Abstract
To investigate the management of Meckel’s diverticulum in children and the feasibility of using laparoscopic and laparoscopically assisted Meckel’s diverticulum resection and intestinal anastomosis according to the different subtypes classified laparoscopically. 55 symptomatic Meckel’s diverticulum cases were classified into two categories, the simple and the complex types depending on Meckel’s diverticulum appearance upon laparoscopic exploration. Forty-one cases of simple Meckel’s diverticulum were treated with simple diverticulectomy during laparoscopy, and 14 cases of complicated Meckel’s diverticulum were treated with laparoscopically assisted Meckel’s diverticulum resection and intestinal anastomosis. The operation time for the laparoscopically assisted was significant longer than laparoscopic-only surgeries [45~123 min (54.57 ± 20.17min) vs 29~78min (38.85 ± 9.75 min)], P = 0.013. Among the 55 cases, Just one child with simple type MD during laparoscopic exploration, and presented a diverticulum with a base that was considered to be in the mesangial margin. The remaining 54 patients were cured, and follow-up for 4~36 months revealed that they did not present abdominal pain, and no hematochezia occurred as a complication. Surgery selection either laparoscopy only or transumbilical laparoscopically assisted intestinal resection and intestinal anastomosis by laparoscopic exploration for Meckel’s diverticulum treatment, based on the type of Meckel’s diverticulum in children, is safe, feasible, and effective.
Keywords: Meckel’s diverticulum, diverticulum types, laparoscopy surgical procedures, child
Introduction
Meckel’s diverticulum (MD) is regarded as one of the most common congenital anomalies of the gastrointestinal tract. Complications that arise due to MD include bleeding, obstruction, inflammation and, less commonly, perforation. MD may remain asymptomatic and, in both paediatric and adult patients, cases are generally diagnosed incidentally or upon investigating unexplained gastrointestinal bleeding, perforation, inflammation, or obstruction [1-3].
The rule of two traditionally describes the characteristics of the MD, and refers to its 2% prevalence rate in the general population, a 2:1 male-to-female ratio, a 2% incidence rate of symptomatic MD, the presence of symptoms before the age of 2 years, a location 2 feet away from the ileocecal valve, a 2-inch diverticular length, and two types of common ectopic tissues [4-6].
The preoperative diagnosis of Meckel’s diverticulum is challenging, despite the availability of modern imaging. CT and Technetium-99m radionuclide imaging are widely used, but the correct diagnosis is often established only at the time of laparotomy or laparoscopy. This is particularly the case for Meckel’s diverticulum in children, as symptoms are often nonspecific and complications are likely to occur at the time of the presentation [7-9].
Hence, a diagnosis of symptomatic MD demands a high degree of suspicion, as an accurate preoperative clinical diagnosis is often difficult to make. Therefore, in the current study, we reported our experience with the targeted selection of surgery through laparoscopic exploration. This study is intended to provide useful information for selecting a surgical method based on the appearance of the MD through laparoscopic exploration.
Methods
Patients
We conducted a retrospective study of pediatric patients (< 18 years of age) with MD who were diagnosed and treated at Wuhan Children’s Hospital Between January 2010 and January 2013. Age at the onset of symptoms, sex, clinical features, treatment, imaging studies, surgical findings, and histopathological reports were reviewed and analyzed. According to the clinical features, patients with symptomatic MD were further categorized into three groups, based on the presence of gastrointestinal bleeding, abdominal pain, and intestinal obstruction.
The patients were classified into two categories, the simple and the complex types, depending on the appearance of MD upon laparoscopic exploration. The simple type of Meckel’s diverticulum presented a clear base, and the basal tissue was the same as in the normal bowel. The complex type was defined as having an unclear base or basal edema, with stiffness, scarring, and abnormal intestinal tissue, or diverticular gangrene.
Surgical process
The children were placed in the supine position with tracheal intubation anesthesia, a 3.5 or 5.5 mm incision was performed on the navel, CO2 pneumoperitoneum (8~12 mmHg pressure) was established, and the sleeve and laparoscope were inserted into the umbilical wheel to an approximately 55 mm depth to find the ileocecal region. Under laparoscopy, we further sought MD toward the proximal region of the small intestine (Figures 1, 2 and 3).
Figure 1.
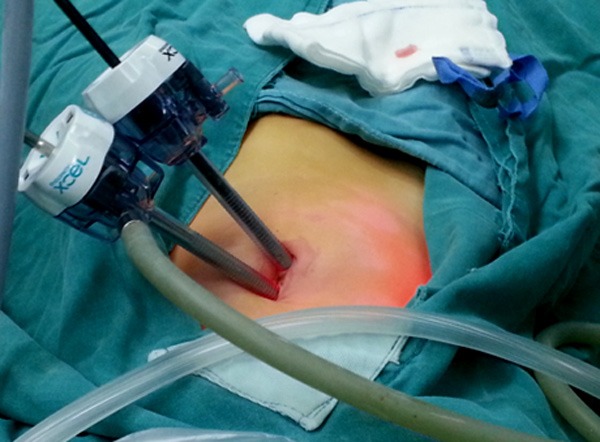
Incision of 3.5 or 5.5 mm at the navel.
Figure 2.
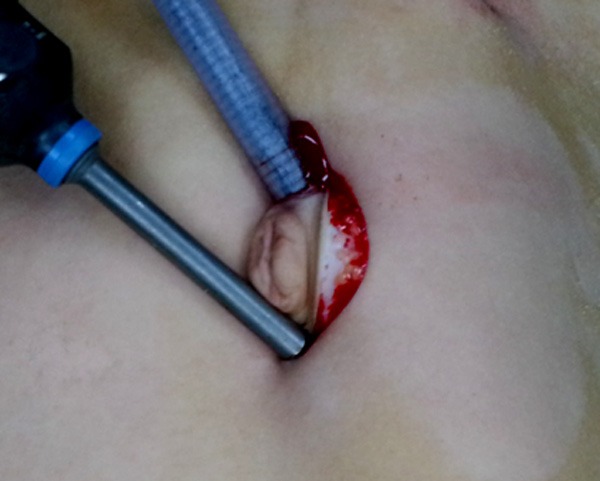
Prolonging the umbilical incision to 2-3 cm.
Figure 3.
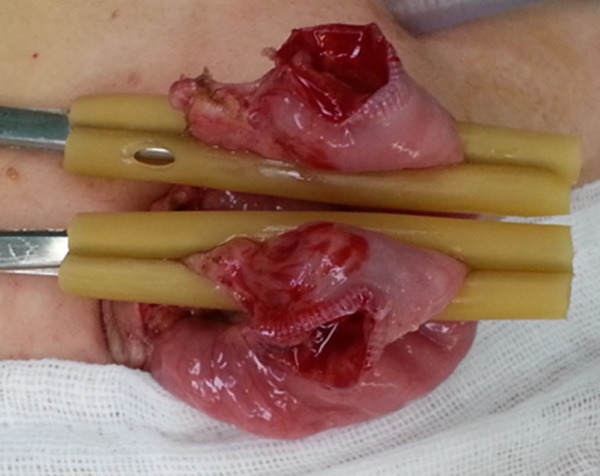
Taking the diverticulum out to the peritoneal.
The children were treated by transumbilical laparoscopically assisted surgery by prolonging the umbilical incision to 2-3 cm (Figure 2) for taking the diverticulum out to the peritoneal layman and resecting the diverticulum and the adjacent intestine and to perform intestinal anastomosis (Figures 3, 4) if complicated MD was found upon laparoscopic exploration. Children with simple MD were treated with laparoscopy only to perform the resection. The right lower abdominal or lower abdominal midline (Figure 5) was placed into a 5.5 mm casing, the blood vessels of the MD were separated and cut off with an ultrasonic knife, and the basal part of the MD was then clamped and resected with the ultrasonic knife (Figure 6), making sure that the dissection points were kept closed. The intestinal wall was sutured in order to avoid overflow, traction and suspension were applied from the lower right, and another layer of suture was then started at the margin with 5-0 absorbable wire (Figure 7), tied back with a seromuscular layer continuous suture (Figure 8), and the anastomotic stoma and vessels were checked to make sure that no leakage occurred.
Figure 4.
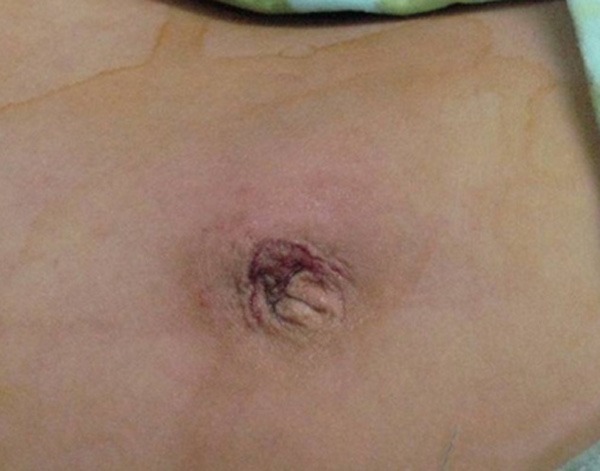
The appearance after umbilical incision closed layman and resecting the diverticulum and the adjacent intestinal.
Figure 5.
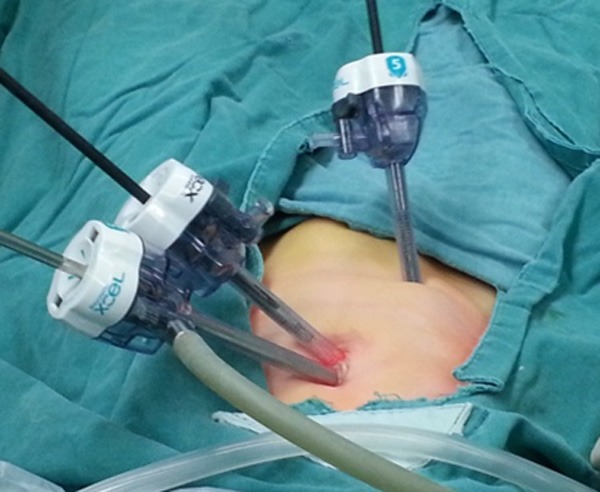
Setting up 3 puncture devices.
Figure 6.
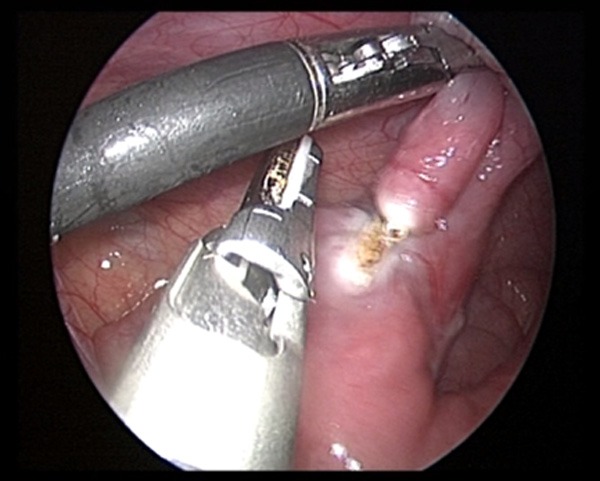
Resecting the MD with the ultrasonic knife.
Figure 7.
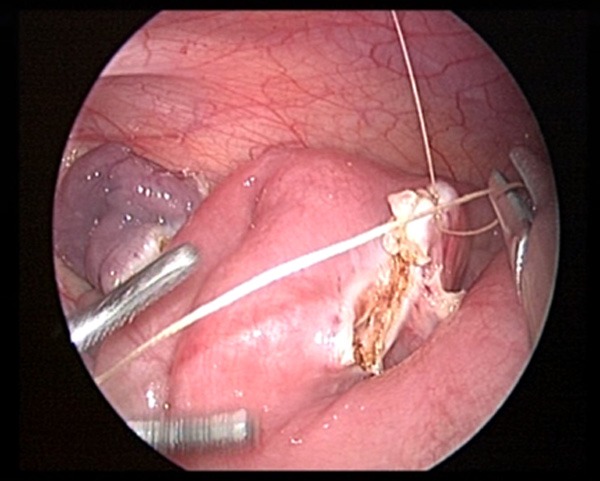
Whole layer suture.
Figure 8.
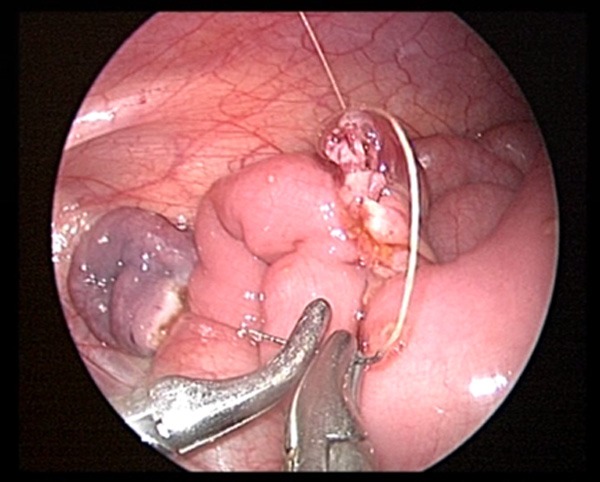
Seromuscular layer continuous suture.
Pathological examination
The resected specimens were fixed by regular 10% formalin, paraffin embedded, sliced to a thickness of 4 μm, and HE staining and immunohistochemistry were performed for anti-alpha smooth muscle actin (anti-actin) detection. The primary antibody, a mouse anti-human smooth muscle actin monoclonal antibody, clone IA4, was used for the identification of smooth muscle actin, a product of Zymed company, USA (Offered by Beijing Zhongshan Biological Technology Co., Ltd.). The Power Vision (PV9000) method was used for immunohistochemistry. The reagents (Immuno-2Bridge+) for the two-step immunohistochemical detection were provided by the GBI company, USA. Pathologic sampling was based on an uninterrupted method from the top to the bottom of the diverticulum, longitudinally.
Statistical analysis
The SPSS 13.0 software package was used for the statistical analysis of the results. The data were represented as mean ± standard deviation (± s); the two groups were compared using the mean rank sum test, with P < 0.05 being used for significant differences.
Results
Patients’ characteristics
Between January 2010 and January 2013, 55 symptomatic patients were enrolled in the current study. The demographic characteristics of the patients are listed in Table 1. A total of 55 children (38 boys, 17 girls) with a mean age of 4.59 ± 2.49 years (range, 1.5 to 17.83 years) were included. The participants were further categorized into three groups: 9 in the bowel obstruction group, 38 in the gastrointestinal bleeding group, and 9 in the infection group (see Table 1).
Table 1.
Patient’s characteristics
| Characteristics | No. of patients |
|---|---|
| Age | |
| Range | 1.5-13 y |
| Mean ± SD | 4.59 ± 2.49 |
| Sex | |
| Boy | 38 |
| Girl | 17 |
| Clinical presentation | |
| Bleeding | 38 (34 cases presented bleeding twice) |
| Infection | 8 |
| Intestinal obstruction | 9 |
Surgical procedures for the simple and complex types of MD
The patients were classified into two categories, including those who presented the simple and the complex types of MD, based on the external appearance of the diverticulum upon laparoscopic exploration. The simple type of MD meant that the diverticulum presented a clear base and the basal tissue was the same as the one found in the normal bowel. The complex type of MD was defined as presenting an unclear base or basal edema, with stiffness, scarring, and abnormal intestinal tissue, or diverticular gangrene.
Forty-one cases of simple MD were treated with simple diverticulectomy by laparoscopy only, and 14 cases of complicated MD were treated with transumbilical laparoscopy assisted by prolonging the umbilical incision for taking the diverticulum out into the peritoneal layman and for resecting the diverticulum and the adjacent intestine and perform intestinal anastomosis. The operation times for the laparoscopically assisted procedure and for laparoscopy only were 45~123 min (54.57 ± 20.17 min) and 29~78 min (38.85 ± 9.75 min), respectively (see Table 2).
Table 2.
The operation time of laparoscopically assisted and laparoscopic only procedures
| Laparoscopic only (n = 41) | Laparoscopically assisted (n = 14) | P | ||
|---|---|---|---|---|
| Operation time (min) | 38.85 ± 9.75 | 54.57 ± 20.17 | Z = 2.482 | 0.013 |
Pathological results
Gastric or pancreatic tissue were found in 41 cases on the top of simple MD, and the basal mucosa was not abnormal. Different degrees of inflammatory cell invasion and/or ulcer formation were found on the basal mucosa in 14 cases of complex MD, and gastric mucosa or pancreatic tissue, or diverticular gangrene were visible on top of the MD.
Participants were categorized into three groups, 9 in the intestinal obstruction group, and they were further categorized into simple (n = 3), with normal basal of MD and complex (n = 6), with 2 cases of tissue swelling, inflammatory cell infiltration, and 4 cases of diverticular gangrene, respectively. The 38 participants in the gastrointestinal bleeding group were further categorized into simple (n = 36), with normal basis of the MD and complex (n = 2), presenting tissue swelling, inflammatory cell infiltration, and ulcer formation. The 8 participants in the infection group were further categorized into simple (n = 2), with normal basis of the MD and complex (n = 6), with 4 cases of tissue swelling, inflammatory cell infiltration, and 2 case of diverticular gangrene (see Table 3).
Table 3.
Clinical classification and pathological results
| Clinical feature | Clinical classification | N | Pathological results (Basal of MD) |
|---|---|---|---|
| Bleed | Simple | 36 | Normal |
| Complex | 2 | 2 cases of tissue swelling, inflammatory cell infiltration, and ulcer formation | |
| Infection | Simple | 2 | Normal |
| Complex | 6 | 4 cases of tissue swelling, inflammatory cell infiltration, 2 cases of diverticular gangrene | |
| Intestinal obstruction | Simple | 3 | Normal |
| Complex | 6 | 2 cases of tissue swelling, inflammatory cell infiltration, 4 cases of diverticular gangrene |
One child with MD was diagnosed with the simple type of MD during laparoscopic exploration, and presented a diverticulum with a base that was considered to be in the mesangial margin. This child presented recurring abdominal pain and gastrointestinal hemorrhage one month after undergoing simple diverticulectomy by laparoscopy. The ECT detection was positive for MD, and the patient recovered after resection of both sides of the intestinal tube of the MD was performed during the surgical exploration. The remaining 54 patients were cured, and follow-up for 4~36 months revealed that they did not present abdominal pain, and no hematochezia occurred as a complication.
Postoperative pathological analysis showed ectopic gastric mucosa residue visible in the MD. Three ectopic gastric mucosa islands were distributed in the top and the body of the MD, although no abnormal findings were noted when reviewing the first MD specimens.
Discussion
MD is usually located in the last 90 cm of the terminal ileum and is formed by all layers of the small intestine. It frequently contains heterotopic tissue, usually gastric mucosa. It is always located in the right lower quadrant or umbilical region and less frequently in the other quadrants. The shape of the MD is conical, with a base that has a broader caliber, a bowel, and an equal or slightly narrow, tapering apex. Sometimes the base is tubular and narrow, like the appendix [10]. MDs range in size from 1-10 cm, generally 3~5 cm, and cases of giant MD (≥ 5 cm) are relatively rare and are associated with more severe forms of complications, especially obstruction. The top of the diverticulum is usually free, but sometimes there is a cord connected with the umbilicus [11]. However, another study [12] reported one case in which the diverticulum was present in the mesenteric region of the ileum. Histologic examination of the specimen revealed the presence of pancreatic tissue and oxyntic and antral type gastric mucosa, showing chronic peptic ulceration apart from the intestinal mucosa. This case report underlines the need for a revision in our understanding and classification of MD. Our current study also included on case in which the diverticulum was located in the mesenteric aspect of the ileum.
Most patients are asymptomatic, and MD is most frequently diagnosed as an incidental finding during laparotomy. The most frequent complications of MD are intestinal obstruction, rectal bleeding, diverticulitis, and perforation due to peptic ulcer [13].
In the pediatric population, MD is the most common cause of massive lower gastrointestinal (LGI) bleeding, which is characterized by painless, massive melena, usually leading to shock. Severe and characteristic LGI bleeding often implies possible MD with ectopic gastric mucosa. A scan of MD using technetium Tc-99m pertechnetate, which is capable of binding to the gastric mucosa, offers a preoperative diagnosis for bleeding MD [14-16]. In our current study, the main symptom of LGI bleeding was found in 69.1% (38/55) of the MD cases. Among children with LGI bleeding, the detection rate was reported to be 90%, while the sensitivity and specificity were reported to be 0.6-0.75, with a positive predictive value 1.0 and a negative predictive value 0.77. Therefore, the preoperative diagnosis of MD is a challenge, despite the availability of modern imaging. CT and Technetium-99m radionuclide imaging are widely used, but the correct diagnosis is often established only at the time of laparotomy or laparoscopy. This is especially the case for Meckel’s diverticulum in children, as symptoms are often nonspecific and complications are likely to occur at the time of the presentation. Even if non-specific, false-positive findings are not common, potential causes include intestinal obstructive disease, intussusception, and inflammatory disease [17-19], and it is therefore very important to make a decision with regard to the indications of laparoscopic exploration.
With laparoscope, both extracorporeal and intracorporeal resection of MD may be performed. laparoscopic-assisted transumbilical has some additional benefits than that of laparoscopic only surgery. In laparoscopic-assisted transumbilical Meckel’s diverticulectomy, a transumbilical trocar is inserted in an open fashion, then using operative laparoscope, the terminal ileum is exteriorized through umbilicus with an atraumatic instrument and then diverticulectomy or segmental resection can be performed. Thus this technique also allow palpation of Meckel’s diverticulum which aids in ruling out any mass or thickening of base thus providing the more complete assessment for presence of any ectopic gastric mucosa. This technique also prevents the use of costly staplers making it more cost effective [8]. Here, we presented a study on the laparoscopic technique, including the laparoscope-only and laparoscopically assisted diagnosis and treatment of MD. All the 55 cases from the current study presented positive laparoscopic exploration results, which avoided any treatment delays or negative results from unnecessary routine surgical exploration. The indications for laparoscopic exploration are the following: for the patients with LGI, we decided to conduct laparoscopic exploration if any of the following situations occurred: ECT-positive; GLI bleeding more than twice; poor efficacy with non-surgical treatment and accompanied by obvious anemia; any other indications for open surgery. For the MDs presenting with infection and intestinal obstruction, the laparoscopic exploration indication followed the indications for general appendicitis and intestinal obstruction; and for the appropriate choice of operating methods, the patients were classified into two categories, the simple and complex types, based on the appearance of the MD upon laparoscopic exploration. The simple type of MD means having a clear base and basal tissue identical to the one found in the normal bowel. The complex type was defined as presenting an unclear base or basal edema, stiffness, scarring, and abnormal intestinal tissue, or diverticular gangrene. The patients with the simple type of MD were treated with simple diverticulectomy by laparoscopy only, and the patients with the complex type of MD were treated with laparoscopically assisted diverticulectomy and adjacent intestinal resection and intestinal anastomosis. In our current study, the selection of surgical methods by laparoscopic exploration yielded good results: Among the 55 cases, Just one child with simple type MD during laparoscopic exploration, and presented a diverticulum with a base that was considered to be in the mesangial margin. The remaining 54 patients were cured, and follow-up for 4~36 months revealed that they did not present abdominal pain, and no hematochezia occurred as a complication.
The pathological results obtained postoperatively (Table 2) further confirmed our selection of the operating methods: 54 patients were cured after the operation, but one child with MD, whose MD base was considered to be in the mesangial margin, was diagnosed with the simple type during laparoscopic exploration. This child presented recurrent abdominal pain and gastrointestinal hemorrhage one month after simple diverticulectomy laparoscopy. The ECT detection was positive for MD, and the patient recovered after performing resection of both sides of the intestinal tube of the MD by surgical exploration. Postoperative pathological analysis showed ectopic gastric mucosa residue visible in the intestinal base of the MD. The three ectopic gastric mucosa islands were distributed at the top and the body of the MD, although no abnormal findings were observed in the basis when reviewing the first MD specimens. Considering this special case, we suggest that the MD located in the mesenteric region of the ileum should be classified into the complex group, and it is better to perform rapid frozen pathological analysis during surgery to decide the proper extent of the excision.
Conclusion
In conclusion, selection of surgical methods by laparoscopic exploration with laparoscopic only in simple type MD and laparoscopically assisted methods in comples typle MD will be a good choice.
Acknowledgements
Thanks to the support of clinical medical research projects in 2011 of health bureau, Wuhan (WX11D10).
Disclosure of conflict of interest
None.
References
- 1.Elsayes KM, Menias CO, Harvin HJ, Francis IR. Imaging manifestations of Meckel’s diverticulum. AJR Am J Roentgenol. 2007;189:81–88. doi: 10.2214/AJR.06.1257. [DOI] [PubMed] [Google Scholar]
- 2.Hill M. Gastrointestinal Tract Abnormalities [M] UNSW Embryology. 2010 [Google Scholar]
- 3.Gokhan Y, Sadettin C, Turgut T. Perforation of Meckel’s diverticulum by a chicken bone, a rare complication: Report of a case. Surg Today. 2004;34:606–608. doi: 10.1007/s00595-004-2765-x. [DOI] [PubMed] [Google Scholar]
- 4.Poley JR, Thielen TE, Pence JC. Bleeding Meckel’s diverticulum in a 4-month-old infant: treatment with laparoscopic diverticulectomy. A case report and review of the literature. Clin Exp Gastroenterol. 2009;2:37–40. doi: 10.2147/ceg.s3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan KW. Perforation of Meckel’s diverticulum caused by a chicken bone: A case report. J Med Case Rep. 2009;3:48. doi: 10.1186/1752-1947-3-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kleinman R. Atlas of Pediatric Gastrointe stinal Disease. Chapter 29. Ontario: B.C. Decker; 1998. p. 113. [Google Scholar]
- 7.Lee KH, Yeung CK, Tam YH, Ng WT, Yip KF. Laparascopy for definitive diagnosis and treatment of gastrointestinal bleeding of obscure origin in children. J Pediatr Surg. 2000;35:1291–1293. doi: 10.1053/jpsu.2000.9299. [DOI] [PubMed] [Google Scholar]
- 8.Sharma R, Jain V. Emergency surgery for Meckel’s diverticulum. World J Emerg Surg. 2008;3:27. doi: 10.1186/1749-7922-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palepu S. Axial volvulus of a giant Meckel’s diverticulum. Abd Surgery. 2007;20:65–66. [Google Scholar]
- 10.Uz Zaman M, Fatima N, Zaman U, Sajjad Z. Wandering Meckel’s diverticulum on 99m technetium-pertechnetate scintigraphy: A diagnostic dilemma? Indian J Nucl Med. 2014;29:30–31. doi: 10.4103/0972-3919.125767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akbulut S, Yagmur Y. Giant Meckel’s diverticulum: An exceptional cause of intestinal obstruction. World J Gastrointest Surg. 2014;6:47–50. doi: 10.4240/wjgs.v6.i3.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manukyan MN, Kebudi A, Midi A. Mesenteric Meckel’s diverticulum: A case report. Acta Chir Belg. 2009;109:510–512. doi: 10.1080/00015458.2009.11680472. [DOI] [PubMed] [Google Scholar]
- 13.Bubnjar J. Meckel’s diverticulitis in the early postoperative course after appendectomy. Acta Med Croatica. 2013;67:65–68. [PubMed] [Google Scholar]
- 14.Blevrakis E, Partalis N, Seremeti C, Sakellaris G. Meckel’s diverticulum in paediatric practice on Crete (Greece): a 10-year review. Afr J Paediatr Surg. 2011;8:279–282. doi: 10.4103/0189-6725.91665. [DOI] [PubMed] [Google Scholar]
- 15.Sinha CK, Pallewatte A, Easty M, De Coppi P, Pierro A, Misra D, Biassoni L. Meckel’s scan in children: a review of 183 cases referred to two paediatric surgery specialist centres over 18 years. Pediatr Surg Int. 2013;29:511–517. doi: 10.1007/s00383-013-3270-3. [DOI] [PubMed] [Google Scholar]
- 16.Menezes M, Tareen F, Saeed A, Khan N, Puri P. Symptomatic Meckel’s diverticulum in children: a 16-year review. Pediatr Surg Int. 2008;24:575–577. doi: 10.1007/s00383-007-2094-4. [DOI] [PubMed] [Google Scholar]
- 17.Rho JH, Kim JS, Kim SY, Kim SK, Choi YM, Kim SM, Tchah H, Jeon IS, Son DW, Ryoo E, Cho KH, Choi DY, Kim YM. Clinical Features of Symptomatic Meckel’s Diverticulum in Children: Comparison of Scintigraphic and Non-scintigraphic Diagnosis. Pediatr Gastroenterol Hepatol Nutr. 2013;16:41–48. doi: 10.5223/pghn.2013.16.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pampal A, Aksakal ED. Littre hernia in childhood: a case report with a brief review of the literature. Afr J Paediatr Surg. 2011;8:221–224. doi: 10.4103/0189-6725.86068. [DOI] [PubMed] [Google Scholar]
- 19.Rangarajan M, Palanivelu C, Senthilkumar R, Madankumar MV. Laparoscopic surgery for perforation of Meckel’s diverticulum. Singapore Med J. 2007;48:e102–105. [PubMed] [Google Scholar]


