Abstract
Background: Adalimumab is used in an attempt to maintain remission for Ulcerative colitis. This study was to evaluate the efficacy and adverse events of adalimumab compared with placebo in inducing remission of Ulcerative colitis. Methods: MEDLINE, EMBASE, the Cochrane Controlled Trials Register, OVID, BIOSIS, CNKI, and Google were searched. All randomized trials comparing adalimumab with placebo in inducing remission of moderate-to-severe ulcerative colitis were included. Results: Two randomized controlled trials with a total of 754 participants met the inclusion criteria. The pooled risk ratio (RR) of clinical remission was 1.85 (95% confidence interval (CI) 1.26 to 2.72) following adalimumab treatment. RR of clinical response was 1.40 (95% CI 1.19 to 1.65) while that of mucosal healing was 1.23 (95% CI 1.03 to 1.47). RR of any adverse events was 1.00 (95% CI 0.93 to 1.09). Conclusion: Compared with placebo, administration of adalimumab may increase the proportion of patients with moderate-to-severe ulcerative colitis attaining clinical remission, clinical response and mucosal healing. Adalimumab is also tolerated well in these patients.
Keywords: Adalimumab, ulcerative colitis, meta-analysis, efficacy, safety
Introduction
Ulcerative colitis (UC), one of the two major forms of inflammatory bowel disease (IBD), is a chronic, non specific, relapsing disorder. Tumor necrosis factor-α (TNF-α) is a proinflammatory cytokine and it is one of the most important factors in the pathogenesis of UC [1]. Data show that, TNF-α is abundantly expressed in the gastrointestinal tracts of patients with IBD [2,3]. Over the past decade, treatments aimed at reducing the activity of TNF-α have been used in patients with CD [4] and some autoimmune diseases [5,6]. But the efficacy and safety of adalimumab (ADA), one of the anti-TNF medications, for inducing remission of UC is still unclear. It needs to be confirmed in more randomized controlled trials (RCTs) [7,8].
UC frequently recurs after effective induction therapy. Although 5-aminosalicylate compounds are widely used in UC, this approach is not effective in approximately 50% patients after 1 year [9]. Antimetabolites, including azathioprine and 6-mercaptopurine, are moderately effective in high-risk patients. However, these drugs might cause significant adverse effects (AEs).
Safety of anti-TNF medications have led to new support for the possible use of ADA in inducing remission of UC. However, it is still a controversy as to whether ADA is superior to placebo (PBO) in the inducing remission of UC. The aim of this meta-analysis was to evaluate the effect and adverse events of ADA compared with PBO in inducing remission of UC.
Methods
Search strategy
All RCTs were searched in the following databases: MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials, OVID, BIOSIS and Conference Papers Index to identify comparative studies of ADA in inducing remission of UC. The key words ‘ulcerative colitis’ and ‘adalimumab’, ‘ulcerative colitis’ and ‘Humira’ were used as the search terms.
Selection criteria
Besides RCTs, studies in abstract form or conference report, without publication of the full paper were also included in the analyses. Studies with two different strategies: control group receiving only PBO or PBO with 5-ASA, steroids and/or azathioprine for maintenance of remission, while experimental group having received ADA or ADA plus the same control treatment, were eligible for inclusion.
According to the trials, moderate-to-severe UC is defined as: UC at least 3 months with a Mayo score of 6 to12 points (endoscopy subscore of at least 2). Finally, there are 2 papers meet the criteria mentioned above (Figure 1).
Figure 1.
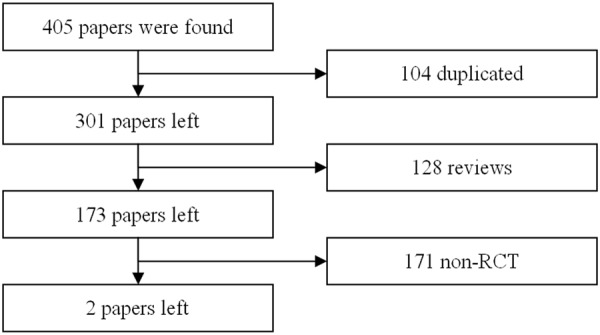
The flow chart of literatures searching and selection.
Data extraction
All papers were examined independently for eligibility by two reviewers (Jin and Zhou). Disagreements were resolved by consulting a third reviewer (Sushil). Quality of evidence was assessed using the software GRADE 3.6 (Cochrane Collaboration, Oxford, UK). The information extracted from included studies were demonstrated on Table 1.
Table 1.
Information extracted from included studies
| Papers | N | Clinical remission (8 weeks) | Clinical remission (52 weeks) | ||
|---|---|---|---|---|---|
|
| |||||
| ADA | PRO | ADA | PRO | ||
| Clinical remission | |||||
| Sandborn 2012 | 248 | 248×16.5% | 248×9.3% | 248×17.3% | 248×8.5% |
| Reinisch 2010 | 130 | 130×18.5% | 130×9.2% | — | — |
| Mucosal healing | |||||
| Sandborn 2012 | 248 | 248×41.1% | 248×31.7% | 248×17.3% | 248×8.5% |
| Reinisch 2010 | 130 | 130×46.9% | 130×41.5% | — | — |
| Clinical response | |||||
| Sandborn 2012 | 248 | 248×50.4% | 248×34.6% | — | — |
| Reinisch 2010 | 130 | 130×54.6% | 130×44.6% | — | — |
| Malignancy (n) | |||||
| Sandborn 2012 | 223 | 2 | 0 | — | — |
| Reinisch 2010 | 130 | 0 | 2 | — | — |
| Opportunistic infection (n) | |||||
| Sandborn 2012 | 223 | 5 | 3 | — | — |
| Reinisch 2010 | 130 | 1 | 0 | — | — |
| Serious infection (n) | |||||
| Sandborn 2012 | 223 | 4 | 5 | — | — |
| Reinisch 2010 | 130 | 0 | 3 | — | — |
| Injection sito reacton (n) | |||||
| Sandborn 2012 | 223 | 31 | 10 | — | — |
| Reinisch 2010 | 130 | 13 | 7 | — | — |
| Withdraw (n) | |||||
| Sandborn 2012 | 223 | 23 | 34 | — | — |
| Reinisch 2010 | 130 | 12 | 12 | — | — |
Outcomes and statistical analysis
Primary outcome of the intervention was defined as ‘clinical remission’ (Mayo score to < 2 points at week 8). Two secondary outcomes were defined as ‘clinical response’ (decrease in Mayo score at least 3 points and at least 30% with an accompanying decrease in rectal bleeding subscore of at least 1 point or an absolute rectal bleeding subscore of 0 or 1 by weeks 8), ‘mucosal healing’ (Mayo endoscopic score of 0 or 1 by weeks 8).
Malignancy, serious infection, opportunistic infection (excluding tuberculosis (TB), and injection site reaction (ISR) were evaluated in this analysis.
We calculated the risk ratios (RRs) with 95% confidence intervals (CI) for dichotomous outcomes, and fixed-effect model was used to examine the RRs. P < 0.05 (two sided) was considered to be significant. Tests of heterogeneity were also carried out. Statistical analysis was conducted with software RevMan 5.2 (Cochrane Collaboration, Oxford, UK). Methodological quality of each included study was assessed using ‘Risk of bias’ tool in RevMan.
Results
Study characteristics
All citations and abstracts obtained from searches of published work were identified. Two RCTs that enrolled a total of 754 participants published in 2011 and 2012 respectively were found to meet the including criteria [10,11]. In Sandborn’s study, ADA was administrated with a dose of 160 mg at week 0, 80 mg at week 2 and then 40 mg every other week beginning at week 4. The outcomes were observed in week 8 (ADA160/80). In addition to ADA160/80 group, there is a lose dose group, ADA80/40, in Reinisch’s study, the data of ADA80/40 group was not included in this analysis.
Effect of ADA use in UC
Data were available for 754 patients, of whom 378 received ADA treatment and 376 received PBO treatment. There were 65 patients in ADA group reached the criteria of ‘clinical remission’ compared to 35 patients in PBO group, Meta-analysis showed a significant difference in the proportion of patients with remission between the interventions (RR 1.85; 95% CI 1.26 to 2.72; Figure 2).
Figure 2.
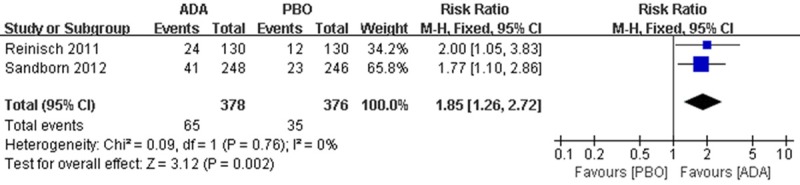
RR for clinical remission in ADA compared with PBO in all pooled trials. RR: risk ratios, CI: confidence intervals.
There were 196 patients in the ADA group reached ‘clinical response’ compared to 132 patients in PBO group. Meta-analysis showed a significant difference in clinical response between interventions (RR 1.40; 95 % CI 1.19 to 1.65; Figure 3).
Figure 3.
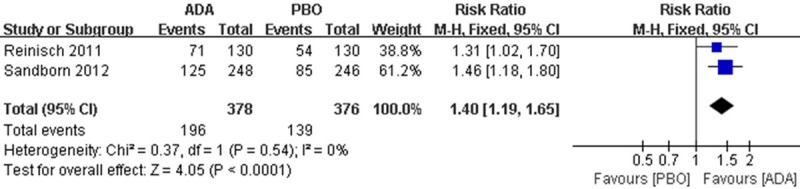
RR for clinical response in ADA compared with PBO in all pooled trials. RR: risk ratios, CI: confidence intervals.
There were 163 patients in the ADA group reached ‘mucosal healing’ compared to 139 patients in PBO group, Pooled risk showed a difference in the incidence of mucosal healing between interventions (RR 1.23; 95% CI 1.03 to 1.47; Figure 4).
Figure 4.
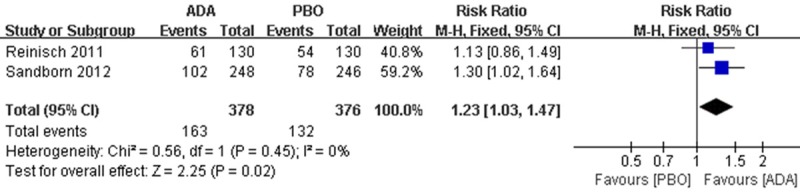
RR for mucosal healing in ADA compared with PBO in all pooled trials. RR: risk ratios, CI: confidence intervals.
There was no heterogeneity between the two studies in the analysis of the outcomes mentioned above (P = 0.76, I2 = 0% for clinic remission; P = 0.54, I2 = 0% for clinic response; P = 0.45, I2 = 0% for mucosal healing).
Adverse events
Generally, AEs were observed in 325 patients in ADA group compared to 326 patients in PRO group, there is no difference between the interventions (RR 1.00; 95% CI 0.93 to 1.09; Figure 5).
Figure 5.
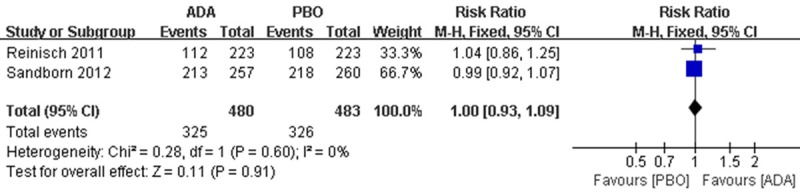
RR for any adverse events in ADA compared with PBO in all pooled trials. RR: risk ratios, CI: confidence intervals.
Specifically, AEs including malignancy, serious infection, opportunistic infection (excluding TB) and ISR were also analysed and the results indicated that, few serious AEs such as malignancy (2 patients in each group) and serious infection (4 patients in ADA group, 8 patients in PBO group) were observed in pooled data, but they are not considered to be trial related (Figures 6, 7).
Figure 6.
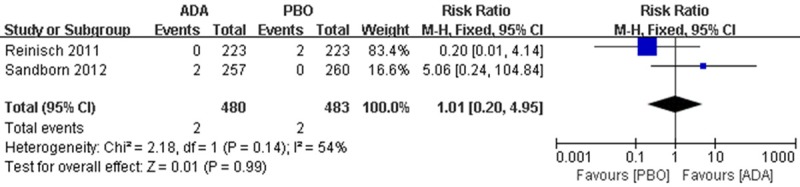
RR for malignancy in ADA compared with PBO in pooled trials. RR: risk ratios, CI: confidence intervals.
Figure 7.
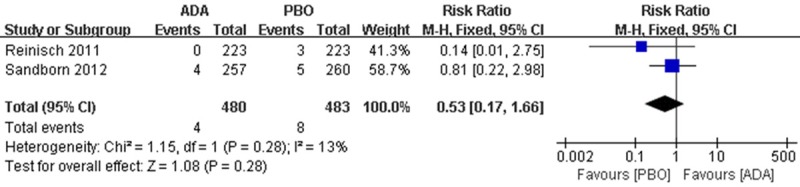
RR for serious infection in ADA compared with PBO in pooled trials. RR: risk ratios, CI: confidence intervals.
For opportunistic infection (excluding TB), 6 patients were found in ADA group and 3 patients in PBO group, there is no difference between the interventions (RR 1.87; 95% CI 0.52 to 6.82) (Figure 8).
Figure 8.
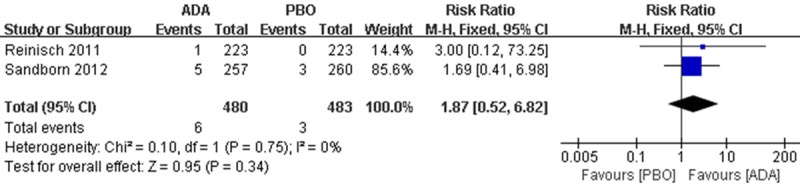
RR for opportunistic infection (excluding TB) in ADA compared with PBO in pooled trials. RR: risk ratios, CI: confidence intervals.
For ISRs, 44 patients in ADA group compared to 17 patients in PBO group were found, pooled risk showed the favour of ADA (RR 2.61; 95% CI 1.51 to 4.50) (Figure 9).
Figure 9.
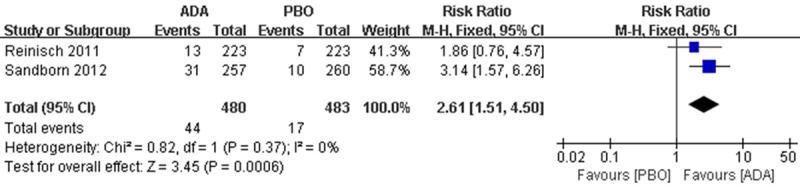
RR for injection site reaction in ADA compared with PBO in pooled trials. RR: risk ratios, CI: confidence intervals.
Discussion
ADA has just be approved to use for UC treatment in Europe and USA, And the efficacy and safety need to be further identified. Thus, we focused our emphasis only on RCTs. The overall quality of the evidence assessed by the GRADE approach was high, and quality of efficacy evidence at 8 weeks follow-up, quality of evidence of malignancy , serious infection, and ISR was also judged to be high, indicating much confidences in this effect estimate. But quality of evidence of opportunistic infection (excluding TB) is judged to be moderate, indicating not so much confidences in this effect estimate.
We did not identify any potential biases in the review process. A comprehensive search was performed and eligible studies were identified by two independent authors. Data extraction was performed independently by two authors. We restricted the included studies to RCTs as they provide the strongest level of evidence available.
Compared with PBO, administration of ADA for UC was effective in inducing the clinical remission, clinical response, and mucosal healing, although minor AE were observed. The dose of curcumin used in trials was ADA160/80. ADA is also effectiveness for Patients with active UC after failure or intolerance of infliximab therapy [12-15]. Those studies are neither controlled trials nor with Mayo score, and the dose of ADA are not coincident between studies as well as the meaning of endpoint, so they are not included in our analysis.
In our analysis, there is a difference suggested between ADA and PBO inducing mucosal healing in patients with UC. It is identical to recent study indicating how anti-TNF-α therapy contributes to epithelial restitution and tissue repair in IBD [16]. Open-label study shows that, ADA in patients with UC including those with prior loss of response or intolerance to Infliximab showed the rate of mucosal healing was 30% at week 8. And mucosal healing were similar in infliximab-naive and previously exposed patients [15].
Recently research found that, anti-TNF agents is associated with an increased risk of carcinoma in patients with IBD [17,18]. There are 2 malignancies in PBO group of Reinisch’s study and 2 in ADA group of Sanborn’s study respectively. It is considered to be non-trial-correlated.
IBD patients after anti-TNF-α therapy will develop serious infection [19]. And there is also a ‘paradoxical’ AEs of anti TNF-α therapy have been recorded: flares or new onset IBD [20]. In our analysis, there is no difference between the groups in serious infection (Figure 7), but UC was also reported to be the most common AE leading to discontinuation in 4.0% of PBO, 3.8% of ADA 80/40, and 3.6% of ADA 160/80 patients [11].
Various and severe opportunistic infections were reported after anti TNF-α treatment [21,22], including in patients with CD. Monoclonal anti-TNF antibody use > 10 mg/day are independently associated with opportunistic infections [23]. In our analysis, there is no difference between the groups in opportunistic infection (Figure 8), but the quality of the evidence is moderate, indicate that further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
ISR of anti-TNF inhibitors including several types of reaction will develop many problems [24]. There is a broad spectrum of adverse cutaneous reactions occurs more frequently and later in ADA therapy for IBD compared with other indications. High incidence of dermatological reaction were reported [25]. Both our including studies, ISRs tends to be favour of ADA rather than PBO (9.17% vs. 3.52%) (Figure 9), it is consistent with previous studies.
Conclusions
Administration of ADA as inducing remission for moderate-to-severe UC is efficacious in short term (8 weeks), it is also tolerated well in these patients we have included in the analysis. Compared with PBO, ADA will not induce malignancy and serious AEs.
Acknowledgements
This study was supported by Medical Research Foundation of Guangdong Province (B2014294), and Scientific Funds of Guangdong Medical College (M2013054).
Disclosure of conflict of interest
None.
References
- 1.Bosani M, Ardizzone S, Porro GB. Biologic targeting in the treatment of inflammatory bowel diseases. Biologics. 2009;3:77–97. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 2.Breese EJ, Michie CA, Nicholls SW, Murch SH, Williams CB, Domizio P, Walker-Smith JA, MacDonald TT. Tumor necrosis factor alpha-producing cells in the intestinal mucosa of children with inflammatory bowel disease. Gastroenterology. 1994;106:1455–1466. doi: 10.1016/0016-5085(94)90398-0. [DOI] [PubMed] [Google Scholar]
- 3.Murch SH, Braegger CP, Walker-Smith JA, MacDonald TT. Location of tumour necrosis factor alpha by immunohistochemistry in chronic inflammatory bowel disease. Gut. 1993;34:1705–1709. doi: 10.1136/gut.34.12.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Assche G, Vermeire S, Rutgeerts P. Adalimumab in Crohn’s disease. Biologics. 2007;1:355–365. [PMC free article] [PubMed] [Google Scholar]
- 5.Garces S, Demengeot J, Wolbink G, Aarden L, Benito-Garcia E. The Immunogenicity of Infliximab, Adalimumab and Etanercept in Rheumatoid Arthritis, Ankylosing Spondylitis, Psoriatic Arthritis, Crohn‘s Disease and Ulcerative Colitis-a Quantitative and a Qualitative Review. Arthritis and Rheumatism. 2011;63:S178–S178. [Google Scholar]
- 6.Voulgari PV, Kaltsonoudis E, Papagoras C, Drosos AA. Adalimumab in the treatment of rheumatoid arthritis. Expert Opini Biol Ther. 2012;12:1679–1686. doi: 10.1517/14712598.2012.721771. [DOI] [PubMed] [Google Scholar]
- 7.Fiorino G, Peyrin-Biroulet L, Repici A, Malesci A, Danese S. Adalimumab in ulcerative colitis: hypes and hopes. Expert Opin Biol Ther. 2011;11:109–116. doi: 10.1517/14712598.2011.541435. [DOI] [PubMed] [Google Scholar]
- 8.Danese S. Adalimumab for ulcerative colitis: A little is better than none? Inflamm Bowel Dis. 2012;18:793–4. doi: 10.1002/ibd.21773. [DOI] [PubMed] [Google Scholar]
- 9.Gisbert JP, Gomollon F, Mate J, Pajares JM. Role of 5-aminosalicylic acid (5-ASA) in treatment of inflammatory bowel disease: a systematic review. Dig Dis Sci. 2002;47:471–488. doi: 10.1023/a:1017987229718. [DOI] [PubMed] [Google Scholar]
- 10.Sandborn WJ, van Assche G, Reinisch W, Colombel JF, D’Haens G, Wolf DC, Kron M, Tighe MB, Lazar A, Thakkar RB. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2012;142:257–265. e251–253. doi: 10.1053/j.gastro.2011.10.032. [DOI] [PubMed] [Google Scholar]
- 11.Reinisch W, Sandborn WJ, Hommes DW, D‘Haens G, Hanauer S, Schreiber S, Panaccione R, Fedorak RN, Tighe MB, Huang B, Kampman W, Lazar A, Thakkar R. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: results of a randomised controlled trial. Gut. 2011;60:780–787. doi: 10.1136/gut.2010.221127. [DOI] [PubMed] [Google Scholar]
- 12.Afif W, Leighton JA, Hanauer SB, Loftus EV Jr, Faubion WA, Pardi DS, Tremaine WJ, Kane SV, Bruining DH, Cohen RD, Rubin DT, Hanson KA, Sandborn WJ. Open-Label Study of Adalimumab in Patients with Ulcerative Colitis Including Those with Prior Loss of Response or Intolerance to Infliximab. Inflamm Bowel Dis. 2009;15:1302–1307. doi: 10.1002/ibd.20924. [DOI] [PubMed] [Google Scholar]
- 13.Singh A, Mahadevan U, Yen E, Terdiman J. Adalimumab for Patients with Ulcerative Colitis Who have Lost Response or are Intolerant of Infliximab: Initial Response Rates are High, But the Response May Not be Durable. Am J Gastroenterol. 2009;104:S438–S438. [Google Scholar]
- 14.Taxonera C, Estelles J, Fernandez-Blanco I, Merino O, Marin-Jimenez I, Barreiro-de Acosta M, Saro C, Garcia-Sanchez V, Gento E, Bastida G, Gisbert JP, Vera I, Martinez-Montiel P, Garcia-Moran S, Sanchez MC, Mendoza JL. Adalimumab induction and maintenance therapy for patients with ulcerative colitis previously treated with infliximab. Aliment Pharmacol Ther. 2011;33:340–348. doi: 10.1111/j.1365-2036.2010.04531.x. [DOI] [PubMed] [Google Scholar]
- 15.Afif W, Leighton JA, Hanauer SB, Loftus EV Jr, Faubion WA, Pardi DS, Tremaine WJ, Kane SV, Bruining DH, Cohen RD, Rubin DT, Hanson KA, Sandborn WJ. Open-label study of adalimumab in patients with ulcerative colitis including those with prior loss of response or intolerance to infliximab. Inflamm Bowel Dis. 2009;15:1302–1307. doi: 10.1002/ibd.20924. [DOI] [PubMed] [Google Scholar]
- 16.Fischer A, Gluth M, Pape UF, Wiedenmann B, Theuring F, Baumgart DC. Adalimumab prevents barrier dysfunction and antagonizes distinct effects of TNF-alpha on tight junction proteins and signaling pathways in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2013;304:G970–979. doi: 10.1152/ajpgi.00183.2012. [DOI] [PubMed] [Google Scholar]
- 17.Mason M, Siegel CA. Do inflammatory bowel disease therapies cause cancer? Inflamm Bowel Dis. 2013;19:1306–1321. doi: 10.1097/MIB.0b013e3182807618. [DOI] [PubMed] [Google Scholar]
- 18.Deepak P, Sifuentes H, Sherid M, Stobaugh D, Sadozai Y, Ehrenpreis ED. T-Cell Non-Hodgkin's Lymphomas Reported to the FDA AERS With Tumor Necrosis Factor-Alpha (TNF-alpha) Inhibitors: Results of the REFURBISH Study. Am J Gastroenterol. 2013;108:99–105. doi: 10.1038/ajg.2012.334. [DOI] [PubMed] [Google Scholar]
- 19.Lawrance IC, Radford-Smith GL, Bampton PA, Andrews JM, Tan PK, Croft A, Gearry RB, Florin TH. Serious infections in patients with inflammatory bowel disease receiving anti-tumor-necrosis-factor-alpha therapy: an Australian and New Zealand experience. J Gastroenterol Hepatol. 2010;25:1732–1738. doi: 10.1111/j.1440-1746.2010.06407.x. [DOI] [PubMed] [Google Scholar]
- 20.Braun J, Baraliakos X, Listing J, Davis J, Van Der Heijde D, Haibel H, Rudwaleit M, Sieper J. Differences in the incidence of flares or new onset of inflammatory bowel diseases in patients with ankylosing spondylitis exposed to therapy with anti-tumor necrosis factor (alpha) agents. Arthritis Rheum. 2007;57:639–647. doi: 10.1002/art.22669. [DOI] [PubMed] [Google Scholar]
- 21.Fraison JB, Guilpain P, Schiffmann A, Veyrac M, Le Moing V, Rispail P, Le Quellec A. Pulmo-nary cryptococcosis in a patient with Crohn's disease treated with prednisone, azathioprine and adalimumab: exposure to chicken manure as a source of contamination. J Crohns Colitis. 2013;7:e11–14. doi: 10.1016/j.crohns.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 22.Kump PK, Hogenauer C, Wenzl HH, Petritsch W. A case of opportunistic skin infection with Mycobacterium marinum during adalimumab treatment in a patient with Crohn's disease. J Crohns Colitis. 2013;7:e15–18. doi: 10.1016/j.crohns.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 23.Salmon-Ceron D, Tubach F, Lortholary O, Chosidow O, Bretagne S, Nicolas N, Cuillerier E, Fautrel B, Michelet C, Morel J, Puechal X, Wendling D, Lemann M, Ravaud P, Mariette X. Drug-specific risk of non-tuberculosis opportunistic infections in patients receiving anti-TNF therapy reported to the 3-year prospective French RATIO registry. Ann Rheum Dis. 2011;70:616–623. doi: 10.1136/ard.2010.137422. [DOI] [PubMed] [Google Scholar]
- 24.Murdaca G, Spano F, Puppo F. Selective TNF-alpha inhibitor-induced injection site reactions. Expert Opin Drug Saf. 2013;12:187–193. doi: 10.1517/14740338.2013.755957. [DOI] [PubMed] [Google Scholar]
- 25.Baumgart DC, Grittner U, Steingraber A, Azzaro M, Philipp S. Frequency, phenotype, outcome, and therapeutic impact of skin reactions following initiation of adalimumab therapy: experience from a consecutive cohort of inflammatory bowel disease patients. Inflamm Bowel Dis. 2011;17:2512–2520. doi: 10.1002/ibd.21643. [DOI] [PubMed] [Google Scholar]


