Abstract
To assess bone augmentation based on a non-critical defect, 1.5 mm deep cobalt-chromium (Co-Cr) barrier membranes were placed on seven adult California rabbits with three different grafting situations: whole blood, whole blood with tricalcium phosphate (TCP), and TCP mixed with bone marrow cells. Macroscopic assessment of the animals was performed once a week and densitometric studies were performed once a month. Three months post-surgery, after detaching the membranes, tibias were sectioned and followed the routine laboratory processing for decalcified sections, with inclusion in paraffin and staining by hematoxylin and eosin technique. Bone augmentation was observed for each animal, even sometimes over the Co-Cr membranes. Compact bone was mostly observed for every situation, with a higher cellular activity on those samples with bone grafts. This could be due to the presence of graft remains at the growth area. It could be concluded that blood supply to the site providing growth factors by the blood clot formation, and the placement of an osteoconductive non-resolvable membrane that favors osseoinduction, may be sufficient elements to achieve bone augmentation in a period of three months in rabbit tibia.
Keywords: Bone augmentation, cobalt-chromium, bone marrow cells, tricalcium phosphate
Introduction
An adequate quantity and quality of bone are some of the prerequisites of good long-term prognosis in implant dentistry. Insufficient bone volume may be due to congenital, post-traumatic, post-surgical defects or the result of disease processes. In order to ensure sufficient bone at implant sites, bone grafts, augmentation of the maxillary sinus floor, guided bone regeneration and bone augmentation techniques have been developed and performed [1].
The biologic mechanisms of osteogenesis, osteoconduction, and osteoinduction can be used to optimize therapeutic approaches to bone augmentation. Some or all of the following principles are used to enhance bone augmentation outcomes. Osteogenesis involves the direct transfer of vital cells originating from the bone graft material to the area where new bone will be regenerated. Osteoconduction occurs when the graft acts as a scaffold for the cellular and biochemical events progressing to bone formation. Osteoinduction concerns the stimulation of pluripotential, mesenchymal-derived cells to differentiate along an osteoblast pathway with the subsequent bone formation [2].
Lately, the promotional effects of growth factors in bone grafting have been studied. The potential of mesenchymal stem cells to differentiate into osteoblast-like cells and their efficacy in bone regenerative procedures have been verified by assay [3]. As the transplantation and culture of undifferentiated bone marrow cell are expensive and difficult procedures, the use of bone marrow aspirates on the site to be regenerated or augmented has been studied as an alternative with the purpose of filling bone defects, stimulating fracture healing, and treating pseudoarthrosis [4].
Autogenous bone grafts are considered as the gold standard for bone replacement. This type of graft is obtained by intraoral or extraoral harvesting procedures, being preferred the first one for the treatment of localized bone defects in partially edentulous jaws [5]. But, the main disadvantage of this kind of filling material is that it requires the removal of bone from the same patient, thus increasing the complexity of surgery since bone availability is not always sufficient in intraoral areas [6]. Therefore, to overcome this drawback, different bone fillers such as allogenic or xenogenic grafts or alloplastic bone substitutes were developed as an option, and used in combination with barrier membranes [7]. Hydroxyapatite (HA) ceramics come in naturally occurring, called xenografts, and synthetic forms. Clinically available, naturally occurring forms of HA include the coral-based products as well as bovine derived products [8]. The osteoconductive properties offered by natural bone substitutes from animal origin such as bovine HA, overcome some of the autografts’ limitations [9]. Tricalcium phosphate is a synthetic compound more soluble than HA due to its small granule size and porosity [8] showing a higher resorption rate in vivo13. Researches demonstrate that TCP has no adverse effect on cell count, viability and morphology, and this material provides a matrix that favors limited cell proliferation [10]. It has been reported that among the bone substitutes considered, and as far as degradation and substitution are concerned, tricalcium phosphate showed a significantly higher percentage of bone fill at 24 weeks of healing [7].
A principle of tissue healing was discovered in the early 1980’s. It was observed that by the placement of a barrier membrane, migration of undesired cells into the wound site could be prevented, and the transfer of desired cells allowed. This was a mean to control the kind of proliferating cells in the wound, which determine the type of tissue regenerating in that space [11].
In the first studies of guided bone regeneration, flexible membranes were applied. Among these, collagen and aliphatic polyesters, such as polyglycolide or polylactide, are best known for their medical applicability [12]. The main advantage of this sort of material lies in its resorption by the body, thus eliminating the need for second-stage removal surgery, and reducing the risk of morbidity and tissue damage. However the unpredictable degree of resorption is one of the disadvantages of resorbable materials, and it may alter the amount of bone formation [13]. Moreover, research studies reported inflammatory processes in the adjacent tissue caused by the placement of some resorbable membranes, and quickly degradation of the material by enzymatic activity of macrophages and neutrophils [14].
Alternatively, non-resorbable membranes include polytetrafluoroethylene (PTFE) and titanium mesh. Certainly, this type of membranes has more advantages than disadvantages. One drawback is that it must be removed with a second-stage surgical procedure. However, these have the capability to provide an efficient barrier function in terms of biocompatibility, maintenance of the space intended for bone regeneration during time, prediction of their behavior and a reduced risk of long-term complications [13]. Also, cobalt-chromium based alloys have been widely used in orthopedics because of its excellent long-term clinical results, the chance of coating its entire surface, and its resistance to abrasive wears [15]. Furthermore, these have been used as an alternative to titanium alloys and alumina ceramics due to its superior stiffness and toughness. However, metallic ions release could disturb bone homeostasis at the bone-implant interface, leading to bone resorption and aseptic loosening of the implant, inhibiting proliferation/mineralization of bone marrow cells [16]. This release is produced because cobalt alloys can easily suffer from corrosion by friction [17] when sliding over another metal surface. As dental implants, these alloys are placed in the bone; thus, its biological behavior is of interest in dentistry. However, the less favorable scenario happens when the alloy acts as an articulating surface of both metal on metal and metal on polyethylene artificial joint implants, producing a large number of metal wear particles [18]. Thereby, orthopedic implants cannot be compared with dental implants as they generally have bigger surfaces under friction and wear. So, information from the orthopedic literature is useful but cannot be directly applied in dentistry. In this study, the membrane was used for a short period being removed afterwards, and it is not in contact with other metallic surface, being unlikely to happen ions release. Moreover, it was found that supplying osteogenic function to the Co-Cr implants prior to their implantation the bone loss could be prevented and a tight fixation could be obtained just 3 weeks after implantation [16].
The aim of the present study was to assess bone augmentation using bone marrow cells and whole blood, and tricalcium phosphate graft with a microfixed Co-Cr membrane.
Materials and methods
Study design
Approval from the Superior Council of the National University of Entre Rios was obtained previous to the start of the study. Seven adult California rabbits weighing between 2 and 2.8 kg were used as experimental animals. The animals were accommodated at 18-21°C with 50-55% humidity. Each rabbit was housed in an individual cage. They were fed a standard commercial rabbit chow. Water and food were available ad libitum. The rabbits were divided into three groups of two each (except the control rabbit). Group 1 had whole blood as grafting material, group 2 had tricalcium phosphate (TCP, 63-250 um, CERASORB PARO®, Curasan, Germany) with whole blood, and group 3 had TCP and bone marrow cells. To provide the space for the future newly formed bone, 1.5 mm deep Co-Cr membranes were used.
Surgical procedure
General anesthesia was induced by an intra-muscular 5 ml dose of ketamine and 1 ml dose xylazine; and local anesthesia was 1.5 ml dose of carticaine L-adrenaline. Before the intervention, digital radiographies were taken from both tibias. Proximal metaphysis’ medial ridge of both left and right tibia were shaved and disinfected with a povidoneiod solution before the operation. This was followed by a full thickness skin incision and flap elevation exposing the tibial bone. For groups 1 and 2, few microperforations were performed through a 1 mm diameter drill, and the membrane was placed above them and fixed with a vitallium microscrew. In the case of group 3, instead of the microperforations, a 3 mm not critical sized osteotomy was performed from which bone marrow was aspirated. For groups 2 and 3, grafts were included between the membrane and the perforations. To the control animal, a non critical osteotomy was performed on its right tibia, which was not covered by a membrane, neither graft was added. The flaps were repositioned and sutured with Vicryl 3.0 and Nylon 3.0 (Ethilon, Belgium), and an antibiotic (RIFOCINA®, Sanofi-Aventis, Rifamycin SV.) was placed at the injury. The animals were sacrificed 12 weeks after the intervention with an overdose of sodium pentobarbital IV (Dolethals; Vetoquinol, Lure, France).
Results
Bone dimensions measurement
Three months after the intervention, bone augmentation was observed on every animal of each group, even sometimes over the Co-Cr membrane (Figure 1). Height, width and length of the newly formed bone were measured from images taken with a stereoscopic binocular microscope (Arcano®) at 2× and 4× magnification, in order to obtain the vertical bone height and the bone volume for each group (Table 1), except for the control rabbit which had no bone augmentation.
Figure 1.
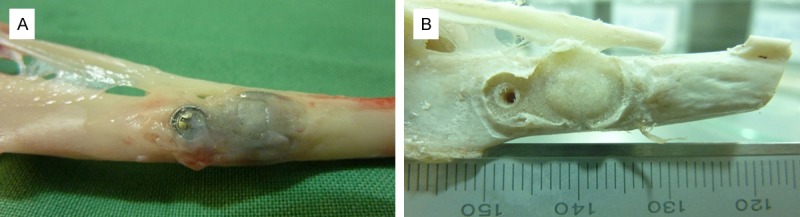
Macroscopic images of the rabbit tibia showing: A. The Co-Cr membrane covered with newly formed bone. B. The augmented bone under the membrane.
Table 1.
Augmented bone dimensions
| Group | Vertical bone height, mm | Bone volume, mm3 |
|---|---|---|
| Group 1 | 1.6 | 155 |
| Group 2 | 1.33 | 137 |
| Group 3 | 1.33 | 143 |
Densitometric measurement
Macroscopic assessment of the animals was performed once a week. Densitometric studies were performed once a month on each specimen using the Digora System® (Soredex Finndent, Tuusula, Finland) for digital radiography, for the evaluation of optical density assessed as pixel intensity (gray value) unit. To determine bone density, different neighboring areas of the membrane were measured: before the microscrew, between the microscrew and membrane, middle, immediately after and after the membrane (Figure 2).
Figure 2.
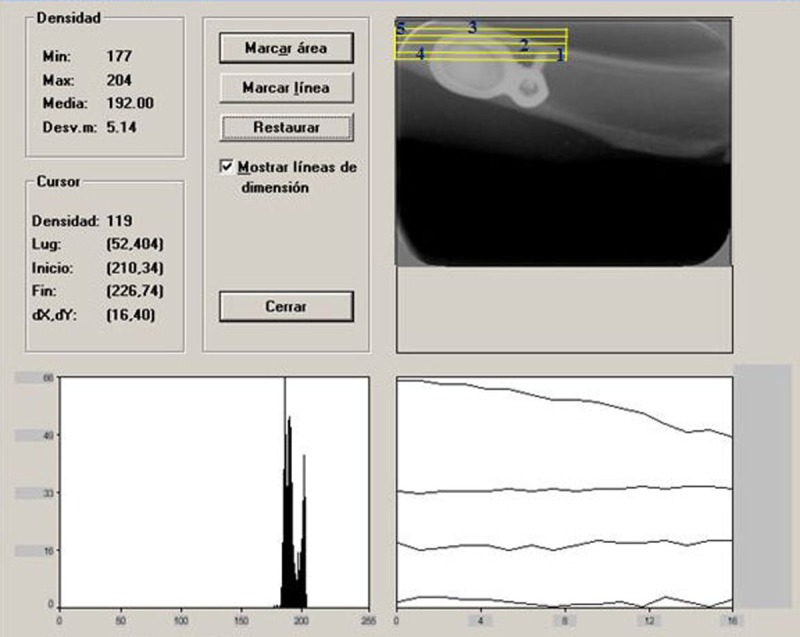
Interface of the Digora System® used to determine density evolution. The different measurement areas are highlighted with red numbers.
Post-mortem, after detaching the membranes, tibias were sectioned and the augmented tissue was measured using a stereo microscope and the Motic Images Plus® 2.0 software. Then the samples were fixed in formaldehyde 10% for further analysis. The tissue samples containing the newly formed bone were removed and followed the routine laboratory processing for decalcified sections, with inclusion in paraffin. After inclusion, the blocks were set on slides, stained with hematoxylin and eosin technique and visualized through an optical microscope NIKON E 200. Microphotographies were taken with a digital camera Nikon Coolpix S 4.
The selected area for measurement was the surrounding bone of the microscrew and the membrane, reporting the maximum, minimum and mean density of the considered tissue (Figure 3). Group 1 exhibited the highest density value for every measure. After three months, group 1 demonstrated the highest mean density (Figure 4).
Figure 3.
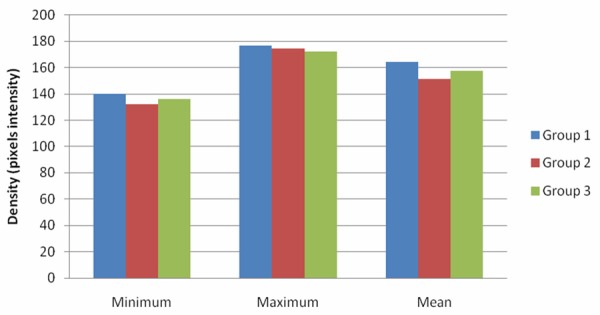
Maximum, minimum and mean density for each group.
Figure 4.
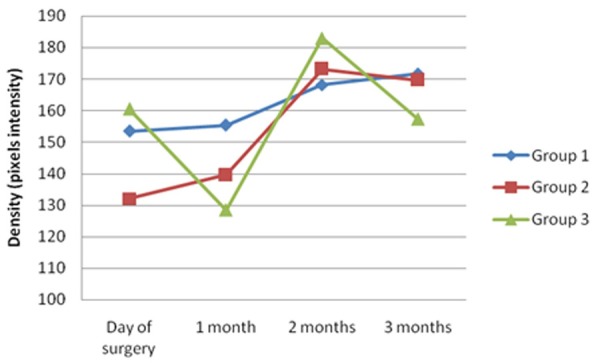
Mean density evolution over time for each testing group.
Histological examination
Osteocytes and blood vessel count was carried out to an average of 36 samples per group (Table 2). Three months after the intervention, high cellular activities were detected in groups 2 and 3, observing compact bone with a high number of osteoblastic cells. In those samples of the group with only the membrane and whole blood, compact bone was observed, with a lower osteocytes count (Figures 5, 6 and 7). Concerning the control rabbit compact bone with a very low osteocytic activity could be noticed.
Table 2.
Osteocytes and blood vessels count per mm2
| Group | Osteocytes/mm2 | Vessels/mm2 |
|---|---|---|
| Group 1 | 995.74 | 21.48 |
| Group 2 | 1660.3 | 32.91 |
| Group 3 | 2382.78 | 56.85 |
Figure 5.
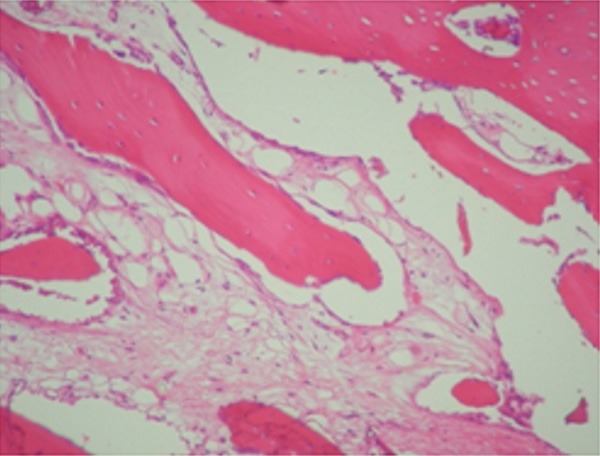
Newly formed trabeculae surrounded by active osteoblasts. (Original magnification 100×; hematoxylin and eosin stain).
Figure 6.
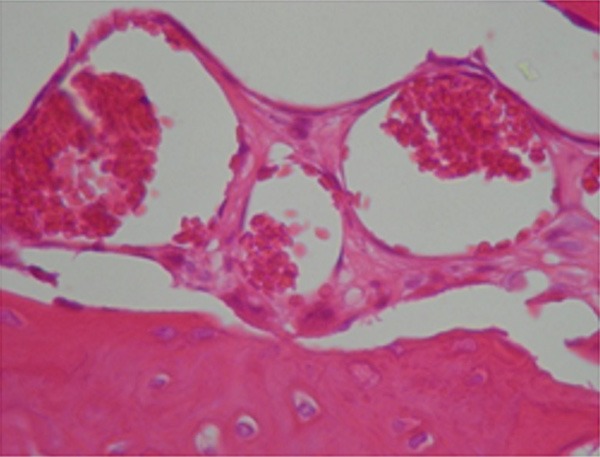
Augmented bone with ectatic blood vessels (Original magnification 400×; hematoxylin and eosin stain).
Figure 7.
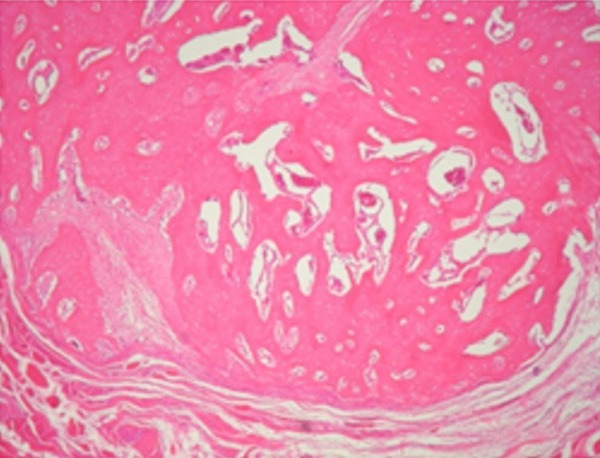
Newly bone augmentation. (Original magnification 40×; hematoxylin and eosin stain).
From the count results (Table 2) and histological images could be concluded that, after three months, compact bone was mostly observed, with a higher cellular activity on those samples with bone substitute. This could be due to the presence of grafts remaining at the growth area.
Discussion
Current strategies for enhancing bone augmentation are based on advances concerning natural bone recovery processes, scaffold techniques, growth factors, signaling molecules, mesenchymal progenitor cells, autogenous tissue, organic and synthetic substitutes. The development of these new methods is linked to the technological progress in materials science, bioengineering, biotechnology, biochemistry and bioinformatics.
Since the 70’s osseointegrated implants were used for lost tooth replacement in total and partially edentulous patients. One of the main requirements for long term success in the rehabilitation with implants are an adequate quality and quantity of bone at the site; considering bone quality to be defined on the basis of bone density, trabecular bone volume, cortical bone density and mineral bone content [19,20].
It has been reported that standard diameter implants with a length lower than 10 mm exhibit worse prognosis [21], meaning that for example, implants of 3.75 mm diameter requires at least 10 mm height and 6 mm bone width. Otherwise, these patients need graft materials or augmentation procedures, in order to reach suitable height and width to host the implant [21]. Frequently, bone grafts are used, being autologous bone the Gold Standard because of meeting-besides osteogenesis, osteoinduction and osteocconduction potentials-a group of optimal properties, such as their histocompatibility, low antigenicity and infection risk, achieving satisfactory results. Conversely, these grafts have certain disadvantages as the availability and morbidity of donor site.
When bone resource is compromised some substitutes of biologic (xenografts) or synthetic derivation can be used in combination with membranes. These act as a barrier that prevents non-osteogenic cells passage, and allow osteogenic and angiogenic cells migration to the injury location [7], stabilizing at the same time the blood clot and bone grafts. Simion et al. supported that membranes as occlusive barrier, seem not to provide additional value to growth effects; on the contrary, they could complicate injury healing. A possible explanation comes from a research study in which is reported that growth factors (rhPDGF-BB) should have stimulated more strongly bone formation from the periosteal surface than from the residual native bone. Li M et al. observed that the placement of a membrane could have prevented the osteoblastic differentiation stimulated by the periostium. To use this stimulation in favor of the desired augmentation, the flap technique performed in the present study, does not clear the periosteum from the bone surface, it is rather preserved doing the microperforations over it, thus using the periosteum as a bone formation enhancer. Moreover, it has been demonstrated that the placement of occlusive osteoconductive non-resolvable membranes ease a significant bone formation [22,23], facilitating the augmentation of bone defects, favoring the induction of bone regeneration and augmentation, and improving the outcomes of techniques that utilizes bone substitutes [13].
In the present study, the purpose was to stimulate vertical growth based on a non-critical defect [24] by the combination of grafting techniques, bone marrow cells and whole blood with microfixed non-resolvable Co-Cr membranes. The membrane microfixation was done to avoid its displacement and to stabilize the blood clot, which is important because it acts as an extracellular matrix where cell can growth, and besides it contains growth factors [25].
From our results it can be seen that vertical augmentation was achieved in each group. Group 1, in which only blood coming from the microperforations was used, showed macroscopic and histological results similar to the other groups. Rompen et al. [18] demonstrated that promoting bone supply and bone forming cells access through the microperforations in rats skulls, new bone formation was enhanced. When microperforations are performed, blood comes in contact with the membrane triggering the coagulation cascade [26], producing thrombin and turning plasma fibrinogen into fibrin. Thrombin stimulates platelets and these release factors that produce vasoconstriction and benefit osteoblast activation. The formed clot holds chemotactic and mitogenic growth factors (GF), as the platelet derived GF (PDGF), insulin-like GF (ILGF), transforming GF beta (TGF-β), and the fibroblasts GF (FGF), all of these promote revascularization and bone formation. The insulin-like factor has an important role in the homeostasis, and in the synthesis balance of the proteoglycans from the granulation tissue. TGF-β acts on immune and inflammatory processes, because it inhibits the differentiation and growth of several immune cell lineages, including T and B cells, by autocrine and paracrine communication. The Bone Morphogenetic Proteins (BMP) interact with specific receptors located at the cell surface, known as BMP receptors, bonding to serine-threonine-kinase transmembrane receptors type I and II and forming receptor complexes type II. As a result of the complex phosphorylation, a transduction of the signal is produced, which mobilizes by proteins phosphorylation the Smad family, particularly Smad 1, 5 and 8 heterodimers that allow the stimulation of osteogenic activity [27], whereas β1, β2, β3 proceed in combination to regulate osteoclast generation and survival, through the induction of osteoprotegerin (OPG), protein that inhibits osteoclast formation and function [28]. PDGF is a potent mitogen and chemotactic of mesenchymal cells which originate fibroblasts, osteoblasts and chondroblasts, and it is involved in the formation and restoration of bone tissue.
In group 2 tricalcium phosphate was used. Okazaki et al. [29] said that clinical and experimental studies have shown that the presence of a clot with the graft promotes bone regeneration. The TCP placed at the site to be regenerated, suffers from a degradation process by the osteoclasts which acidify the extracellular compartment with a consequent pH decrease [30]. This process could decelerate bone regeneration in the early stages of restoration, in comparison with autologous bone as graft [9]. However, TCP has a high rate of resorption in vivo [8] due to its porosity and small granule size [31], determinants of nutrients and oxygen diffusion, insertion, migration and differentiation of cells. Handschel et al. [32] reported that although at the early stages of bone healing autologous bone shows a higher total bone volume, over time bone substitutes approach this result, and after 9 months no statistical differences are found between bone grafts.
In group 3, bone marrow cells (BMC) in combination with TCP as scaffold, were used as bone graft. BMC have been used for the reconstruction of hard tissue defects in combination with graft materials which act as scaffolds. Among BMC, mesenchymal progenitor cells (MPC) are found. Once transplanted, and under optimal conditions, cells differentiate and form a mineralized matrix on the scaffold which then is replaced by new bone. MPC can differentiate into osteoblasts, chondroblasts, lipoblasts and stromal cells, depending on local factors, oxygen tension and mechanical stimuli [33]. If there is a good vascularization, close contact of bone edges and stability, bone regeneration occurs by intramembranous ossification. Defect instability and low oxygen tension favor MPC differentiation towards the chondrogenic linage. This tissue is then replaced with bone by endochondral ossification. Betoni et al. [4] studied the use of centrifuged bone marrow aspirates on the rabbit tibia, comparing with the control group that only had blood clot, and they concluded that there was no difference in the bone repair of periimplant cervical defect with or without the use of centrifuged bone marrow 60 days after surgery. They also reported that the spontaneously formed clot at the bone defect in the control group was enough to achieve adequate bone regeneration on periimplant bone defects. This is in accordance to our results in which, for the period under consideration, there were no differences between groups.
Concerning the membranes manufactured for this study, bone formation is possible due to their design and topography. An important issue is if the membrane architecture and topography could influence osteoclast generation at the surface or in its vicinity. It has been demonstrated that the surface topography alters OPG expression trough adherents MG63 cells [34], which produce transduction signaling, transcription, regulation, proliferation and apoptosis of the cell cycle, and cytoskeleton formation. Davis et al. [35] reported that cultivated human mesenchymal stem cells express high OPG levels when are placed in contact with complex microtopographical surfaces. A surface topography could affect osteoclast number and activity showing that progenitor cells populations are influenced by the membrane surface. In addition, the surface oxide could have a favorable effect on the extracellular matrix architecture and on the cellular activity around the tissue. Osteoblasts to implant fixation is an important condition for a good tissue compatibility and thus, for an optimal bone formation. Thull reported the possibility that the protein layer which adheres to the material surface is of critical importance for this processes [36]. Current evidence suggests that the absorption of extracellular matrix proteins in a way that preserves their natural conformation is more conducive to cellular adhesion and osseointegration, in comparison to the change of this conformation after absorption. From the literature it is observed that in the future not only osteoblasts generation could be enhanced, but also will be possible to control osteoclast generation at the surrounding bone.
It could be concluded that blood supply to the site providing growth factors by the blood clot formation, and the placement of an osteoconductive non-resolvable membrane that favors osseoinduction, may be sufficient elements to achieve bone augmentation in a period of 3 months in rabbit tibia.
In conclusion, bone augmentation has been accomplished just by providing the tissue an adequate space through the use of a Co-Cr membrane. This together with the blood supply to the site, delivering growth factors by the blood clot formation, may be sufficient elements to achieve bone augmentation in a period of 3 months in rabbit tibia. This might lead to the exercise of less traumatic augmentation techniques by decreasing tissue response to bone grafts.
Disclosure of conflict of interest
None.
References
- 1.Watzinger F, Luksch J, Millesi W, Schopper C, Neugebauer J, Moser D, Ewers R. Guided bone regeneration with titanium membranes: a clinical study. Br J Oral Maxillofac Surg. 2000;38:312–315. doi: 10.1054/bjom.1999.0228. [DOI] [PubMed] [Google Scholar]
- 2.McAllister BS, Haghighat KJ. Bone augmentation techniques. Periodontol. 2007;78:377–396. doi: 10.1902/jop.2007.060048. [DOI] [PubMed] [Google Scholar]
- 3.Khojasteh A, Eslaminejad MB, Nazarian H, Morad G, Dashti SG, Behnia H, Stevens M. Vertical bone augmentation with simultaneous implant placement using particulate mineralized bone and mesenchymal stem cells: a preliminary study in rabbit. J Oral Implantol. 2013;39:3–13. doi: 10.1563/AAID-JOI-D-10-00206. [DOI] [PubMed] [Google Scholar]
- 4.Betoni W Jr, Queiroz TP, Luvizuto ER, Valentini-Neto R, Garcia-Júnior IR, Bernabé PF. Evaluation of centrifuged bone marrow on bone regeneration around implants in rabbit tibia. Implant Dent. 2012;21:481–485. doi: 10.1097/ID.0b013e31826916b6. [DOI] [PubMed] [Google Scholar]
- 5.Hämmerle CH, Jung RE, Yaman D, Lang NP. Ridge augmentation by applying bioresorbable membranes and deproteinized bovine bone mineral: a report of twelve consecutive cases. Clin Oral Implants Res. 2008;19:19–25. doi: 10.1111/j.1600-0501.2007.01407.x. [DOI] [PubMed] [Google Scholar]
- 6.Jardini MA, De Marco AC, Lima LA. Early healing pattern of autogenous bone grafts with and without e-PTFE membranes: a histomorphometric study in rats. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:666–673. doi: 10.1016/j.tripleo.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 7.Buser D, Hoffmann B, Bernard JP, Lussi A, Mettler D, Schenk RK. Evaluation of filling materials in membrane-protected bone defects. A comparative histomorphometric study in the mandible of miniature pigs. Clin Oral Implants Res. 1998;9:137–150. doi: 10.1034/j.1600-0501.1998.090301.x. [DOI] [PubMed] [Google Scholar]
- 8.Pryor LS, Gage E, Langevin CJ, Herrera F, Breithaupt AD, Gordon CR, Afifi AM, Zins JE, Meltzer H, Gosman A, Cohen SR, Holmes R. Review of bone substitutes. Craniomaxillofac Trauma Reconstr. 2009;2:151–160. doi: 10.1055/s-0029-1224777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tamimi FM, Torres J, Tresguerres I, Clemente C, López-Cabarcos E, Blanco LJ. Bone augmentation in rabbit calvariae: comparative study between Bio-Oss and a novel b-TCP/DCPD granulate. J Clin Periodontol. 2006;33:922–928. doi: 10.1111/j.1600-051X.2006.01004.x. [DOI] [PubMed] [Google Scholar]
- 10.Aybar B, Bilir A, Akcakaya H, Ceyhan T. Effects of tricalcium phosphate bone graft materials on primary cultures of osteoblast cells in vitro. Clin Oral Implants Res. 2004;15:119–125. doi: 10.1111/j.1600-0501.2004.01002.x. [DOI] [PubMed] [Google Scholar]
- 11.Hämmerle CH, Jung RE. Bone augmentation by means of barrier membranes. Periodontol 2000. 2003;33:36–53. doi: 10.1046/j.0906-6713.2003.03304.x. [DOI] [PubMed] [Google Scholar]
- 12.Hutmacher D, Hürzeler MB, Schliephake H. A review of material properties of biodegradable and bioresorbable polymers and devices for GTR and GBR applications. Int J Oral Maxillofac Implants. 1996;11:667–678. [PubMed] [Google Scholar]
- 13.Rakhmatia YD, Ayukawa Y, Furuhashi A, Koyano K. Current barrier membranes: titanium mesh and other membranes for guided bone regeneration in dental applications. J Prosthodont Res. 2013;57:3–14. doi: 10.1016/j.jpor.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Monteiro AS, Macedo LG, Macedo NL, Balducci I. Polyurethane and PTFE membranes for guided bone regeneration: histopathological and ultrastructural evaluation. Med Oral Pathol Oral Cir Bucal. 2010;15:e401–406. doi: 10.4317/medoral.15.e401. [DOI] [PubMed] [Google Scholar]
- 15.Jinno T, Goldberg VM, Davy D, Stevenson S. Osseointegration of surface-blasted implants made of titanium alloy and cobalt-chromium alloy in a rabbit intramedullary model. J Biomed Mater Res. 1998;42:20–29. doi: 10.1002/(sici)1097-4636(199810)42:1<20::aid-jbm4>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 16.Horner K, Devlin H. The relationship between mandibular bone mineral density and panoramic radiographic measurements. J Dent. 1998;26:337–343. doi: 10.1016/s0300-5712(97)00020-1. [DOI] [PubMed] [Google Scholar]
- 17.Kawalec JS, Brown SA, Payer JH, Merritt K. Mixed-metal fretting corrosion of Ti6Al4V and wrought cobalt alloy. J Biomed Mater Res. 1995;29:867–873. doi: 10.1002/jbm.820290712. [DOI] [PubMed] [Google Scholar]
- 18.Haynes DR, Crotti TN, Haywood MR. Corrosion of and changes in biological effects of cobalt chrome alloy and 316L stainless steel prosthetic particles with age. J Biomed Mater Res. 2000;49:167–175. doi: 10.1002/(sici)1097-4636(200002)49:2<167::aid-jbm3>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 19.Rompen EH, Biewer R, Vanheusden A, Zahedi S, Nusgens B. The influence of cortical perforations and of space filling with peripheral blood on the kinetics of guided bone generation. A comparative histometric study in the rat. Clin Oral Implants Res. 1999;10:85–94. doi: 10.1034/j.1600-0501.1999.100202.x. [DOI] [PubMed] [Google Scholar]
- 20.Alhava EM. Bone density measurements. Calcif Tissue Int. 1991;49(Suppl):S21–3. doi: 10.1007/BF02555082. [DOI] [PubMed] [Google Scholar]
- 21.Haas R, Mensdorf-Pouilly N, Mailath G, Watzek G. Survival of 1928 IMZ implants followed for up to 100 months. Int J Oral Maxillofac Implants. 1966;11:581–588. [PubMed] [Google Scholar]
- 22.Miyamoto I, Tsuboi Y, Takahashi K, Hyon SH, Iizuka T. Enhancement of bone volume in guided bone augmentation by cell transplants derived from periosteum: an experimental study in rabbit calvarium bone. Clin Oral Implants Res. 2004;15:308–314. doi: 10.1111/j.1600-0501.2004.01011.x. [DOI] [PubMed] [Google Scholar]
- 23.Engelke W, Deccó O, Cura AC, Borie E, Beltrán V. Rigid occlusive titanium barriers for alveolar bone augmentation: two reports with 24-month follow-up. Int J Clin Exp Med. 2014;7:1160–1165. [PMC free article] [PubMed] [Google Scholar]
- 24.Schmitz JP, Hollinger JO. The critical size defect as an experimental model for craniomandibulofacial nonunions. Clin Orthop Relat Res. 1986;205:299–308. [PubMed] [Google Scholar]
- 25.Ishidou Y, Kitajima I, Obama H, Maruyama I, Murata F, Imamura T, Yamada N, ten Dijke P, Miyazono K, Sakou T. Enhanced expression of type I receptors for bone morphogenetic proteins during bone formation. J Bone Miner Res. 1995;10:1651–1659. doi: 10.1002/jbmr.5650101107. [DOI] [PubMed] [Google Scholar]
- 26.Ogawa M, Tohma Y, Ohgushi H, Takakura Y, Tanaka Y. Early Fixation of Cobalt-Chromium Based Alloy Surgical Implants to Bone Using a Tissue-engineering Approach. Int J Mol Sci. 2012;13:5528–5541. doi: 10.3390/ijms13055528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thor A, Rasmusson L, Wennerberg A, Thomsen P, Hirsch JM, Nilsson B, Hong J. The role of whole blood in thrombin generation in contact with various titanium surfaces. Biomaterials. 2007;28:966–974. doi: 10.1016/j.biomaterials.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 28.Kretzschmar M, Liu F, Hata A, Doody J, Massagué JO. The TGF-beta family mediator Smad1 is phosphorylated directly and activated functionally by the BMP receptor kinase. Genes Dev. 1997;11:984–95. doi: 10.1101/gad.11.8.984. [DOI] [PubMed] [Google Scholar]
- 29.Thirunavukkarasu K, Miles RR, Halladay DL, Yang X, Galvin RJ, Chandrasekhar S, Martin TJ, Onyia JE. Stimulation of osteoprotegerin (OPG) gene expression by transforming growth factor-beta (TGF-beta). Mapping of the OPG promoter region that mediates TGF-beta effects. J Biol Chem. 2001;276:36241–36250. doi: 10.1074/jbc.M104319200. [DOI] [PubMed] [Google Scholar]
- 30.Okazaki K, Shimizu Y, Xu H, Ooya K. Blood-filled spaces with and without deproteinized bone grafts in guided bone regeneration. A histomorphometric study of the rabbit skull using non-resorbable membrane. Clin Oral Implants Res. 2005;16:236–243. doi: 10.1111/j.1600-0501.2004.01095.x. [DOI] [PubMed] [Google Scholar]
- 31.Detsch R, Mayr H, Ziegler G. Formation of osteoclast-like cells on HA and TCP ceramics. Acta Biomater. 2008;4:139–148. doi: 10.1016/j.actbio.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 32.Jensen SS, Broggini N, Hjørting-Hansen E, Schenk R, Buser D. Bone healing and graft resorption of autograft, anorganic bovine bone and beta-tricalcium phosphate. A histologic and histomorphometric study in the mandibles of minipigs. Clin Oral Implants Res. 2006;17:237–243. doi: 10.1111/j.1600-0501.2005.01257.x. [DOI] [PubMed] [Google Scholar]
- 33.Handschel J, Simonowska M, Naujoks C, Depprich RA, Ommerborn MA, Meyer U, Kübler NR. A histomorphometric meta-analysis of sinus elevation with various grafting materials. Head Face Med. 2009;5:12. doi: 10.1186/1746-160X-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 35.Lossdörfer S, Schwartz Z, Wang L, Lohmann CH, Turner JD, Wieland M, Cochran DL, Boyan BD. Microrough implant surface topographies increase osteogenesis by reducing osteoclast formation and activity. J Biomed Mater Res A. 2004;70:361–369. doi: 10.1002/jbm.a.30025. [DOI] [PubMed] [Google Scholar]
- 36.Davies JE, Schupbach P, Cooper L. In: The Implant Surface and Biological Response. Jokstad A, editor. Iowa, USA: Wiley-Blackwell, Ames; 2009. pp. 213–223. [Google Scholar]
- 37.Thull R. Physicochemical principles of tissue material interactions. Biomol Eng. 2002;19:43–50. doi: 10.1016/s1389-0344(02)00009-6. [DOI] [PubMed] [Google Scholar]


