Abstract
Objective: A meta-analysis was carried out to summarize published data on the relationship between breast cancer and dietary factors. Methods: Databases in Chinese (China National Knowledge Infrastructure [CNKI], China Biology Medicine [CBM], WanFang, VIP) and in English (PubMed and Web of Science) were searched for articles analyzing vegetable, fruit, soy food and fat consumption and breast cancer risk published through June 30, 2013. Random effects models were used to estimate summary odds ratios (OR) based on high versus low intake, and subgroup analysis was conducted according to region, study design, paper quality and adjustment for confounding factors to detect the potential source of heterogeneity. Every study was screened according to the inclusion criteria and exclusion criteria, evaluated in accordance with the Newcastle-Ottawa Scale. RevMan 5.2 software was used for analysis. Results: Of 785 studies retrieved, 22 met inclusion criteria (13 in Chinese and 9 in English), representing 23,201 patients: 10,566 in the experimental group and 12,635 in the control group. Thirteen included studies showed vegetables consumption to be a relevant factor in breast cancer risk, OR = 0.77 (95% CI [confidence interval] 0.62-0.96). Eleven studies showed fruits consumption to be relevant, OR = 0.68 (95% CI 0.49-0.93). Significant differences were also found between those who consumed soy foods, OR = 0.68 (95% CI 0.50-0.93) and those who ate a high-fat diet, OR = 1.15 (95% CI 1.01-1.30). Conclusion: This analysis confirms the association between intake of vegetables, fruits, soy foods and fat and the risk of breast cancer from published sources. It’s suggested that high consumption of vegetables, fruits and soy foods may reduce the risk of breast cancer, while increasing fat consumption may increase the risk.
Keywords: Breast cancer, dietary factor, meta-analysis
Introduction
Breast cancer, the most common malignant tumor in women, seriously threatens the physical and mental health of women throughout the world. The incidence of breast cancer is high in developed North American and Northern European countries, but lower in some Asian countries, including China and Japan. Breast cancer accounts for 16.81% of all malignant tumors in women, the most for any type [1]. Long-term trend studies and migration studies show that the descendants of people who emigrate from low-incidence countries to high-incidence countries have an incidence of breast cancer similar to that of local residents. This result suggests that environmental factors induce changes in breast cancer-related risk factors and affect the level of exposure to risk factors.
Although the influence of lifestyle and environment factors on breast cancer seems very strong, no clear epidemiological data support the effect of diet on human breast cancer. At present, internationally accepted risk factors for breast cancer primarily include age, family history of breast cancer, medical history of benign breast tumor, dietary factors, reproductive factors, social factors and hormone levels. However, most studies have involved only women in Western countries. In China, few studies have been conducted on the causes of breast cancer. In addition, comparing the results of these studies is complicated by their different subject characteristics, study designs, sample sizes and statistical methods. For example, according to a report from the World Cancer Research Fund (WCRF), only 11 prospective studies and 23 case-control studies have analyzed the relationship between fat in the diet and breast cancer. However, 10 of the 11 prospective studies reported that higher total fat intake did not significantly increase the risk of breast cancer and only one study (including 99 cases) showed a significantly decreased risk of breast cancer in the group with the lowest quartile of total fat intake [2]. Many studies consider that fruits, vegetables and soy foods are protective factors for the human body. Moreover, it has been reported that a high fiber diet can inhibit intestinal reabsorption of estrogen and increase fecal excretion of estrogen [3]. However, studies differ in their results about the relationship between the amount of dietary fiber and the risk of breast cancer. In an early meta-analysis of 12 case-control studies showed a negative correlation between the amount of fiber intake and the risk of breast cancer (odds ratio [OR] = 0.85) [4]. However, most prospective studies do not find that a large amount of fruits, vegetables and soy foods in the diets of adult women have a protective effect on the occurrence of breast cancer [5-21].
At present, it is not clear whether the different results of various studies can be explained by the amount of dietary fiber or by the low statistical efficiency of individual studies, by the differences between studies in the demographic characteristics of subjects, by measurement error in prospective studies or by selection and recall biases in case-control studies. The authoritative book Food, Nutrition, Physical Activity and the Prevention of Cancer published in 2007 by the WCRF indicates that the correlation between the amount of fiber intake and the risk of breast cancer is very limited or that the conclusions are inconsistent [22]. Since the release of this report, eight prospective studies have reported on the correlation between diet and breast cancer [14-21,23]. Therefore, the aim of the current paper is to collect the peer-reviewed articles on studies of the link between dietary factors and breast cancer, carry out statistical meta-analysis of the studies using Chinese women as subjects and investigate the correlation between dietary factors and breast cancer in Chinese women.
Materials and methods
Search strategy
We searched major domestic and international databases, including databases in Chinese (i.e., China National Knowledge Infrastructure [CNKI], VIP, WanFang and China Biology Medicine [CBM]) and databases in English (i.e., PubMed and the Web of Science). We also used literature review and manual search methods to collect papers on the correlation between breast cancer and diet in Chinese women. The end date of the literature search is June 2013.
The retrieval strategy for Chinese papers included selecting those with a subject of breast cancer or breast tumor and with one or more of the following keywords: diet, soy, legume, soy food, vegetable, fruit, dietary fat or fatty acid, case-control study, cohort study, randomized controlled trial, prospective studies, controlled clinical trial, risk factor, and influencing factor.
The retrieval strategy for English papers included selecting those with a subject of breast cancer or breast neoplasm or breast tumor or breast adenocarcinoma and one or more of the following keywords: dietary, diet, food, vegetable, fruit, bean, soy, legume, isoflavone, risk factor, case-control study, cohort study, randomized controlled trial, RCT, controlled clinical trial, Chinese women or Chinese female.
Inclusion criteria and exclusion criteria
Inclusion criteria
(1) Analytical epidemiological studies on the risk of breast cancer and dietary factors published in China or abroad, including case-control studies, cohort studies, randomized controlled trials, prospective studies, and controlled clinical trials. (2) The content of the study includes risk factors for breast cancer and dietary factors; and the study provides complete contingency tables or OR/relative risk (RR) values for the highest intake compared with the lowest (or these can be calculated using the data provided in the paper). (3) Subjects are Chinese women or contain Chinese women. (4) Studies published in Chinese or English.
Exclusion criteria
(1) Reviews (including systematic reviews), comments, case reports or other anecdotal papers. (2) Case-control studies with less than 100 cases in the sample size and studies with incomplete data or those being published repeatedly. (3) Studies in which breast cancer cannot be separated from other cancers.
Data abstraction and quality evaluation
Two reviewers searched all the literature, collected data and related information after evaluating the quality of the study. For different papers based on the same research, we selected the paper with the largest sample size or the most recently published paper or one with sufficient data for analysis. Collected data included first author, year of publication, study type, sample size of cases/controls, quantity, source of cases/controls, and contingency table or OR/RR value related to the risk factors. Among these, OR/RR value was obtained from the result of univariate analysis only, not from the result of adjusted multivariate analysis. The Newcastle-Ottawa Scale (NOS) recommended by the Cochrane Collaboration was used, and eight criteria were established to evaluate the quality of the included case-control studies and cohort studies [24], such being the case, studies could receive a score of 0-9 points, based on the three criteria of subject selection, comparability between groups and measurement of exposure factors.
Statistical analysis
RevMan 5.2 (http://ims.cochrane.org/revman) was used to analyze the included studies. To evaluate the study results conservatively, we applied a random effects model to calculate effect sizes (OR), corresponding 95% confidence intervals (CI) and P-values for heterogeneity. A funnel plot was used to check for the presence of bias. A symmetric inverted funnel shape indicates the absence of publication bias; an asymmetric or incomplete funnel indicates the possible presence of publication bias [25]. To identify the sources of heterogeneity across studies, subgroup analyses were conducted, which includes 4 factors (study design, region, study quality and adjustment for confounders). This analysis focused on the association between vegetables, soy foods, fruits intake and breast cancer risk, because the greater number of studies permits investigation of further sub-groups. We also conducted a sensitivity analysis excluding one study in turn to confirm the stability of the study.
Results
Characteristics of studies
Our search initially identified 785 relevant studies. Among these, 464 studies were excluded after we read the title and abstract. After we read the full text, 25 of the remaining 321 studies were selected according to the above-mentioned inclusion and exclusion criteria. Six of 25 of included studies were different papers from the same studies written by the same authors, including, Rong SY, Xu GF, Tao MH. Of these, the one paper with the largest sample sizes was selected [26], another two papers with greater data were selected when the sample size was the same [27,28]. Therefore, a total of 22 studies were included in our final systematic review [26-47], which contained at least a fourfold table reflecting the relationship between breast cancer and vegetables, fruits, soy foods or dietary fat, 9 in English and 13 in Chinese (Figure 1). These studies represent a total of 23,201 patients, 10,566 in the experimental group and 12,635 in the control group; the basic information for each study is shown in Table 1. Among the included studies, both by Zhang CX [39,40] were included because the subjects of two studies were different. NOS scores ranged from 4 to 9: 4 points for 1 study, 5 for 5, 6 for 5, 7 for 5, 8 for 5 and 9 for 1 study.
Figure 1.

Flow diagram for search results.
Table 1.
Characteristics of included studies
| First author, Year | Region | Type of study | Exposure | Quantity | Number of cases | Number of controls | NOS |
|---|---|---|---|---|---|---|---|
| Rong SY 2008 [26] | Hebei | Case-control study | Soy foods | ≥3 times/month vs. ≤1 times/month | 175 | 175 | 6 |
| Vegetables | ≥3 times/week vs. ≤1 times/week | ||||||
| Fruits | ≥3 times/week vs. ≤1 times/week | ||||||
| Xu GF 1998 [27] | Shangdong | Case-control study | Soy foods | >100 g/d vs. <10 g/d | 186 | 186 | 7 |
| Vegetables | >400 g/d vs. <50 g/d | ||||||
| Fat | >100 g/d vs. <50 g/d | ||||||
| Tao MH 2001 [28] | Shanghai | Case-control study | Soy foods | >3631.75 g/year vs. ≤1182.6 g/year | 356 | 925 | 5 |
| Vegetables | >9000 g/month vs. ≤6000 g/month | ||||||
| Fruits | >5250 g/month vs. ≤1040 g/month | ||||||
| Ren XN 2008 [29] | Liaoning | Case-control study | Soy foods | >3 times/week vs. <2 times/week | 200 | 200 | 5 |
| Vegetables | >14 times/week vs. ≤7 times/week | ||||||
| Fruits | ≥14 times/week vs. <7 times/week | ||||||
| Wang YQ 2006 [30] | Zhejiang | Cohort study | Soy foods | >4.8 kg/year vs. ≤0.799 kg/year | 84 | 269 | 9 |
| Vegetables | 36 kg/year vs. ≤7.2 kg/year | ||||||
| Zhao YB 1999 [31] | Sichuang | Case-control study | Soy foods | Occasional vs. regular | 265 | 265 | 5 |
| Vegetables | >250 g/day vs. <250 g/day | ||||||
| Fat | More vs. less | ||||||
| Fruits | Occasional vs. regular | ||||||
| Zou L 2003 [32] | Hubei | Case-control study | Soy foods | Occasional vs. regular | 112 | 112 | 5 |
| Vegetables | >250 g/day vs. <250 g/day | ||||||
| Fat | More vs. less | ||||||
| Fruits | Occasional vs. regular | ||||||
| Zhou L 2009 [33] | Jiangsu | Case-control study | Soy foods | >6500 g/year vs. ≤2500 g/year | 206 | 214 | 5 |
| Fruits | >54750 g/year vs 36500 g/year | ||||||
| Wang LY 2008 [34] | Beijing | Case-control study | Soy foods | >3 times/week vs. 0 | 429 | 781 | 4 |
| Cai SR 1996 [35] | Ningxia | Case-control study | Soy foods | Occasional vs. regular | 100 | 100 | 6 |
| Yuan JM 1987 [36] | Shanghai | Case-control study | Fat | >72.5 g/day vs. <38 g/day | 534 | 534 | 6 |
| Fu XJ2012 [37] | Guangdong | Case-control study | Vegetables | Occasional vs. regular | 419 | 372 | 7 |
| Fruits | Occasional vs. regular | ||||||
| Zhang JC 2003 [38] | Henan | Case-control study | Soy foods | Occasional vs. regular | 300 | 300 | 6 |
| Vegetables | >250 g/day vs. <250 g/day | ||||||
| Fat | More vs. less | ||||||
| Fruits | Occasional vs. regular | ||||||
| Zhang CX 2011 [39] | Guangdong | Case-control study | Fat | Quartiles of intake Q4 vs. Q1 | 438 | 438 | 7 |
| Zhang CX 2009 [40] | Guangdong | Case-control study | Vegetables | Quartiles of intake Q4 vs. Q1 | 438 | 438 | 7 |
| Fruits | Quartiles of intake Q4 vs. Q1 | ||||||
| Shannon 2005 [41] | Shanghai | Case-control study | Soy foods | ≥1.1 servings/day vs. ≤2.6 servings/week | 378 | 1070 | 8 |
| Vegetables | ≥2.6 servings/day vs. ≤1.5 servings/day | ||||||
| Fruits | ≥1.2 servings/day vs. ≤3.9 servings/week | ||||||
| Shen YP 2006 [42] | Shanghai | Case-control study | Vegetables | >73000 g/year vs. ≤73000 g/year | 282 | 298 | 8 |
| Fruits | >41600 g/year vs. ≤41600 g/year | ||||||
| Yu ZG 2012 [43] | Shangdong | Case-control study | Soy foods | Nearly every day vs. never | 103 | 309 | 7 |
| Vegetables | Nearly every day vs. never | ||||||
| Lee 2005 [44] | Taiwan | Case-control study | Soy foods | >341 g/day vs. <114 g/day | 250 | 219 | 6 |
| Fat | >98 g/day vs. <56 g/day | ||||||
| Kallianpur 2008 [45] | Shanghai | Case-control study | Fat | Quartiles of intake Q4 vs. Q1 | 3452 | 3474 | 8 |
| Wang Q 2010 [46] | Sichuang | Case-control study | Fat | ≥77 g/day vs. <77 g/day | 400 | 400 | 8 |
| Dai Q 2001 [47] | Shanghai | Case-control study | Soy foods | >91 g/week vs. ≤35 g/day | 1459 | 1556 | 8 |
NOS, Newcastle-Ottawa Scale.
Odds ratios estimates of vegetables consumption and breast cancer
Thirteen studies [26-32,37,38,40-43] reported the relationship between vegetables consumption and breast cancer. These represented a total of 8,174 cases: 3,275 in the experimental group and 4,899 in the control group. Comprehensive evaluation of vegetables consumption and breast cancer using the meta-analysis showed a significant difference between the experimental and control groups (P = 0.02), which suggests the presence of correlation between high versus low vegetables intake and breast cancer. The combined OR value was 0.77 (95% CI: 0.62-0.96, I2 = 65%) (Figure 2). Funnel plot analysis showed a basically symmetrical funnel plot, suggesting no publication bias in these studies (Figure 3).
Figure 2.

The Odd Ratio of vegetables and breast cancer risk in Chinese women (high vs. low intake).
Figure 3.

The funnel plot of related articles on vegetables.
Odds ratio estimates of fruits consumption and breast cancer
Eleven studies [28,29,31-33,35,37,38,40-42] reported a correlation between fruits consumption and breast cancer. These studies included a total of 7,457 cases: 3,108 in the experimental group and 4,349 in the control group. Comprehensive evaluation of fruits consumption and risk of breast cancer using the meta-analysis showed significant differences between the experimental and control groups (P = 0.02), suggesting a correlation between the higher fruits consumption and the lower breast cancer risk. The studies had a combined OR value of 0.68 (95% CI: 0.49-0.93, I2 = 89%) (Figure 4). Funnel plot analysis revealed a basically symmetrical funnel plot, suggesting no significant publication bias among the included studies (Figure 5).
Figure 4.
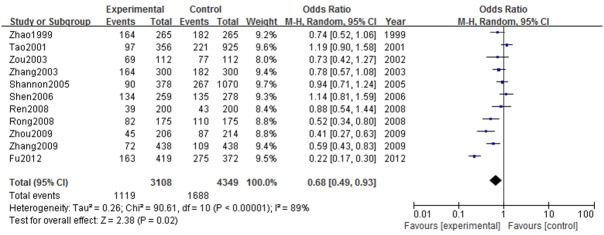
The Odd Ratio of fruits and breast cancer risk in Chinese women (high vs. low intake).
Figure 5.

The funnel plot of related articles of fruits.
Odds ratio estimates of soy foods consumption and breast cancer
Fifteen studies [26-35,38,41,43-44,47] reported a correlation between soy foods consumption and breast cancer. These studies included a total of 11,283 cases: 4,602 in the experimental group and 6,681 in the control group. Comprehensive evaluation of soy foods consumption and risk of breast cancer using the meta-analysis showed a significant difference between the experimental and control groups (P = 0.02), and the combined OR value is 0.68 (95% CI: 0.50-0.93, I2 = 89%) (Figure 6), suggesting a correlation that high soy foods consumption have a comparable protective effect on breast cancer. Funnel plot analysis revealed a basically symmetrical funnel plot, indicating no significant publication bias among the included studies (Figure 7).
Figure 6.

The Odd Ratio of soy foods and breast cancer in Chinese women (high vs. low intake).
Figure 7.

The funnel plot of related articles of soy foods.
Odds ratio estimates of fat consumption and breast cancer
Nine studies [27,31-32,36,38,39,44-46] reported a correlation between high dietary fat intake and breast cancer. These studies included a total of 10,683 cases: 4,302 in the experimental group and 6,381 in the control group. Comprehensive evaluation of high dietary fat intake and risk of breast cancer using the meta-analysis showed a significant difference between the experimental and control groups (P = 0.03), suggesting a correlation between high dietary fat intake and increased risk of breast cancer. The combined OR value was 1.15 (95% CI: 1.01-1.30, I2 = 36%) (Figure 8), indicating high-fat food consumption may increase the risk of breast cancer. Funnel plot analysis revealed a basically symmetrical funnel plot, suggesting no significant publication bias among included studies (Figure 9).
Figure 8.

The Odd Ratio of intake of fat and breast cancer risk in Chinese women (high vs. low intake).
Figure 9.

The funnel plot of related articles of fat.
Subgroup and sensitivity analysis
In the subgroup analysis (Table 2), the association between high versus low vegetables, fruits and soy foods and breast cancer risk was inverse in most strata, although usually not statistically significant. Thirteen studies were included in the high versus low analysis of vegetables and fifteen studies were included for soy foods intake, the summary associations were mostly similar except in studies that adjusted or not for study design and body mass index, weight, waist-to-hip ratio. According to study design, there was a suggestion of a difference in the results for vegetables and soy foods among case-control study and cohort study, however, the summary OR were approaching 1, which indicates no association, and only one study was among cohort study [41]. Meanwhile, after stratified according to body mass index, weight, waist-to-hip ratio, a difference in the results between studies of vegetables intake was suggested, with approximately no association among eight studies (OR = 1.04, 95% CI: 0.89-1.22; P heterogeneity = 0.93) without any variability [29-32,38,41-43], but an inverse association among the studies which did not. When it refers to fruits, the summary associations were similar in different subgroups. After excluding each study, we performed a sensitivity analysis of the remaining studies by using vegetables, fruits and soy foods between highest and lowest consumption as the observation indicators. The results of analysis revealed these ORs were approximately close from the results before the study was excluded. This result suggests that the results of evaluation in the current study are stable.
Table 2.
Subgroup analyses of vegetables, soy foods, fruits intake and breast cancer risk
| Vegetables | Soy foods | |||||||
|
|
||||||||
| Possible heterogeneity factors | No.of studies | OR (95% CI) | I2 (%) | Ph a | No. of studies | OR (95% CI) | I2 (%) | Ph a |
|
| ||||||||
|---|---|---|---|---|---|---|---|---|
| All studies | 13 | 0.77 (0.62-0.96) | 65% | 0.0006 | 15 | 0.68 (0.50-0.93) | 89% | <0.00001 |
| Region | ||||||||
| Eastern areas | 10 | 0.74 (0.56-0.98) | 73% | 0.0001 | 11 | 0.68 (0.46-1.02) | 91% | <0.00001 |
| Middle areas | 2 | 0.93 (0.64-1.35) | 0 | 0.92 | 2 | 0.57 (0.43-0.76) | 0 | 0.65 |
| Western areas | 1 | 0.86 (0.46-1.60) | 2 | 0.84 (0.30-2.40) | 84% | 0.01 | ||
| Paper quality | ||||||||
| Score ≥7 | 7 | 0.78 (0.54-1.14) | 78% | 0.0001 | 5 | 0.87 (0.60-1.26) | 70% | 0.009 |
| Score <7 | 6 | 0.74 (0.60-0.93) | 18% | 0.29 | 10 | 0.62 (0.41-0.93) | 91% | <0.00001 |
| Study design | ||||||||
| Case control study | 12 | 0.76 (0.60-0.96) | 68% | 0.0004 | 14 | 0.66 (0.48-0.92) | 89% | <0.00001 |
| Cohort study | 1 | 1 (0.52-1.92) | 1 | 1.09 (0.60-1.98) | ||||
| Population based study | 6 | 0.79 (0.59-1.06) | 45% | 0.11 | 7 | 0.56 (0.32-0.98) | 92% | <0.00001 |
| Hospital based study | 7 | 0.76 (0.53-1.07) | 76% | 0.0003 | 8 | 0.81 (0.58-1.15) | 82% | <0.00001 |
| Adjustment for confounders | ||||||||
| Age at menarche | ||||||||
| Yes | 5 | 0.67 (0.42-1.07) | 76% | 0.002 | 4 | 0.45 (0.18-1.14) | 95% | <0.00001 |
| No | 8 | 0.84 (0.66-1.06) | 48% | 0.06 | 11 | 0.80 (0.61-1.04) | 78% | <0.00001 |
| Age at first birth | ||||||||
| Yes | 4 | 0.77 (0.49-1.21) | 70% | 0.02 | 4 | 0.68 (0.53-0.87) | 36% | 0.20 |
| No | 9 | 0.77 (0.58-1.02) | 67% | 0.002 | 11 | 0.68 (0.45-1.05) | 92% | <0.00001 |
| BMI, Weight, WHR | ||||||||
| Yes | 5 | 0.54 (0.43-0.68) | 26% | 0.25 | 4 | 0.59 (0.44-0.78) | 42% | 0.16 |
| No | 8 | 1.04 (0.89-1.22) | 0 | 0.93 | 11 | 0.73 (0.49-1.11) | 91% | <0.00001 |
| Energy intake | ||||||||
| Yes | 4 | 0.78 (0.45-1.37) | 86% | 0.0001 | 4 | 0.97 (0.67-1.43) | 81% | 0.001 |
| No | 9 | 0.78 (0.63-0.98) | 40% | 0.10 | 11 | 0.59 (0.40-0.89) | 89% | <0.00001 |
| Education | ||||||||
| Yes | 7 | 0.85 (0.69-1.05) | 17% | 0.30 | 8 | 0.57 (0.35-0.94) | 91% | <0.00001 |
| No | 6 | 0.72 (0.48-1.09) | 81% | <0.0001 | 7 | 0.86 (0.63-1.17) | 75% | 0.0005 |
|
| ||||||||
| Fruits | ||||||||
|
|
||||||||
| Possible heterogeneity factors | No.of studies | OR (95% CI) | I2 (%) | Ph a | ||||
|
| ||||||||
| All studies | 11 | 0.68 (0.49-0.93) | 89% | <0.00001 | ||||
| Region | ||||||||
| Eastern areas | 8 | 0.65 (0.42-1.01) | 92% | <0.00001 | ||||
| Middle areas | 2 | 0.77 (0.58-1.02) | 0 | 0.83 | ||||
| Western areas | 1 | 0.74 (0.52-1.06) | ||||||
| Paper quality | ||||||||
| Score ≥7 | 4 | 0.61 (0.30- 1.26) | 95% | <0.00001 | ||||
| Score <7 | 7 | 0.73 (0.55-0.96) | 72% | 0.001 | ||||
| Study design | ||||||||
| Case control study | 11 | 0.68 (0.49-0.93) | 89% | <0.00001 | ||||
| Cohort study | 0 | |||||||
| Population based study | 7 | 0.74 (0.63-0.87) | 96% | <0.00001 | ||||
| Hospital based study | 4 | 0.54 (0.25-1.14) | 23% | 0.25 | ||||
| Adjustment for confounders | ||||||||
| Age at menarche | ||||||||
| Yes | 4 | 0.51 (0.24-1.09) | 95% | <0.00001 | ||||
| No | 7 | 0.82 (0.69-0.98) | 36% | 0.15 | ||||
| Age at first birth | ||||||||
| Yes | 3 | 0.73 (0.43-1.25) | 87% | 0.0006 | ||||
| No | 8 | 0.66 (0.44-1.00) | 90% | <0.00001 | ||||
| BMI, Weight, WHR | ||||||||
| Yes | 4 | 0.54 (0.25-1.14) | 95% | <0.00001 | ||||
| No | 7 | 0.79 (0.63-0.99) | 61% | 0.02 | ||||
| Energy intake | ||||||||
| Yes | 3 | 0.88 (0.60-1.29) | 80% | 0.007 | ||||
| No | 8 | 0.61 (0.40-0.92) | 89% | <0.00001 | ||||
| Education | ||||||||
| Yes | 7 | 0.66 (0.40-1.07) | 93% | <0.00001 | ||||
| No | 4 | 0.72 (0.53-0.97) | 61% | 0.05 | ||||
BMI, body mass index; WHR, waist-to-hip ratio.
The P for heterogeneity within each subgroup.
Discussion
The higher living standards and urban lifestyle of modern life have resulted in increased attention to the relationship between tumors and modifiable health behaviors such as diet and lifestyle. It is proved that genetic factors account for only 10%-15% of breast cancer. Therefore, environmental factors, including dietary, chemical products and lifestyle are supposed to play an important role in the pathogenesis of breast cancer. Environmental factors, especially dietary ones, have been reported as the cause of 70% of sporadic breast cancers. Number of studies have shown that women with breast cancer risk in Asia make up about one-sixth of American women, and the assumption of soy is a major factor [48,49]. According to the study from nutritionists, there is a big difference between Asia and Western countries’ diet. Most European and American countries live off animal food, while the dietary structure of Asian countries have been proved consistent, such as Chinese, Korean, Japanese, Singaporeans, who are living by plant food or plant-animal food balanced diet. Compared with western countries, Asian countries have lower incidence of breast cancer. With the development of international trade and imports, not only did the eating habits change, but also the incidence rate increase. Therefore, an in-depth study of the risk factors for breast cancer provides a scientific basis for establishing effective strategies to prevent and control the disease. We carried out a meta-analysis of dietary factors related to breast cancer to evaluate the effect of dietary factors on the onset of this disease and provide a theoretical basis to guide public health efforts to prevent breast cancer for Chinese women, also it is applicable to other Asian women based on the similar dietary pattern.
The results of studies about the relationship between breast cancer and fruits or vegetables intake have not been consistent. Our review shows that higher fruits and vegetables consumption, as a protective measure, can indeed reduce the incidence of breast cancer in Chinese women. The vitamin C and carotene in vegetables and fruits are dietary antioxidants which can prevent breast cancer by inhibiting the oxidation of polyunsaturated fatty acids [50]. However, some studies have found no protective effect against breast cancer associated with a large intake of fruits [51]. The stratified analysis according to study design has to be considered, only one cohort study was included that indicated no association between vegetables intake and breast cancer.
Since 1991, the cancer research center of American first put forward that soybeans could reduce incidence of breast cancer, this thesis has been repeatedly demonstrated over 20 years. It is difficult to study the correlation between dietary factors and breast cancer, and the results of various studies often differ. However, basic research has provided some valuable evidence. Soy has been proven to contain isoflavones, which can inhibit in vitro proliferation of blood vessels in tumor tissues and restrict the occurrence of DMBA or radiation-induced breast cancer in mice [52]. So far, more than 30 epidemiological studies and a number of animal experiments explore the relationship between soybean and breast cancer, these studies have demonstrated that the Asia women’s soy foods intake is greater than which in the Europe and America, which may be one of the factors leading to difference of breast cancer incidence between eastern and western regions. For example, in 1992, Lee et al reported Chinese Singapore females who eat traditional food rich in soy have a lower incidence of breast cancer. In 1996, Wu et al found that it can decrease the onset of breast cancer in Asian women eating soy among menopausal and post-menopausal. Furthermore, Yamamoto, Iswasaki et al show that the soy rich in isoflavones has a protective effect against breast cancer based on cohort studies from women in Japan. However, Horn-Ross, Peterson, Keinan-Boker, Touillaud and other authors’s studies do not support the protective role of soy foods about multi-ethnic non-Asian women and European women. Our review shows that soy foods consumption is a protective factor against breast cancer, and higher soy foods consumption induces a 69% reduction in breast cancer risk. In the heterogeneity analysis, little heterogeneity was observed among adjusted studies for BMI, age at first birth and studies performed in middle areas [32,38]. It is suggested that the differences in geographic region might relate to proportions of the observed heterogeneity between the individual studies. The results from other stratifications were heterogenous. According to the above analysis, we known Asian women have a similar dietary structure, although our topic focus on Chinese female, the results can generalization to Asian women from Chinese results.
Whether a high-fat diet is a risk factor for breast cancer remains controversial. Animal experiments, ecology studies and case-control studies have all shown that a high-fat diet is a risk factor for breast cancer; however, in cohort studies of humans, the result tend to be irrelevant or mildly relevant [53]. The hypothesis of a correlation between dietary fat and breast cancer was a focus of much debate in the 1980s and 1990s. Difference in the results of cohort and case-control studies may be caused by a bias in the different methods. The WCRF report concluded that the results of various analytic epidemiology studies do not strongly support an etiological relationship between total fat intake and breast cancer in adults [2]. Our results with little heterogeneity showed that high-fat food consumption may increase the risk of breast cancer. We did not perform a subgroup analysis of fat type due to the limited information available from the studies. We suggest that further targeted research should be carried out to clarify the role of each potential contributing factor.
There are several limitations that should be discussed. Substantial stratifications were conducted in our analysis. As a result, the negative association was detected for high versus low intake of vegetables, fruits, soy foods and breast cancer, and the summary effect value was similar, respectively. However, many confounding factors having not been adjusted are possible contributed to the observed association. On the other hand, the information acquired about diet among majority of studies was difficult for semi-quantified into quantifying, and different units were used, such being the case, an additional limitation of dose-response analysis was not taken into account.
In conclusion, our study shows the strengths also. It is based on a large number of participants, which enhance the statistical efficiency. It suggests that higher consumption of fruits, vegetables and soy plays a protective role in breast cancer while a high-fat diet may increase the risk of the onset of breast cancer. We used conservative estimation methods in the current study, and carried out analysis using a random effect model, which makes the results more reliable. Owing to limitation of meta-analysis itself is that parameters of the various studies- such as participants, intervention measures and heterogeneity or diversity of results-themselves affect the result of analysis. Therefore, our conclusions should be verified by high quality future studies with large sample sizes.
Acknowledgements
The authors thank Prof. Zhong-Li Ma (Ministry of Health of Air Logistics Department, Beijing, China) and Prof. Zhi-Kang Zou (Chinese General Hospital of The Air Force) for their helpful discussion and suggestions.
Disclosure of conflict of interest
None.
References
- 1.Hao J, Chen WQ. Chinese Cancer Report-2012. Beijing: Military Medical Science Press; 2012. p. 50. [Google Scholar]
- 2.Diet, Nutrition and the Prevention of Cancer: A Global Perspective. Washington DC: American Institute for Cancer Research; 1997. World Cancer Research Fund/American Institute for Cancer Research. [Google Scholar]
- 3.Rose DP, Goldman M, Connolly JM, Strong LE. High-fiber diet reduces serum estrogen concentrations in premenopausal women. Am J Clin Nutr. 1991;54:520–525. doi: 10.1093/ajcn/54.3.520. [DOI] [PubMed] [Google Scholar]
- 4.Howe GR, Hirohata T, Hislop TG, Iscovich JM, Yuan JM, Katsouyanni K, Lubin F, Marubini E, Modan B, Rohan T. Dietary factors and risk of breast cancer: combined analysis of 12 case-control studies. J Natl Cancer Inst. 1990;82:561–569. doi: 10.1093/jnci/82.7.561. [DOI] [PubMed] [Google Scholar]
- 5.Kushi LH, Sellers TA, Potter JD, Nelson CL, Munger RG, Kaye SA, Folsom AR. Dietary fat and postmenopausal breast cancer. J Natl Cancer Inst. 1992;84:1092–1099. doi: 10.1093/jnci/84.14.1092. [DOI] [PubMed] [Google Scholar]
- 6.Graham S, Zielezny M, Marshall J, Priore R, Freudenheim J, Brasure J, Haughey B, Nasca P, Zdeb M. Diet in the epidemiology of postmenopausal breast cancer in the New York State Cohort. Am J Epidemiol. 1992;36:1327–1337. doi: 10.1093/oxfordjournals.aje.a116445. [DOI] [PubMed] [Google Scholar]
- 7.Verhoeven DT, Assen N, Goldbohm RA, Dorant E, van’t Veer P, Sturmans F, Hermus RJ, van den Brandt PA. Vitamins C and E, retinol, beta-carotene and dietary fibre in relation to breast cancer risk: a prospective cohort study. Br J Cancer. 1997;75:149–155. doi: 10.1038/bjc.1997.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Terry P, Jain M, Miller AB, Howe GR, Rohan TE. No association among total dietary fiber, fiber fractions, and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2002;11:1507–1508. [PubMed] [Google Scholar]
- 9.Sieri S, Krogh V, Muti P, Micheli A, Pala V, Crosignani P, Berrino F. Fat and protein intake and subsequent breast cancer risk in postmenopausal women. Nutr Cancer. 2002;42:10–17. doi: 10.1207/S15327914NC421_2. [DOI] [PubMed] [Google Scholar]
- 10.Horn-Ross PL, Hoggatt KJ, West DW, Krone MR, Stewart SL, Anton H, Bernstei CL, Deapen D, Peel D, Pinder R, Reynolds P, Ross RK, Wright W, Ziogas A. Recent diet and breast cancer risk: the California Teachers Study (USA) Cancer Causes Control. 2002;13:407–415. doi: 10.1023/a:1015786030864. [DOI] [PubMed] [Google Scholar]
- 11.Cho E, Spiegelman D, Hunter DJ, Chen WY, Colditz GA, Willett WC. Premenopausal dietary carbohydrate, glycemic index, glycemic load, and fiber in relation to risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2003;12:1153–1158. [PubMed] [Google Scholar]
- 12.Holmes MD, Liu S, Hankinson SE, Colditz GA, Hunter DJ, Willett WC. Dietary carbohydrates, fiber, and breast cancer risk. Am J Epidemiol. 2004;159:732–739. doi: 10.1093/aje/kwh112. [DOI] [PubMed] [Google Scholar]
- 13.Giles GG, Simpson JA, English DR, Hodge AM, Gertig DM, Macinnis RJ, Hopper JL. Dietary carbohydrate, fibre, glycaemic index, glycaemic load and the risk of postmenopausal breast cancer. Int J Cancer. 2006;118:1843–1847. doi: 10.1002/ijc.21548. [DOI] [PubMed] [Google Scholar]
- 14.Cade JE, Burley VJ, Greenwood DC. Dietary fibre and risk of breast cancer in the UK Women’s Cohort Study. Int J Epidemiol. 2007;36:431–438. doi: 10.1093/ije/dyl295. [DOI] [PubMed] [Google Scholar]
- 15.Sonestedt E, Gullberg B, Wirfalt E. Both food habit change in the past and obesity status may influence the association between dietary factors and postmenopausal breast cancer. Public Health Nutr. 2007;10:769–779. doi: 10.1017/S1368980007246646. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki R, Rylander-Rudqvist T, Ye W, Saji S, Adlercreutz H, Wolk A. Dietary fiber intake and risk of postmenopausal breast cancer defined by estrogen and progesterone receptor status-a prospective cohort study among Swedish women. Int J Cancer. 2008;122:403–412. doi: 10.1002/ijc.23060. [DOI] [PubMed] [Google Scholar]
- 17.Sonestedt E, Borgquist S, Ericson U, Gullberg B, Landberg G, Olsson H, Wirfält E. Plant foods and estrogen receptor alpha- and beta-defined breast cancer: observations from the Malmo Diet and Cancer cohort. Carcinogenesis. 2008;29:2203–2209. doi: 10.1093/carcin/bgn196. [DOI] [PubMed] [Google Scholar]
- 18.Maruti SS, Lampe JW, Potter JD, Ready A, White E. A prospective study of bowel motility and related factors on breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2008;17:1746–1750. doi: 10.1158/1055-9965.EPI-07-2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lajous M, Boutron-Ruault MC, Fabre A, Clavel-Chapelon F, Romieu I. Carbohydrate intake, glycemic index, glycemic load, and risk of postmenopausal breast cancer in a prospective study of French women. Am J Clin Nutr. 2008;87:1384–1391. doi: 10.1093/ajcn/87.5.1384. [DOI] [PubMed] [Google Scholar]
- 20.Wen W, Shu XO, Li H, Yang G, Ji BT, Cai H, Gao YT, Zheng W. Dietary carbohydrates, fiber, and breast cancer risk in Chinese women. Am J Clin Nutr. 2009;89:283–289. doi: 10.3945/ajcn.2008.26356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shikany JM, Redden DT, Neuhouser ML, Chlebowski RT, Rohan TE, Simon MS, Liu S, Lane DS, Tinker L. Dietary glycemic load, glycemic index, and carbohydrate and risk of breast cancer in the Women’s Health Initiative. Nutr Cancer. 2011;63:899–907. doi: 10.1080/01635581.2011.587227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Food, Nutrition, Physical Activity and the Prevention of Cancer: A Global Perspective. Washington, DC: AICR; 2007. World Cancer Research Fund/American Institute for Cancer Research. [Google Scholar]
- 23.Park Y, Brinton LA, Subar AF, Hollenbeck A, Schatzkin A. Dietary fiber intake and risk of breast cancer in postmenopausal women: the National Institutes of Health-AARP Diet and Health Study. Am J Clin Nutr. 2009;90:664–671. doi: 10.3945/ajcn.2009.27758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ownby RL, Crocco E, Acevedo A, John V, Loewenstein D. Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Arch Gen Psychiatry. 2006;63:530–538. doi: 10.1001/archpsyc.63.5.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fang JQ, Sun ZQ, editors. Health Statistics. Beijing: People’s Health Publishing House; 2004. p. 409. [Google Scholar]
- 26.Rong SY, Li J, Zhang Y. Relationship between dietary factors and female breast cancer: a case-control study. J Environ Health. 2008;25:337–339. [Google Scholar]
- 27.Xu GF, Lin XY, Yu HX, Zhao CF, Zhao XL. The relationship between the dietary and breast cancer: a case-control study. Modern Preventive Medicine. 1998;25:427–429. [Google Scholar]
- 28.Tao MH. Population-based case-control study on risk factors for breast cancer in Shanghai. Fudan University. 2001 [Google Scholar]
- 29.Ren XN. A 1:1 case-control study on risk factors of breast cancer. Dalian Medical University. 2008 [Google Scholar]
- 30.Wang YQ. Case-control study on the risk factors of breast cancer in Jiashan county. Zhejiang University. 2006 [Google Scholar]
- 31.Zhao YB, Shi Z, Liu L. Matched case-control study for detecting risk factors of breast cancer in women living in Chengdu. Chin J Epidemiol. 1999;20:91–94. [PubMed] [Google Scholar]
- 32.Zou L, Tian JR, Wu HS, editors. National Oncology Nursing Academic Conference and Seminar. 2003. A case-control study on risk factors of female breast cancer in Qingshan areas; pp. 56–59. [Google Scholar]
- 33.Zhou L, He TF, Jin YL, Liu N, Shen YP. A case-control study on risk factors of breast cancer. China Cancer. 2009;18:27–30. [Google Scholar]
- 34.Wang LY, Liu L, Tao MF, Qiu LP, Ding H. An investigation of the relationship between diet and breast cancer in women. Maternal and Child Health Care of China. 2008;23:4630–4631. [Google Scholar]
- 35.Cai SR, He LR, Xu XZ, Guo WD. An epidemic factor study of breast cancer in women in Ningxia. 1996;8:1–3. [Google Scholar]
- 36.Yuan JM, Yu MC, Ronald K, Ross , Gao YT, Henderson BE. Risk factors for female breast cancer in Shanghai. Cancer. 1987;7:244–248. [Google Scholar]
- 37.Fu XJ. Environmental factors, physiological factors and DNA repair function related to breast cancer among women in Chaoshan area, China. Shantou University; 2012. [Google Scholar]
- 38.Zhang JC, Xie ZB, Zai SF, Liu JL, Li JF. Matched case-control study for detecting risk factors of breast cancer in women living in Xinjiang, Henan province. Henan Journal of Oncology. 2003;3:201–203. [Google Scholar]
- 39.Zhang CX, Ho SC, Lin FY, Chen YM, Cheng SZ, Fu JH. Dietary fat intake and risk of breast cancer: a case-control study in China. Eur J Cancer Prev. 2011;20:199–206. doi: 10.1097/CEJ.0b013e32834572bb. [DOI] [PubMed] [Google Scholar]
- 40.Zhang CX, Ho SC, Chen YM, Fu JH, Cheng SZ, Lin FY. Greater vegetable and fruit intake is associated with a lower risk of breast cancer among Chinese women. Int J Cancer. 2009;125:181–188. doi: 10.1002/ijc.24358. [DOI] [PubMed] [Google Scholar]
- 41.Shannon J, Ray R, Wu C, Nelson Z, Gao DL, Li W, Hu W, Lampe J, Horner N, Satia J, Patterson R, Fitzgibbons D, Porter P, Thomas D. Food and botanical groupings and risk of breast cancer: a case-control study in Shanghai, China. Cancer Epidemiol Biomarkers Prev. 2005;14:81–90. [PubMed] [Google Scholar]
- 42.Shen YP, Li DK, Wu J, Zhang Z, Gao E. Joint effects of the CYP1A1 MspI, ERalpha PvuII, and ERalpha XbaI polymorphisms on the risk of breast cancer: results from a population-based case-control study in Shanghai, China. Cancer Epidemiol Biomarkers Prev. 2006;15:342–347. doi: 10.1158/1055-9965.EPI-05-0485. [DOI] [PubMed] [Google Scholar]
- 43.Yu ZG, Jia CX, Geng CZ, Tang JH, Zhang J, Liu LY. Risk factors related to female breast cancer in regions of Northeast China: a 1:3 matched case-control population-based study. Chinese Medical Journal. 2012;125:733–740. [PubMed] [Google Scholar]
- 44.Lee MM, Chang IY, Horng CF, Chang JS, Cheng SH, Huang A. Breast cancer and dietary factors in Taiwanese women. Cancer Causes Control. 2005;16:929–937. doi: 10.1007/s10552-005-4932-9. [DOI] [PubMed] [Google Scholar]
- 45.Kallianpur AR, Lee SA, Gao YT, Lu W, Zheng Y, Ruan ZX, Dai Q, Gu K, Shu XO, Zheng W. Dietary animal-derived iron and fat intake and breast cancer risk in the Shanghai Breast Cancer Study. Breast Cancer Res Treat. 2008;107:123–132. doi: 10.1007/s10549-007-9538-3. [DOI] [PubMed] [Google Scholar]
- 46.Wang Q, Li H, Tao P, Wang YP, Yuan P, Yang CX, Li JY, Yang F, Lee H, Huang Y. Soy isoflavones, CYP1A1, CYP1B1, and COMT polymorphisms, and breast cancer: a case-control study in southwestern China. DNA Cell Biol. 2010;30:585–595. doi: 10.1089/dna.2010.1195. [DOI] [PubMed] [Google Scholar]
- 47.Dai Q, Shu XO, Jin F, Potter JD, Kushi LH, Teas J, Gao YT, Zheng W. Population-based case-control study of soyfood intake and breast cancer risk in Shanghai. Br J Cancer. 2001;85:372–378. doi: 10.1054/bjoc.2001.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kang X, Zhang Q, Wang S, Huang X, Jin S. Effect of soy isoflavones on breast cancer recurrence and death for patients receiving adjuvant endocrine therapy. CMAJ. 2010;182:1857–1862. doi: 10.1503/cmaj.091298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cho YA, Kim J, Park KS, Lim SY, Shin A, Sung MK, Ro J. Effect of dietary soy intake on breast cancer risk according to menopause and hormone receptor status. Eur J Clin Nutr. 2010;64:924–932. doi: 10.1038/ejcn.2010.95. [DOI] [PubMed] [Google Scholar]
- 50.Chen JS. Anti-cancer effect of antioxidant micronutrients. China Cancer. 1994;3:17–19. [Google Scholar]
- 51.Smith-Warner SA, Spiegelman D, Yaun SS. Intake of fruits and vegetables and risk of breast cancer: a pooled analysis of cohort studies. JAMA. 2001;285:769–776. doi: 10.1001/jama.285.6.769. [DOI] [PubMed] [Google Scholar]
- 52.Theodore Fotsis, Michael Pepper. Genistein, a dietary derived inhibitor of in vitro angiogenesis. Proc Natl Acad Sci U S A. 1993;90:2690. doi: 10.1073/pnas.90.7.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kumar NB, Cantor A, Allen K, Riccardi D, Cox CE. The specific role of isoflavones on estrogen metabolism in premenopausal women. Cancer. 2002;94:1166–1174. doi: 10.1002/cncr.10320. [DOI] [PMC free article] [PubMed] [Google Scholar]


