5 at 100 μg/ml for the time periods indicated in 5% FBS supplemented medium. For the studies testing the effects of inhibitors, A549 cells were pretreated for 1h with inhibitors, including 3-MA (Sigma, 10 mM), U1026 (Merck, 5 μM), SP6005 (Merck, 50 μM), LY294002 (Merck, 10 μM), SB203580 (Merck, 10 μM), PFT-α (Merck, 30 μM), and then cells were treated with PM2.5 in the presence of the inhibitors. Inhibitors were dissolved in dimethyl sulfoxide (DMSO), and the final concentrations of DMSO in the culture medium did not exceed 0.2%.
Cell viability assays
For cell viability evaluation, the treated cells were stained with 0.25% trypan blue solution and then counted using a hemacytometer (Neubauer Improved, Marienfeld, Germany) under a light microscope.
Acridine orange staining and MDC incorporation assays
A549 cells were treated with 100 µg/ml PM2.5 for 24 h. After rinsing with fresh medium, the cells were stained with 1 µg/mL of acridine orange solution at 37°C for 15 minutes, and the fluorescence signal was examined using a confocal microscope (Leica TCS SP5 II, Germany) with a peak excitation wavelength of 490 nm. MDC staining of autophagic vacuoles was also performed for autophagy analysis. Cells were treated with 100 µg/ml PM2.5 for 24 h and then labeled with 0.05 mmol/L MDC in PBS at 37°C for 10 min. After incubation, the cells were washed three times with PBS and immediately analyzed under a confocal laser scanning microscope (Leica TCS SP5 II, Germany). Fluorescence of MDC was measured at the excitation wavelength 380 nm with an emission filter at 530 nm.
Immunofluorescence staining
Cells were enzymatically removed from the flasks and plated in 35 mm diameter confocal dishes (Coverglass-Bottom Dish), after 24 h in culture; the medium was replaced with 2 ml of fresh medium with PM2.5 at a final concentration 100 µg/ml. PM2.5 remained in contact with the cells for a period of 24 h, after which the medium was changed. After an additional 24 h period, cells were washed with PBS three times and fixed with 3.7% formaldehyde for 30 min. Nonspecific binding was blocked by incubating the cover slips with 5% normal donkey serum in PBS, 1% BSA for 15 min at room temperature, followed by incubation for 90 min with PBS, 1% BSA containing rabbit anti-LC3 primary antibody (1:200 dilution). Dishes were washed with PBS three times for 5 min each wash, and then labeled with a 1:500 dilution of donkey Alexa-Fluor 488 anti-rabbit IgG for 30 min at room temperature in darkness. For visualization of nuclei, coverslip were stained with DAPI at 1:5,000 dilution in PBS for 2 min. Subsequently, dishes were mounted with Prolong Gold Antifade Reagent, and fluorescent images were captured using a confocal microscope (Leica TCS SP5 II, Germany). The images were prepared and labeled using Adobe Photoshop 7.0 software.
Quantitative real-time-PCR (QPCR)
Total RNA was extracted using TRIzol reagent (Invitrogen, USA), and cDNA was prepared using 0.5 μg of oligo-d (T) primers and PrimeScript RT reagent (Takara Bio, Japan) according to the manufacturer’s protocol. Real time quantitative PCR (qPCR) was performed using SYBR Green (Takara Bio, Japan). Each test was carried out in triplicate according to standard protocol. Data were calculated using the 2ΔΔCt method comparing ΔCt of treated A549 cells to ΔCt of control untreated samples. Reactions were incubated in the LightCycler® 480 Real-Time PCR System. Ct values were calculated using the SDS software version 2.3 applying automatic baselines and threshold settings.
Western blotting
After treatment, A549 cells were harvested and lysed with ice-cold cell lysis solution, and the homogenate was centrifuged at 10,000 g for 15 min at 4°C. Total protein in supernatants was quantified using a BCA protein assay kit. Total protein (30 μg) from each sample was separated by 12% SDS-PAGE and transferred to PVDF membrane. The PVDF membrane was placed in wash buffer containing skim milk powder at room temperature and blocked for 2 h. After washing 3 times, primary antibodies were added, and membranes were incubated at 4°C overnight. After 3 additional washes, horseradish peroxidase-conjugated secondary antibody was added for 1 h, and then X-ray film exposure was performed. The AlphaImager HP fluorescence/visible light gel imaging analyzer system was used to analyze band intensities.
Statistical analysis
Data are presented as the mean ± standard deviation (SD) of at least triplicates. Statistical analyses (two group comparisons) were performed using the Student’s t-test. P < 0.05 was considered to be statistically significant.
Results
PM2.5 decreases cell viability and induces apoptosis in A549 cells
To determine the effect of PM2.5 on A549 cell survival, cell viability assays were performed for A549 cells treated with or without PM2.5 over a time course (6, 12, 24, and 48) and over a dose curve (25 to 200 μg/ml PM2.5). PM2.5 significantly decreased cell viability in a time- and dose-dependent manner (Figure 1A). We also examined the effect of PM2.5 on cell apoptosis in A549 cells by PI/Annexin V double staining and TUNEL assay. PM2.5 significantly increased cell apoptosis in a dose-dependent manner (Figure 1B and 1C). Collectively, these data demonstrate that PM2.5 decreases cell viability and induces apoptosis in A549 cells.
Figure 1.
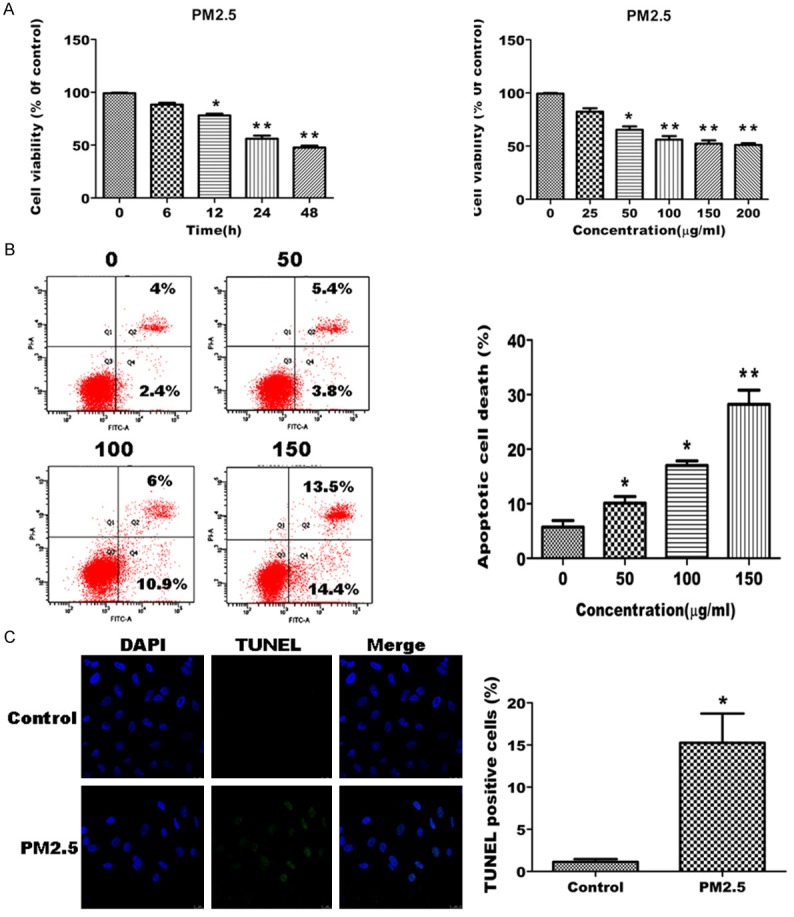
PM2.5 decreases cell viability and induce apoptosis in A549 cells. A. PM2.5 decreases cell viability in a concentration- and time-dependent manner. Cells were treated with 100 μg/ml PM2.5 for variable periods (6, 12, 24 and 48 h; left panel), or different concentrations (25, 50, 100, 150 and 200 μg/ml) of PM2.5 for 24 h (right panel). Cell viability was assessed by trypan blue staining. A representative experiment (left panel) and the mean ± SD from 3 separate experiments (right panel) are shown. Cells from triplicate wells and are representative of 3 independent experiments. B, C. PM2.5 induces apoptosis in A549 cells. B. Cells were treated with increasing concentrations (50,100 and 150 μg/ml) of PM2.5 for 24 h. Cell apoptosis was assessed by PI and Annexin V double staining. A representative experiment (left panel) and the mean ± SD from 3 separate experiments (right panel) are shown. C. Cells were treated with 100 μg/ml PM2.5 for 24 h, and then cell apoptosis was assessed by TUNEL assay. A representative assay (left panel) and the mean ± SD of 3 separate experiments (right panel) are shown, *p < 0.05, **p < 0.01, significant compared to controls.
PM2.5 induces autophagy in A549 cells
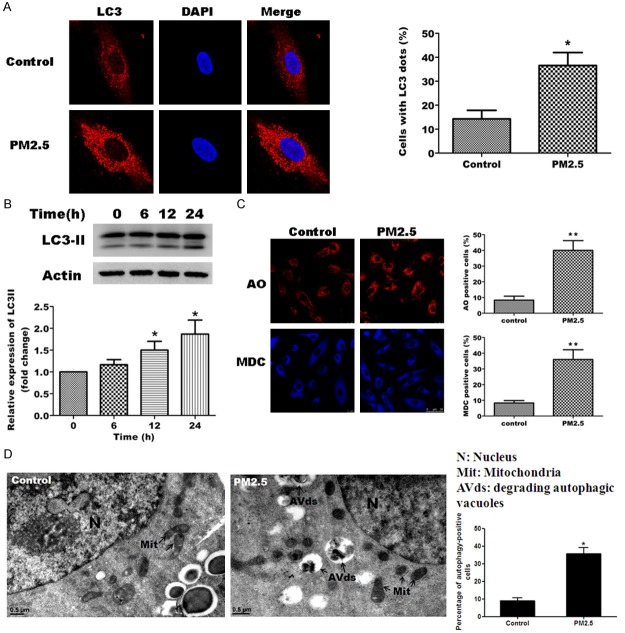
To determine whether PM2.5 induces autophagy in A549 cells, cells were exposed to 100 μg/ml PM2.5 for 24 h. We analyzed the expression of a known autophagy marker, LC3, by immunofluorescence staining and western blot analysis following exposure of A549 cells to 100 μg/ml PM2.5. Consistent with the induction of autophagy, incubation with PM2.5 significantly increased the number of cells with LC3 puncta staining (Figure 2A) and also increased the expression of the relevant form of LC3, LC3B-II as determined by western blotting (Figure 2B).
Figure 2.
PM2.5 induces autophagy in A549 cells. A, B. PM2.5 increases LC3B-II expression in A549 cells. A. A549 cells were left untreated (control) or were treated with 100 μg/ml PM2.5 for 24 h, and LC3 expression was assessed by immunofluorescence staining. DAPI staining of nuclei was performed as a background stain. A representative image is shown of the rather diffuse LC3 staining for the control cells and punctuates staining for the PM2.5 cells (left panel). Quantification of the number of cells with LC3 puncta was performed for > 100 cells from 3 independent experiments (right panel). B. Western blot of LC3 protein expression following treatment with 100 μg/ml PM2.5 for 0, 6, 12 and 24 h (left panel). Expression of LC3B-II was determined relative to actin and standardized to 1 in untreated cells (bottom panel). Results are representative of 3 independent experiments. C. PM2.5 increases autophagic vacuoles in A549 cells. Autophagic vacuoles were observed by Acridine orange (AO) and monodansylcadaverine (MDC) staining in untreated cells (control) or A549 cells treated with 100 μg/ml PM2.5 for 24 h. D. PM2.5 induces ultrastructural features of autophagy. A549 cells were treated with 100 µg/ml of PM2.5 for 24 h and processed for electron microscopy. Note the double membrane structure of the autophagic vacuoles. We indicate the presence of degrading autophagic vacuoles (AVds). N: Nucleus. Mit: Mitochondria. Bars 0.5 μm. Representative images are shown (right panel) and the mean ± SD of data from > 100 cells in 3 separate experiments (right panel). *p < 0.05, **p < 0.01, significant compared to controls.
We also examined autophagic vacuoles in A549 cells treated with or without PM2.5 by acridine orange staining and MDC incorporation assays. PM2.5 significantly increased the number of both acridine orange and MDC positive cells (Figure 2C). This findings support previous findings that PM2.5 induces autophagy in A549 cells [23]. We also investigated morphological change of autophagy in PM2.5-treated A549 cells (Figure 2D). PM2.5 exposure resulted in increased formation of immature and degradative AVs after 24 h exposure in A549 cells, as detected by electron microscopy. Quantification of electron micrographs revealed approximately 3-fold percentage of autophagy-positive cells in PM2.5-exposed A549 cells relative to PM2.5-untreated cells. These data clearly suggest that PM2.5 induces autophagy in A549 cells.
To support a molecular mechanism of PM2.5-induced autophagy in A549 cells, we examined the expression of the autophagy related genes, BECN1, ATG3, ATG5, ATG7 in A549 cells exposed to PM2.5 for variable periods (0.5, 2,4, 12 and 24 h) proteins BECN1, ATG5, ATG7,ULK1 and P-ULK1. QPCR results showed that mRNA expressions of each of these genes reached peak levels in A549 cells exposed to PM2.5 for 24 h (Figure 3A). Protein expressions of autophagy related genes reached peak levels in A549 cells exposed to PM2.5 for 6-12 h (Figure 3B). The increase in autophagy-related genes and proteins by PM2.5 further confirms the function of PM2.5 in stimulating autophagy.
Figure 3.
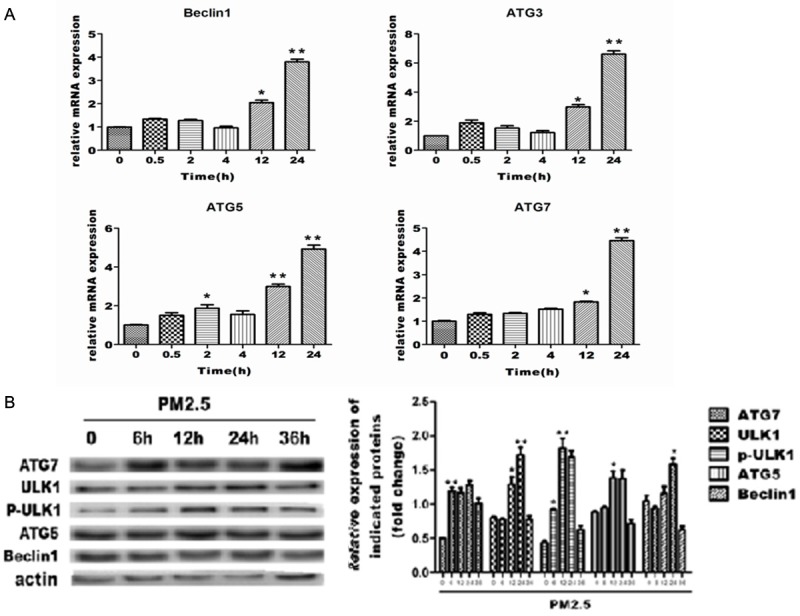
Changes in autophagy related mRNA and protein in A549 cells (respectively). Values are given as fold of change compared to control. Data is displayed as means ± SD. of at least three (n = 6) independent experiments for each time point. A. Quantitative RT-PCR analysis of the total RNAs from A549 cells treated with PM2.5. B. Western blot analysis of the protein expression of ATG7. ULK1. ATG5 and Beclin1 in A549 cells.
The AMPK signaling pathway is required for PM2.5-induced autophagy
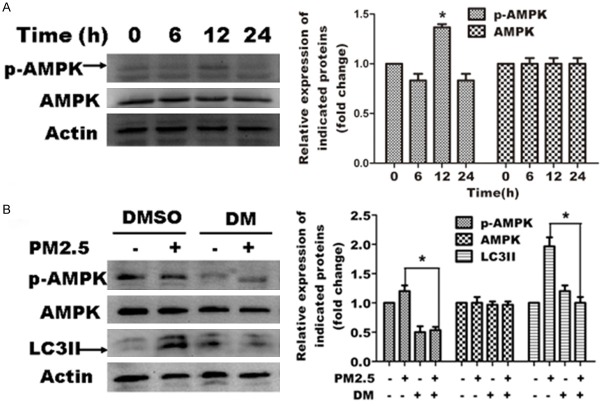
To further explore the mechanism underlying PM2.5-induced autophagy, we examined the expression and phosphorylation of AMPK in PM2.5-exposed A549 cells. Immunoblot analysis revealed that PM2.5 increased the phosphorylation of AMPK at 12 h PM2.5 exposure, but did not significantly change total expression (Figure 4A). To determine whether this increase correlates with AMPK function, we blocked AMPK phosphorylation using the specific AMPK inhibitor, dorsomorphin. Western blot analysis showed that dorsomorphin effectively blocked AMPK activation in A549 cells. Furthermore, PM2.5-induced autophagy was significantly decreased by dorsomorphin as revealed by LC3 expression analysis (Figure 4B). Collectively, these data indicate that AMPK is activated by 12 h PM2.5 exposure and that activation is required for PM2.5-induced autophagy.
Figure 4.
The AMPK signaling pathway positively regulates PM2.5-mediated autophagy in A549 cells. Representative images are shown in the left panels, and quantification relative to β-actin expression (mean ± SD of at least 3 separate experiments) is shown in the right panels. A. PM2.5 activates AMPK in A549 cells. Cells were treated with 100 μg/ml PM2.5 for variable periods (0, 6, 12 and 24 h). AMPK and p-AMPK expression was assessed by western blot analysis. B. Blocking AMPK activation attenuates PM2.5 mediated autophagy. Cells were treated with 100 μg/ml PM2.5 for 24 h following 6 h pretreatment with the AMPK inhibitor (40 μm/L)dorsomorphin (DM). DMSO was tested as a control. AMPK, p-AMPK and LC3 expression was assessed by western blot analysis.
The AKT signaling pathway inhibits PM2.5-induced autophagy in A549 cells
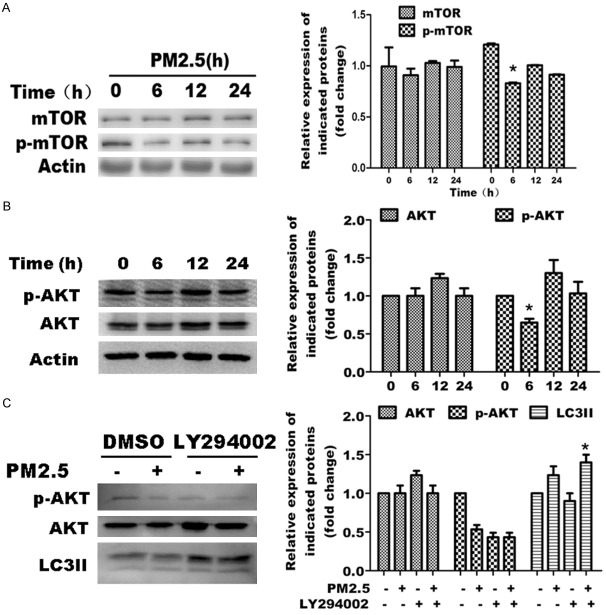
Previous studies also demonstrate an important role of the AKT/mTOR signaling pathway in autophagy. To determine whether this pathway is necessary for PM2.5-induced autophagy, we examined the protein expression and phosphorylation of AKT and mTOR in PM2.5-treated or -untreated A549 cells. PM2.5 did not significantly change the protein expression of total mTOR, though a modest but reproducible reduction in mTOR phosphorylation was observed at 6 h (Figure 5A). Furthermore, PM2.5 did not significantly change the protein expression of total AKT, but caused a decrease in AKT phosphorylation at 6 h, followed by an increase at 12 h (Figure 5B). To further explore the role for this pathway, we blocked AKT activation by using the AKT inhibitor, LY294002 in A549 cells treated with or without PM2.5. LY294002 eliminated p-AKT expression effectively, and simultaneously enhanced LC3B-II accumulation (Figure 5C). These results indicate that AKT activation fluctuates over the course of PM2.5 treatment, but that it displays an overall inhibitory effect on the signaling pathway of PM2.5-induced autophagy.
Figure 5.
The AKT/mTOR signaling pathways inhibits PM2.5-mediated autophagy in A549 cells. For figures A through C, representative images are shown on the left, and quantification of the mean ± SD derived from at least 3 separate experiments is shown on the right. Quantification was relative to β-actin expression in all experiments. A. PM2.5 causes a modest dephosphorylation in mTOR activation in A549 cells at 6 h. Cells were treated with 100 μg/ml PM2.5 for variable periods (0, 6, 12 and 24 h). The expression of mTOR and activated (phosphorylated) mTOR was assessed by western blot analysis. B. PM2.5 causes fluctuation of AKT activation in A549 cells. Cells were treated with 100 μg/ml PM2.5 for variable periods (0, 6, 12 and 24 h). AKT and p-AKT, expression was assessed by western blot analysis.C. AKT activation is not required for PM2.5-mediated autophagy. Cells were treated with 100 μg/ml PM2.5 for 24 h following 6 h pretreatment with AKT inhibitor LY294002(50 µM ). DMSO was tested as a control. AKT, p-AKT and LC3 expression was assessed by western blot analysis.
ERK is not required for PM2.5-induced autophagy in A549 cells
To determine whether the ERK signaling pathway is involved in PM2.5-induced autophagy, we examined the expression and phosphorylation status of ERK1/2 following exposure to PM2.5 in A549 cells. PM2.5 increased the phosphorylation of ERK1/2 with minimal change in total ERK1/2 expression (Figure 6A). We blocked ERK activation by using the ERK inhibitor, U0126, in A549 cells treated with or without PM2.5, and then investigated the effects on PM2.5-mediated autophagy. Western blot analysis showed that U1026 effectively blocked ERK phosphorylation; however, PM2.5-mediated autophagy was not affected as revealed by LC3 expression in the U0126 treated group compared to control group (Figure 6B). These data indicate that ERK is activated by PM2.5 and negatively regulates PM2.5-induced autophagy in part, and is not required for PM2.5-mediated autophagy in A549 cells.
Figure 6.
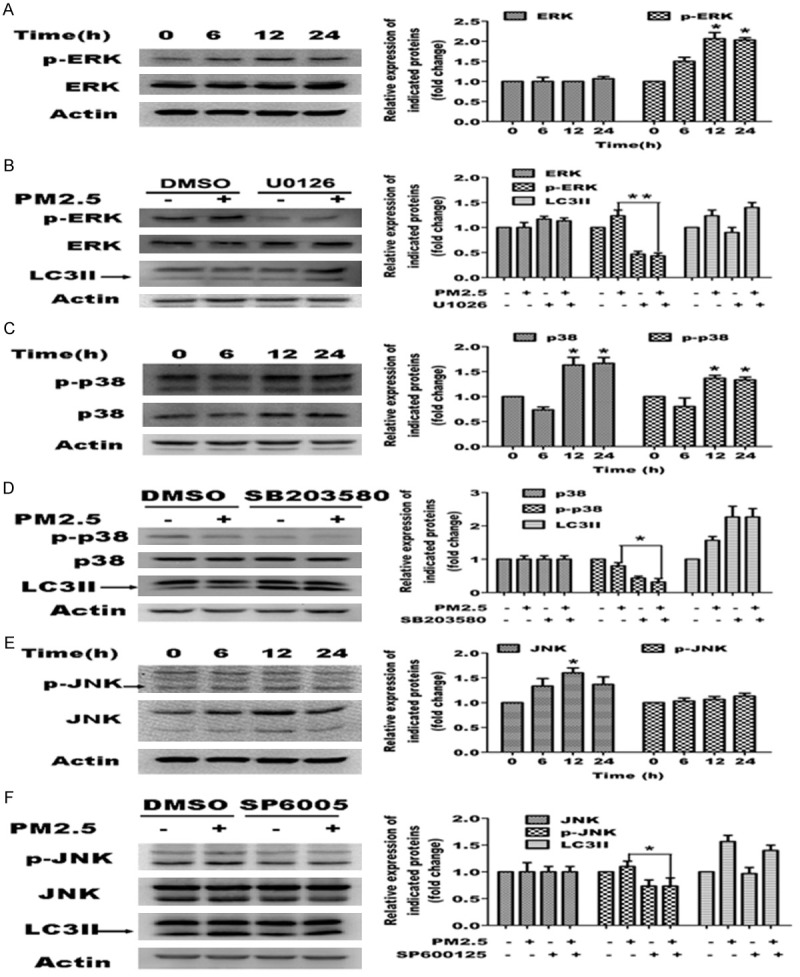
Role of the MAPK signaling pathways in PM2.5-mediated autophagy. Representative images are shown in the left panels, and quantification relative to β-actin expression (mean ± SD of at least 3 separate experiments) is shown in the right panels. A, C, E. PM2.5 activates MAPKs in A549 cells. Cells were treated with 100 μg/ml PM2.5 for variable periods (0, 6, 12 and 24 h). ERK, p-ERK, p38, p-p38, JNK and p-JNK expression was assessed by western blot analysis. B, D, F. Blocking ERK and p38 MAPKs enhances PM2.5-mediated autophagy. Cells were treated with 100 μg/ml PM2.5 for 24 h following 6 h pretreatment with ERK inhibitor U1026 (5 μM), p38 inhibitor SB203580 (10 μM), or JNK inhibitor SP6005 (50 μM). MAPKs and LC3 expression was assessed by western blot analysis.
p38 negatively regulates PM2.5-induced autophagy
To determine whether p38 signaling pathway is also involved in PM2.5-induced autophagy, we examined the expression and phosphorylation of p38 in PM2.5-exposed A549 cells. PM2.5 caused an elevation in total p38 protein expression, which was also reflected in the levels of p-p38 (Figure 6C). We blocked p38 activity using the p38 inhibitor, SB203580, in A549 cells treated with or without PM2.5. Western analysis showed that SB203580 blocked p38 phosphorylation without affecting total levels of p38. Conversely, LC3B-II expression was enhanced by SB203580 for both PM2.5 treated and untreated cells (Figure 6D). These results indicate that p38 expression is elevated by PM2.5 and that p38 negatively regulates PM2.5-induced autophagy in A549 cells.
JNK does not regulate PM2.5-induced autophagy in A549 cells
To determine whether the JNK signaling pathway mediates PM2.5-induced autophagy, we examined the expression and phosphorylation of JNK1/2 in A549 cells following PM2.5 exposure. PM2.5 increased total protein expression significantly, but not the phosphorylation of JNK1/2 (Figure 6E). Furthermore, the JNK1/2 inhibitor, SP600125, reduced the levels of p-JNK, but did not significantly change the LC3B-II expression in A549 cells treated with or without PM2.5 (Figure 6F). Collectively, this data indicates that JNK1/2 signaling does not affect PM2.5-induced autophagy in A549 cells significantly.
PM2.5-mediated autophagy is p53-independant
Previous studies demonstrate a crucial role for p53 in autophagy. To determine whether PM2.5 enhances the expression and phosphorylation of p53, we performed immunoblots of A549 cells following a time-course of exposure to PM2.5. Total protein expression and phosphorylation of p53 increased with maximal expression at 24 h (Figure 7A). To assess the function of p53, we blocked p53 activity using the p53 inhibitor, PFT-α, in A549 cells treated for 24 h with or without PM2.5. Western analysis showed that PFT-α reversed the induction of p53 phosphorylation following PM2.5 treatment, verifying its inhibitory function; however, PFT-α had no obvious effect on PM2.5-induced autophagy as revealed by LC3 expression (Figure 7B). These data indicate that PM2.5-induced autophagy is p53-independant.
Figure 7.
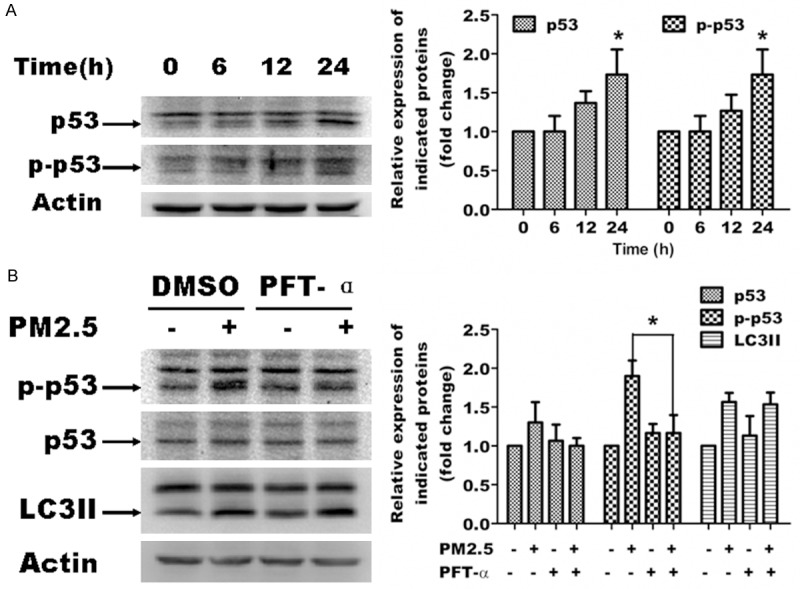
PM2.5-mediated autophagy is p53-independant. Representative images are shown in the left panels, and quantification relative to β-actin expression (mean ± SD of at least 3 separate experiments) is shown in the right panels. A. PM2.5 activates p53 in A549 cells. Cells were treated with 100 μg/ml PM2.5 for variable periods (0, 6, 12 and 24 h). p53 and p-p53 expression was assessed by western blot analysis. B. p53 inhibition does not affect PM2.5 mediated autophagy. Cells were pretreated for 6 h with p53 inhibitor PFT-α, and then treated with 100 μg/ml PM2.5 for 24 h. p53, p-p53 and LC3 expression was assessed by western blot analysis, *P < 0.05, significant compared to controls or without treatment of PFT-α.
Autophagy promotes cell survival of A549 cells following exposure to PM2.5
To determine whether autophagy regulates alveolar epithelial cell survival, rapamycin and 3-MA were used to induce and inhibit autophagy, respectively, in PM2.5 exposed A549 cells. The effect on autophagy by these drugs was confirmed by quantifying LC3 expression and distribution by western blot analysis and immunofluorescence staining and by quantification of AO-stained autophagic vacuoles (data not shown). Though PM2.5 and rapamycin each promoted cell death, the death by the two drugs in combination was significantly less than that of PM2.5 alone (Figure 8A); conversely, the addition of 3-MA significantly increased the cell death by PM2.5 alone (Figure 8B). These data indicate that autophagy protects A549 cells from cell death following exposure to PM2.5.
Figure 8.
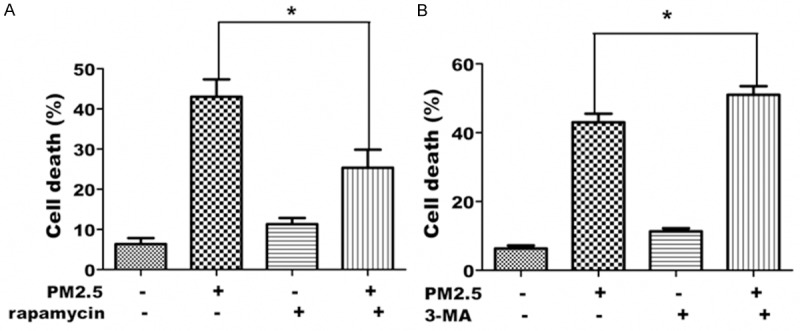
Autophagy is required for cell survival of A549 cells exposed to PM2.5. A. Inducing autophagy by rapamycin decreases PM2.5-induced cell death. A549 cells were treated with 100 μg/ml PM2.5 for 24 h following treatment with rapamycin for 6 h. Cell death was assessed by trypan blue staining. B. Inhibiting autophagy by 3-MA in A549 cells exposed to PM2.5 increases PM2.5-induced cell death. A549 cells were treated with 100 μg/ml PM2.5 for 24 h following treatment with 3-MA for 6 h. Cell death was assessed by trypan blue staining. Data represent the mean ± SD derived from at least 3 separate experiments (> 100 cells per experiment), *P < 0.05, significant compared to without treatment of 3-MA.
Discussion
PM2.5 is ambient airborne particulate matter with an aerodynamic diameter of less than 2.5 μm. It has become a major air pollutant and increases morbidity and mortality rates due to its ability to cause pulmonary and cardiovascular diseases, including COPD. Studies on cigarette smoking demonstrate that autophagy may play an important role in the pathogenesis of COPD, but the mechanism of autophagy in contributing to COPD remains unclear [6,23]. Increased autophagy has been demonstrated in lung tissue from COPD patients by electron microscopy and by the increased activation of autophagic proteins [23]. The aim of our study was to elucidate the molecular mechanisms underlying PM2.5-induced autophagy in A549 cells.
Accumulating evidence indicates that autophagy is a survival mechanism in response to cellular stress. Autophagy maintains homeostasis by eliminating excessive or unnecessary proteins and injured or aged organelles [24]. Our results confirm that autophagy functions as a survival mechanism for PM2.5-induced cytotoxicity in A549 cells: inhibition of autophagy by 3-MA increased cell death significantly, whereas induction of autophagy by rapamycin decreased cell death. Thus, though PM2.5 induces apoptotic cell death, autophagy serves to alleviate the deleterious effects of PM2.5.
Numerous studies indicate a central role of AMPK in the regulation of autophagy [14,25-27]. AMPK is a dual regulator of mTORC1 and ULK1 [13,14,25-27], an ATG1 homolog in mammalians that plays a key role in autophagy initiation stage [26,28]. In fact, AMPK-mediated autophagy serves as a survival mechanism in androgen-dependent prostate cancer cells [29]. Consistent with this role for AMPK, we show that PM2.5 induces phosphorylation of AMPK in A549 cells and that blocking AMPK activation with the AMPK inhibitor, dorsomorphin, eliminates PM2.5-induced activation of the autophagy marker LC3B-II. Our results indicate that activation of AMPK promoted the induction of autophagy in PM2.5-exposed A549 cells, and AMPK, therefore, serves as a positive regulator of autophagy.
Our results also show that PM2.5 activates AKT, the MAPKs and p53. AKT and the MAPKs are upstream regulators of mTOR that mediate responses to various extracellular stimuli. All four categories of MAPKs (ERK, p38, JNK/ SAPK and BMK) have been reported to regulate autophagy, though controversy exists as to whether they promote or inhibit autophagy [11,15]. In this study, we show that AKT, ERK and p38, serve a negatively regulatory role in PM2.5-induced autophagy. However, autophagy is independent of JNK, suggesting a specificity of the MAPK response to PM2.5. The autophagy induced by PM2.5 was also independent of p53. P53 is known to serve a dual role in the control of autophagy [30]: whereas nuclear p53 transactivates proapoptotic, cell cycle-arresting and proautophagic genes, cytoplasmic p53 can operate at the mitochondria to promote cell death and repress autophagy via poorly characterized mechanisms [16,31]. Thus, it is likely that a balance of these pathways contributes to the ultimate result of autophagy and cell death induction in response to PM2.5. Because PM2.5 induces an overall increase in autophagy, it can be presumed that the positive regulatory function of the AMPK pathway is the predominant regulatory pathway in the PM2.5 autophagy network. However, it is likely that other pathways are instrumental in the cell death response.
Apoptosis is implicated in COPD pathogenesis [32,33], and therefore we investigated the potential role of autophagy in PM2.5-induced cell death in A549 cells. Our data show that inhibition of autophagy by 3-MA increased cell death significantly, whereas induction of autophagy by rapamycin decreased cell death. These findings support our hypothesis that autophagy serves a survival mechanism for A549 cells when faced with PM2.5-mediated cytotoxicity.
In summary, a hypothetical model how these signaling might be coordinated is shown in Figure 9. In response to PM2.5-induced increases in cellular ROS [34,35], a self-protective mechanism is elicited by inducing autophagy via the AMPK pathway, AMPK predominates as a positive regulator of PM2.5-induced autophagy, whereas AKT, ERK and p38 serve as negative regulators of autophagy. The balance of this signaling axis mediates A549 cell autophagy, which protects cells from the PM2.5-induced death response. The elucidation of this signaling network advances our understanding of the pathogenic effect of PM2.5-associated chronic airway diseases and may reveal novel drug targets for the development of effective treatments.
Figure 9.
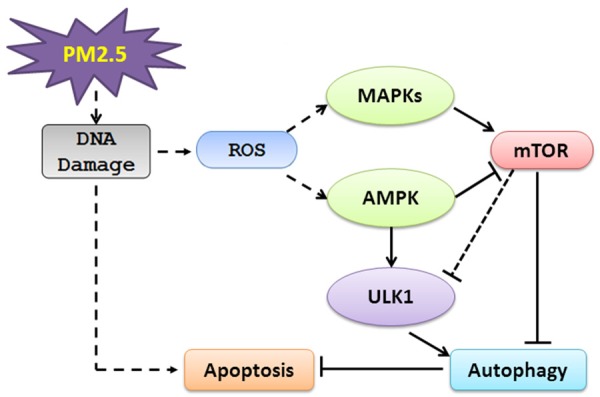
Schematic diagram describing PM2.5 induced autophagy mediated by the AMPK pathway. PM2.5 exposure in A549 cells causes the activation of AMPK, potentially through the ROS signaling pathway, which subsequently leads to autophagy. The AKT, MAPK and p53 signaling pathways are also activated, with an inhibitory effect by the AKT, ERK and p38 pathways and no apparent effect on autophagy by JNK and p53 signaling pathways. These results suggest there is a balance of activating and inhibitory pathways, and that activating pathways (ROS and AMPK) predominately regulate the response to PM2.5 to promote an overall induction of autophagy. The DNA damage induced by PM2.5 also activates apoptosis, which is counteracted in part by the induction of autophagy.
Acknowledgements
This project is supported by Science and Technology Innovation Fund of Guangdong Medical College (STIF201109) and by the Natural Science Foundation of China project (NSFC81172615), Guangdong Natural Science Foundation (S20122010008299), Science and Technology Planning Project of Guangdong Province (2012B031800223).
Disclosure of conflict of interest
None.
Abbreviations
- AMPK
Adenosine monophosphate-activated protein kinase
- AO
acridine orange
- MDC
Monodansylcadaverine
- mTOR
mammalian target of rapamycin
- LC3
microtubule-associated protein1 light chain 3
- MAPK
mitogen-activated protein kinase
- p38
p38 mitogen-activated protein kinase
- ERK
extracellular signal-regulated kinase
- JNK
c-jun-N-terminal Kinase
- siRNA
short interfering RNA
- ROS
reactive oxygen species
- 3-MA
3-methyladenine
References
- 1.Schwartz J. PM10 ozone, and hospital admissions for the elderly in Minneapolis-St. Paul, Minnesota. Arch Environ Health. 1994;49:366–374. doi: 10.1080/00039896.1994.9954989. [DOI] [PubMed] [Google Scholar]
- 2.Sunyer J, Basagaña X. Particles, and not gases, are associated with the risk of death in patients with chronic obstructive pulmonary disease. Int J Epidemiol. 2001;30:1138–1140. doi: 10.1093/ije/30.5.1138. [DOI] [PubMed] [Google Scholar]
- 3.Schikowski T, Mills IC, Anderson HR, Cohen A, Hansell A, Kauffmann F, Krämer U, Marcon A, Perez L, Sunyer J. Ambient air pollution: a cause of COPD. Eur Respir J. 2014;43:250–263. doi: 10.1183/09031936.00100112. [DOI] [PubMed] [Google Scholar]
- 4.Peacock JL, Anderson HR, Bremner SA, Marston L, Seemungal TA, Strachan DP, Wedzicha JA. Outdoor air pollution and respiratory health in patients with COPD. Thorax. 2011;66:591–596. doi: 10.1136/thx.2010.155358. [DOI] [PubMed] [Google Scholar]
- 5.Hussain SN, Sandri M. Role of autophagy in COPD skeletal muscle dysfunction. J Appl Physiol. 2013;114:1273–1281. doi: 10.1152/japplphysiol.00893.2012. [DOI] [PubMed] [Google Scholar]
- 6.Hwang JW, Chung S, Sundar IK, Yao H, Arunachalam G, McBurney MW, Rahman I. Cigarette smoke-induced autophagy is regulated by SIRT1-PARP-1-dependent mechanism: Implication in pathogenesis of COPD. Arch Biochem Biophys. 2010;500:203–209. doi: 10.1016/j.abb.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deng X, Zhang F, Rui W, Long F, Wang L, Feng Z, Chen D, Ding W. PM2.5-induced oxidative stress triggers autophagy in human lung epithelial A549 cells. Toxicol In Vitro. 2013;27:1762–1770. doi: 10.1016/j.tiv.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Webber JL, Tooze SA. Coordinated regulation of autophagy by p38α MAPK through mAtg9 and p38IP. EMBO J. 2010;29:27–40. doi: 10.1038/emboj.2009.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manjithaya R, Jain S, Farré JC, Subramani S. A yeast MAPK cascade regulates pexophagy but not other autophagy pathways. J Cell Biol. 2010;189:303–310. doi: 10.1083/jcb.200909154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jose FMM, Eva PJ, Erwin K. Glucose induces autophagy under starvation conditions by a p38 MAPK-dependent pathway. Biochem J. 2013;449:497–506. doi: 10.1042/BJ20121122. [DOI] [PubMed] [Google Scholar]
- 11.Jemma LW. Regulation of autophagy by p38alpha MAPK. Autophagy. 2010;6:292–293. doi: 10.4161/auto.6.2.11128. [DOI] [PubMed] [Google Scholar]
- 12.Zhou C, Zhou J, Sheng F, Zhu H, Deng X, Xia B, Lin J. The heme oxygenase-1 inhibitor ZnPPIX induces non-canonical, Beclin 1-independent, autophagy through p38 MAPK pathway. Acta Biochim Biophys Sin (Shanghai) 2012;44:815–822. doi: 10.1093/abbs/gms064. [DOI] [PubMed] [Google Scholar]
- 13.Alers S, Löffler AS, Wesselborg S, Stork B. Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: cross talk, shortcuts, and feedbacks. Mol Cell Biol. 2012;32:2–11. doi: 10.1128/MCB.06159-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim J, Guan KL. Regulation of the autophagy initiating kinase ULK1 by nutrients. Cell Cycle. 2011;10:1337–1338. doi: 10.4161/cc.10.9.15291. [DOI] [PubMed] [Google Scholar]
- 15.Hazzalin CA, Mahadevan LC. MAPK-regulated transcription: a continuously variable gene switch? Nat Rev Mol Cell Biol. 2002;3:30–40. doi: 10.1038/nrm715. [DOI] [PubMed] [Google Scholar]
- 16.Abida WM, Gu W. p53-Dependent and p53-independent activation of autophagy by ARF. Cancer Res. 2008;68:352–357. doi: 10.1158/0008-5472.CAN-07-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soberanes S, Panduri V, Mutlu GM, Ghio A, Bundinger GS, Kamp DW. p53 Mediates Particulate Matter-induced Alveolar Epithelial Cell Mitochondria-regulated Apoptosis. Am J Respir Crit Care Med. 2006;174:1229. doi: 10.1164/rccm.200602-203OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scherz-Shouval R, Elazar Z. ROS, mitochondria and the regulation of autophagy. Trends Cell Biol. 2007;17:422–427. doi: 10.1016/j.tcb.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 19.Montoya-Estrada A, Torres-Ramos Y, Flores-Pliego A, Ramirez-Venegas A, Ceballos-Reyes G, Guzman-Grenfell A, Hicks J. Urban PM2. 5 activates GAPDH and induces RBC damage in COPD patients. Front Biosci (Schol Ed) 2012;5:638–649. doi: 10.2741/s396. [DOI] [PubMed] [Google Scholar]
- 20.Lin Z, Liu T, Kamp DW, Wang Y, He H, Zhou X, Li D, Yang L, Zhao B, Liu G. AKT/mTOR and C-Jun N-terminal Kinase (JNK) Signaling Pathways Are Required for Chrysotile Asbestos-Induced Autophagy. Free Radic Biol Med. 2014;72:296–307. doi: 10.1016/j.freeradbiomed.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bølling AK, Totlandsdal AI, Sallsten G, Braun A, Westerholm R, Bergvall C, Boman J, Dahlman HJ, Sehlstedt M, Cassee F. Wood smoke particles from different combustion phases induce similar pro-inflammatory effects in a co-culture of monocyte and pneumocyte cell lines. Part Fibre Toxicol. 2012;9:15. doi: 10.1186/1743-8977-9-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu Z, Xu X, Zhong M, Hotchkiss IP, Lewandowski RP, Wagner JG, Bramble LA, Yang Y, Wang A, Harkema JR. Ambient particulate air pollution induces oxidative stress and alterations of mitochondria and gene expression in brown and white adipose tissues. Part Fibre Toxicol. 2011;8:20. doi: 10.1186/1743-8977-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen ZH, Kim HP, Sciurba FC, Lee SJ, Feghali-Bostwick C, Stolz DB, Dhir R, Landreneau RJ, Schuchert MJ, Yousem SA. Egr-1 regulates autophagy in cigarette smoke-induced chronic obstructive pulmonary disease. PLoS One. 2008;3:e3316. doi: 10.1371/journal.pone.0003316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Der Vaart A, Mari M, Reggiori F. A picky eater: exploring the mechanisms of selective autophagy in human pathologies. Traffic. 2008;9:281–289. doi: 10.1111/j.1600-0854.2007.00674.x. [DOI] [PubMed] [Google Scholar]
- 25.Roach PJ. AMPK -> ULK1 -> autophagy. Mol Cell Biol. 2011;31:3082–3084. doi: 10.1128/MCB.05565-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mao K, Klionsky DJ. AMPK activates autophagy by phosphorylating ULK1. Circ Res. 2011;108:787–788. doi: 10.1161/RES.0b013e3182194c29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, Mair W, Vasquez DS, Joshi A, Gwinn DM, Taylor R. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science. 2011;331:456–461. doi: 10.1126/science.1196371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chhipa RR, Wu Y, Ip C. AMPK-mediated autophagy is a survival mechanism in androgen-dependent prostate cancer cells subjected to androgen deprivation and hypoxia. Cell Signal. 2011;23:1466–1472. doi: 10.1016/j.cellsig.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mayo LD. Directing p53 to induce autophagy. Cell Cycle. 2012;11:3354. doi: 10.4161/cc.21849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang X, Wu Z, Mei Y, Wu M. XIAP inhibits autophagy via XIAP-Mdm2-p53 signalling. EMBO J. 2013;32:2204–2216. doi: 10.1038/emboj.2013.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petrache I, Natarajan V, Zhen L, Medler TR, Richter AT, Cho C, Hubbard WC, Berdyshev EV, Tuder RM. Ceramide upregulation causes pulmonary cell apoptosis and emphysema-like disease in mice. Nat Med. 2005;11:491–498. doi: 10.1038/nm1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kasahara Y, Tuder RM, Taraseviciene-Stewart L, Le Cras TD, Abman S, Hirth PK, Waltenberger J, Voelkel NF. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest. 2000;106:1311–1319. doi: 10.1172/JCI10259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soberanes S, Urich D, Baker CM, Burgess Z, Chiarella SE, Bell EL, Ghio AJ, De Vizcaya-Ruiz A, Liu J, Ridge KM, Kamp DW, Chandel NS, Schumacker PT, Mutlu GM, Budinger GR. Mitochondrial complex III-generated oxidants activate ASK1 and JNK to induce alveolar epithelial cell death following exposure to particulate matter air pollution. J Biol Chem. 2009;284:2176–2186. doi: 10.1074/jbc.M808844200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Torres-Ramos Y, Montoya-Estrada A, Guzman-Grenfell A, Mancilla-Ramirez J, Cardenas-Gonzalez B, Blanco-Jimenez S, Sepulveda-Sanchez J, Ramirez-Venegas A, Hicks J. Urban PM2. 5 induces ROS generation and RBC damage in COPD patients. Front Biosci (Elite Ed) 2010;3:808–817. doi: 10.2741/e288. [DOI] [PubMed] [Google Scholar]
- 36.Yang L, Liu G, Lin Z, Wang Y, He H, Liu T, Kamp DW. Pro-inflammatory response and oxidative stress induced by specific components in ambient particulate matter in human bronchial epithelial cells. Environ Toxicol. 2014 doi: 10.1002/tox.22102. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]