Abstract
Objects: to probe into the effects of PKCε on migration and paracrine functions of stem cells and potential molecular mechanisms. Methods: Bone Marrow mesenchymal stem cells (BMMSCs) were obtained from rat femur and passaged. mRNA and protein levels of capital proteins in PKCε signaling, SDF-1/CXCR4 axis and PI3K/AKT pathway in the MSCs in different conditions treating with PKC agonist, specific PKCε inhibitor, CXCR4 antagonist or PI3K inhibitor for 24 hours were analyzed by real-time PCR and western blot, and migration abilities were observed by migration assay in vitro and the changes of paracrine factors in different treatments were analyzed by protein clips assay. Results: the levels of p-JNK, p-P38MAPK, SDF-1, CXCR4, PI3K and p-AKT increased significantly after treating with PKC agonist (P < 0.05) and decreased obviously after treating with specific PKCε inhibitor. Migration ability and paracrine function of MSCs were enhanced in PMA group and attenuated in PKCε inhibitor group, and inhibiting activity of CXCR4 or PI3K attenuated the effects of PKCε, but not abolished completely. Conclusion: There was cross-talking between PKCε signaling and SDF-1/CXCR4 axis and PI3K/AKT pathway in signal transduction of MSCs. Activating PKCε could improve migration ability and paracrine function of MSCs partly at least independent of SDF-1/CXCR4 axis and PI3K/AKT pathway.
Keywords: PKCε, stem cells, migration, paracrine factors
Introduction
Ischemic heart diseases is worldwide leading death causes, and irreversible and widespread loss of myocardial cells and subsequent ventricular remodeling resulting from acute myocardial infarction (AMI) is still main cause resulting into chronic heart failure and permanent loss of labor force [1]. There are 17.3 million peoples died of ischemic heart diseases in 2008, and the numbers would be 23.6 million by 2030 [2]. Stem cell-based regeneration therapy for AMI had shown encouraging outcomes and great therapeutic potential both in preclinical [3,4] and clinical studies [5-8], and probably become an important routine assisting approach for coronary artery disease in the future soon. However, there are two crucial bottleneck problems to resolve to improve efficiency of stem cells before transplantation therapy is widely used in clinic, i.e. poor homing and survival rate. It is known that SDF-1/CXCR4 axis plays an important role during immigration, proliferation and survival, and researchers had tried many methods to improve homing and survival of stem cells [9-15]. Nevertheless, substantial breakthrough hadn’t still been got on homing and survival of stem cells despite of arduous exploring, and the rates of homing and survival are unsatisfied until now, which suggested that the signal mechanisms of homing and survival may be very complicated and probably there are some unknown mechanisms which play an important role during homing and survival, and investigating thoroughly the mechanisms would probably provide new clues and evidences for enhancing effects of stem cells, and have important clinical significance.
Previous studies [16-18] had shown that Protein Kinase C ε (PKCε) play a unique crucial role in signal transduction during ischemic cardioprotection, and activation of PKCε may be necessary and sufficient condition for ischemic cardioprotection. However, whether it have an effect on homing and survival of stem cells and potential molecular mechanisms involved aren’t clear at all by now. So we performed the study to determine the effects of PKCε on migration and paracrine function of Bone Marrow mesenchymal stem cells (BMMSCs) and potential mechanisms, thus provide new clues for working out reasonable and effective strategy of improving migration and survival of stem cells.
Materials and methods
Conduct of the study
The study was reviewed and approved by the Institutional Ethics Committee of the Anzhen Hospital and Beijing Institute of Heart Lung and Blood Vessel Diseases on Animal Resource and conformed to the guiding principles of “Guide for the Care and Use of Laboratory Animals” (NIH Publication NO. 83-23, revised 1996) during maintaining and using the animals.
Isolation, expansion and passage of rat bone Marrow-Derived Mesenchymal stem cells (BMMSCs)
BMMSCs were separated as previously described [19]. Briefly, BMMSCs were separated from the femurs taken from Sprague Dawley rats (male, weighing 100~120 g, sacrificed by intraperitoneal injection of 3% Pentobarbital Sodium with the dose of 100 mg/kg before their femurs were taken). The cells were centrifuged at 1000 g for 5 minutes and suspended in Dulbecco’s modified Eagle medium with low glucose (GIBCO, Carlsbad, CA) supplemented with 15% fetal bovine serum (Hyclone, Utah, CA). After the cells were vaccinated in culture flask with the density of 2 × 105 cells per cm2 and incubated in the condition of 37°C with 5% CO2 and saturated humidity, the adherent layer was washed once every two day with fresh medium. Cells from Passages 5 to 8 were harvested to be used in subsequent biochemical experiments.
Vitality assay and delineating of growth curve MSCs
MSCs vitality was measured by Trypan blue Staining, and viable and died cells were counted respectively. Cell vitality (%) = [(total cellular score-colouring cell count)/total cellular score] × 100%.
MSCs from Passages 5 were digested with 0.25% trypsogen and vaccinated in 96-well plate with the density of 2 × 104 cells per ml (200 μL per well) and cultured for consecutive 8 days, and the optical density (OD) and cellular counts were measured each day. OD value at 570 nm was measured by incubating with 20 μL 5 mg/ml methyltetrazolium for four hours followed by adding into 150 μL DMSO, and culture solution without cells acted as blank control. Cellular counts were performed by Trypanblau Staining. Growth curves were delineated. Population doubling time (TD) = t [log2/(logNt-logN0)].
Identification of surface markers of MSCs by flow cytometry
After digested with 2.5 g/L trypsin, MSCs were prepared with the density of 1 × 106/ml, and incubated for 30 minutes at 37°C respectively with Monoclonal antibodies of CD29, CD44, CD34, CD45, then incubated for 30 minutes with corresponding FITC-labeled secondary antibody after centrifuging and washing three times with PBS (see attached Supplementary Tables 2 and 3), and homologous IgG and PBS acted as negative control. Then expressions of the surface markers of MSCs were analyzed by flow cytometry.
Experimental protocol
The two days before experiments, MSCs were cultured on Petri dishes at a density of 3000 cells/cm2. To optimize the dose and time for treatment, cells were treated with several doses of PKCε (0-20 μM) for 24 hours. The optimal dose of PKCε (5 μM) determined by MSCs vitality assay was then used to treat cells for a period of time (6-36 hours) to determine an optimal time period.
Then the harvested MSCs were divided into six groups to receive respectively different treatments: MSCs + medium (control group), MSCs + PMA (low-dose PKC agonist, 0.5 uM), MSCs + PMA (high-dose PKC agonist, 5 uM), MSCs + R031-8820 (specific PKCε inhibitor, 1 uM), MSCs + PMA (5 uM) + AMD3100 (CXCR4 antagonist, 2 uM), MSCs + PMA (5 uM) + LY294002 (PI3K inhibitor, 2 uM). MSCs in each group were incubated respectively with above drugs for 24 hours.
Determination of translocation of PKCε in MSCs in different groups
Cytoplasm proteins and membrane proteins of MSCs in each group were extracted and separated with the extraction kit (Beyotime, shanghai, China), and Western Blot was performed as previously described [20] to analyze PKCε expression in different parts of MSCs. Translocation index = content of PKCε in membrane protein/(content of PKCε in membrane protein + content of PKCε in cytoplasm).
Real-time reverse transcription-PCR analysis of mRNA expression of interest proteins in different groups
Total RNA was extracted from the cells in each group with RNA simple Total RNA Kit (TIANGEN, Peking, china). cDNA was prepared using iScript cDNA Synthesis Kit (Bio Rad) and Real time PCR was performed on samples using Exicycler 96 (BIONEER, Korea) according to manufacturer’s protocol, then mRNA expression of each interest protein was analyzed. The primer sequences used for experiments were listed in attached Supplementary Table 1. Amplification and melting curves were obtained, and β-actin was used as the reporter gene, and simple primer reaction products acted as negative control.
Western blot analysis of expressions of interest proteins in different groups
The samples were plated in 24-well dishes (50,000 cells/well) and harvested in 50 µl of sample buffer, boiled and sonicated. Protein lysates were separated on 10% SDS-polyacrylamide gels. After transferring on polyvinylidene fluoride Membranes and blocking with nonfat milk (5% w/v), blots were incubated with primary antibodies (attached Supplementary Table 2) overnight at 4°C. β-actin served as internal reference. Primary antibody binding was detected with an ECL Western Blotting kit, and quantified by laser densitometry using Typhoon 9400 fluorescent scanner together with Image Quant 5.0 software (Amersham Biosciences) as previously described [20].
Migration assay in vitro of MSCs in different groups
MSCs were stained with DAPI solution when they were cultured to 90% confluency in standard media. Then MSCs were digested with 0.25% trypsin and diluted to the density of 1 × 105 cells/ml, and 200 μL of them was added into upper transwell (2 × 104 cells/well) and incubated respectively with different drugs according to the protocol above and allowed to migrate for 24 hours towards 0.125 M SDF-1 (PeproTech) of lower transwell. An uncoated transwell permeable support system with a pore size of 8 μm (Corning, Fisher Scientific) was employed. Transmigrated cells were removed from the lower part of the filter and counted by inversion fluorescence microscope (200 ×). The migratory response to SDF-1 was determined in MSCs in each group. Five non-overlapping visual fields in each sample were selected and means were counted.
Protein clips assays of paracrine factors of MSCs in different groups
MSCs cells were cultured to 90% confluency in standard media and incubated with different drugs for 24 hours according to the protocol. Then the media were collected and concentrated by using the Millipore Amicon Centricon YM-3 system (Bedford, MA), and protein array assays (Biotin label-based Rat antibody array 1, Ray Biotech, Inc) were performed as directed by manufacturer’s instructions. After developing, the membranes were scanned, and the images were processed with the analysis-tool software (Ray Biotech, Inc). The mean signal intensity for each cytokine was obtained from measuring for three times in each chip and normalized to the internal controls, and the ratio of cytokine levels in MSCs in each group was calculated.
Statistical analysis
All values were expressed as means ± SEM. Differences in continuous variables between two groups were analyzed via the Student’s t test, and differences between 3 or more groups were evaluated via 1-way ANOVA with Bonferroni correction; differences in categorical data were assessed by chi-square test, in case of low cell counts (< 5), Fisher’s exact test was used instead of Χ2 test; P < 0.05 was considered to be significant.
Results
Growth and expansion of rat bone marrow-derived MSCs
MSCs obtained from rat femurs were expanded and passaged (Figure 1), and MSCs vitality measured by Trypanblau Staining was 91.76% ± 3.29%. The growth curves were delineated according to their OD value at 570 nm and the counts of MSCs for consecutive eight days (shown as Figure 1), and population doubling time was respectively 0.83, 0.85, 0.97, 1.12, 1.30, 1.51, 1.76, and the mean of the time was 1.19.
Figure 1.
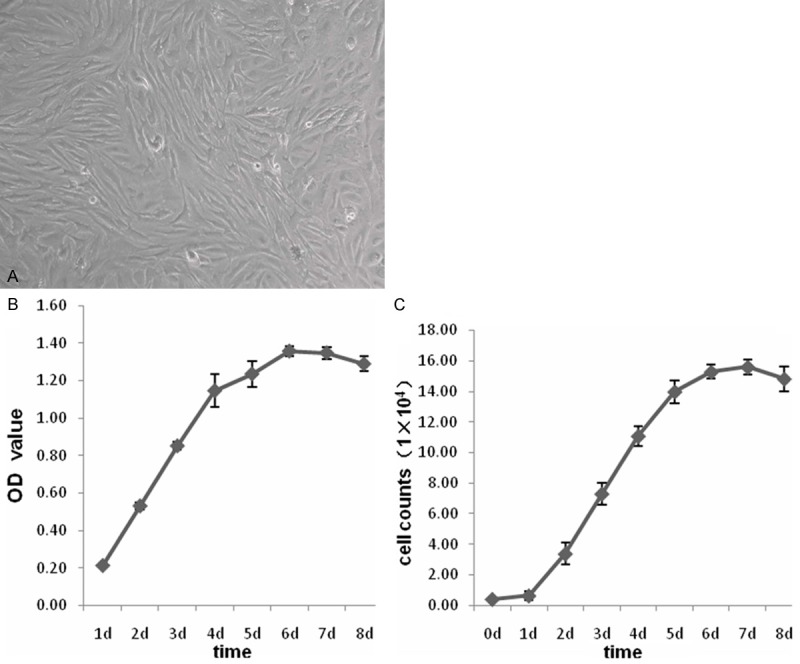
Growth and expansion of rat Bone Marrow-Derived MSCs. A: Representative image of MSCs of passage three. B: The curves delineated according to the OD value at 570 nm of MSCs for consecutive eight days. C: The growth curves delineated according to the counts of MSCs for consecutive eight days. Data were represented as means ± SD for three different experiments.
Phenotype characterization of BMMSCs
Analysis performed by flow cytometry showed that CD29 and CD44 were expressed by 98.04% and 98.73% of the cultured rat BMMSCs, respectively. Positive rates of expressions of CD34 and CD45 of the cells were respectively 5.50% and 5.35% (Figure 2).
Figure 2.
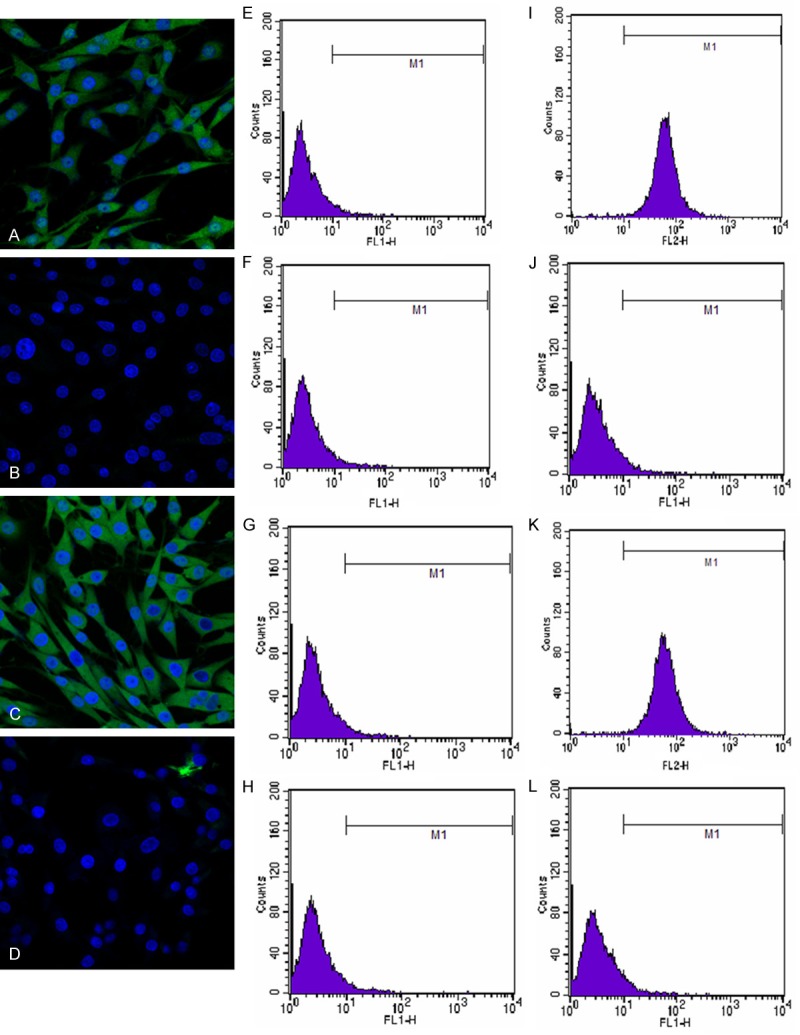
Identification of surface markers of MSCs. A-D: Immunofluorescence images of expressions of CD29, CD34, CD44, CD45 on the surfaces of MSCs; E-H: The results of homeotype control of CD29, CD34, CD44, CD45 measured by flow cytometry; I-L: The results of CD29, CD34, CD44, CD45 of MSCs measured by flow cytometry. Data were represented as means ± SD for three different experiments.
Expression levels of PKCε in different groups
Expressions of PKCε both in the cell membrane and cytoplasm increased significantly after MSCs were treated with PKC activator (P < 0.05), however, increase of PKCε level in cell membrane was greater than that in cytoplasm. PKCε level in cell membrane of MSCs decreased significantly in specific PKCε inhibitor group, and translocation index in each group was calculated (Figure 3), which indicated PKCε translocation increased significantly and PKCε was activated in PKC agonist group, in contrast, translocation and activity of PKCε decreased significantly in PKCε inhibitor group. The activations of PKCε were moderately attenuated after receiving CXCR4 antagonist or PI3K inhibitor (Figure 3), which suggested that the activity of CXCR4 or PI3K pathway could affect the activity of PKCε.
Figure 3.
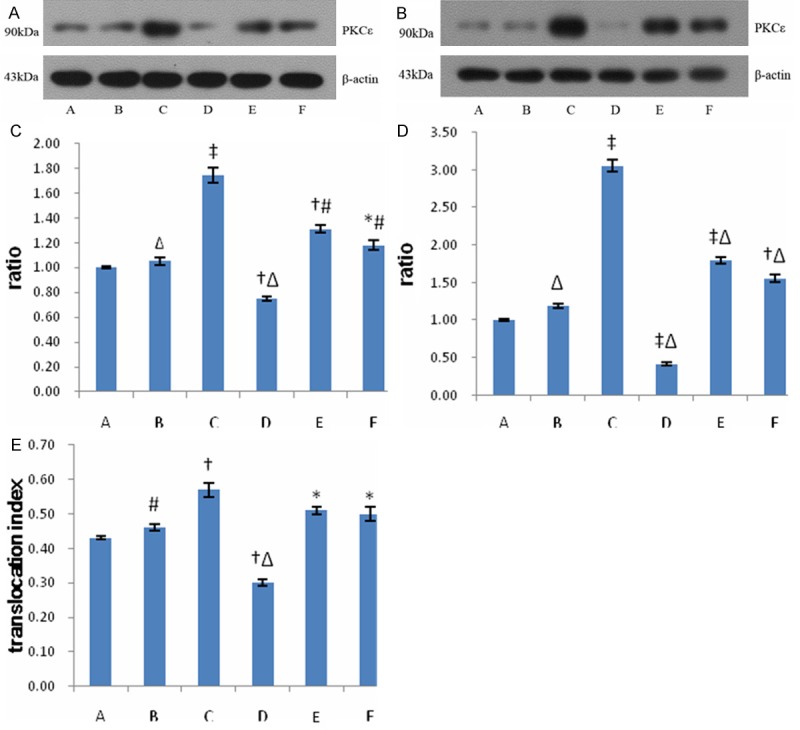
Expressions of PKCε in each group. A: Western blots of PKCε in the cytoplasms of MSCs in each group; B: Western blots of PKCε in the cell membranes of MSCs in each group; C: Relative expressions of PKCε in the cytoplasms of MSCs in each group; D: Relative expressions of PKCε in the cell membranes of MSCs in each group; E: Translocation index of PKCε of MSCs in each group. Data were represented as means ± SD for three different experiments. Group A: MSCs + medium (control group); B: MSCs + PMA (0.5 μM); C: MSCs + PMA (5 μM); D: MSCs + R031-8820 (1 μM); E: MSCs + PMA (5 μM) + AMD3100 (2 μM); F: MSCs + PMA (5 μM) + LY294002 (2 μM). *P < 0.05 vs. the control; †P < 0.01 vs. the control; ‡P < 0.001 vs. the control; #P < 0.05 vs. group C; ΔP < .01 vs. group C.
mRNA levels of capital signal proteins in PKCε, SDF-1/CXCR4 and PI3K/AKT pathway in different groups
After MSCs were treated with PKC activator, mRNA levels of PKCε and its downstream signal proteins, JNK and P38MAPK, increased significantly (P < 0.05), and mRNA levels of SDF-1, CXCR4, PI3K in the MSCs also increased significantly (P < 0.05) in PMA group. However, mRNA level of PKCε, JNK, P38MAPK, SDF-1, CXCR4, PI3K and of AKT decreased significantly in PKCε inhibitor group (P < 0.05) (Figure 4). These indicated activation of PKCε could enhance expression of the signal proteins in SDF-1/CXCR4 axis and PI3K/AKT pathway.
Figure 4.
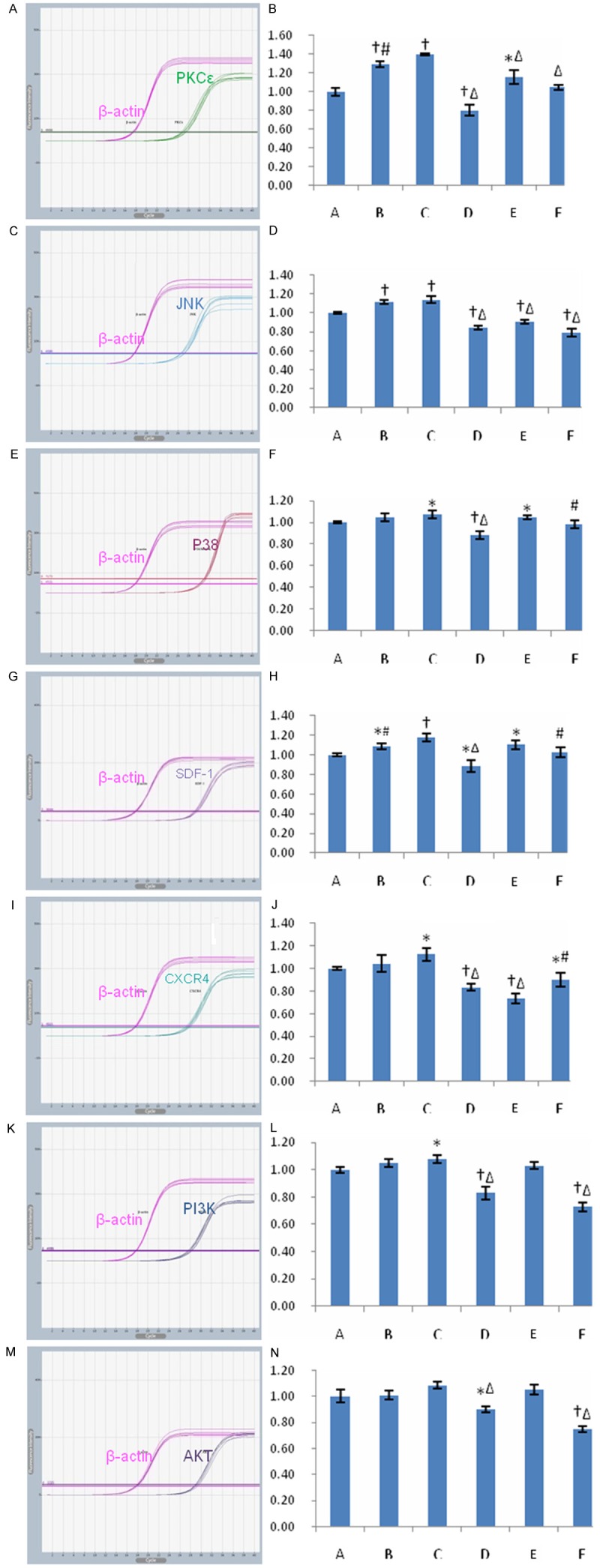
Transcripts for capital signal proteins in PKCε pathway, SDF-1/CXCR4 axis and PI3K/AKT pathway in different groups. A-N: Representative tracings and quantitative data of PKCε, JNK, P38MAPK, SDF-1, CXCR4, PI3K and AKT were shown. Bar graphs showing relative mRNA levels of PKCε, SDF-1, CXCR4, PI3K over β-actin, and β-actin was used as internal reference. Data were represented as means ± SD for five different experiments. A: control group; B: low-dose PMA group; C: high-dose PMA group; D: PKCε inhibiting group; E: CXCR4 antagonist group; F: PI3K inhibiting group. *P < 0.05 vs. the control; †P < 0.01 vs. the control; ‡P < 0.001 vs. the control; #P < 0.05 vs. group C; ΔP < 0.01 vs. group C.
Expressions of capital signal proteins in PKCε, SDF-1/CXCR4 and PI3K/AKT pathway in different groups
After MSCs were treated with PKC activator, PKCε level and phosphorylation of its downstream signal proteins, JNK and P38MAPK, increased significantly (P < 0.05). Expression of SDF-1, CXCR4, PI3K and phosphorylation of AKT also increased significantly in PMA group (P < 0.05). In contrast, expression of PKCε, SDF-1, CXCR4, PI3K and phosphorylated JNK, P38MAPK and AKT decreased significantly in PKCε inhibitor group (P < 0.05) (Figure 5). These results indicated that activation of PKCε pathway could contribute to activations of SDF-1/CXCR4 axis and PI3K/AKT pathway, thus enhance the functional activities of the two pathways, and there were cross-talking and positive interactions between PKCε signaling and SDF-1/CXCR4 axis and PI3K/AKT pathway.
Figure 5.
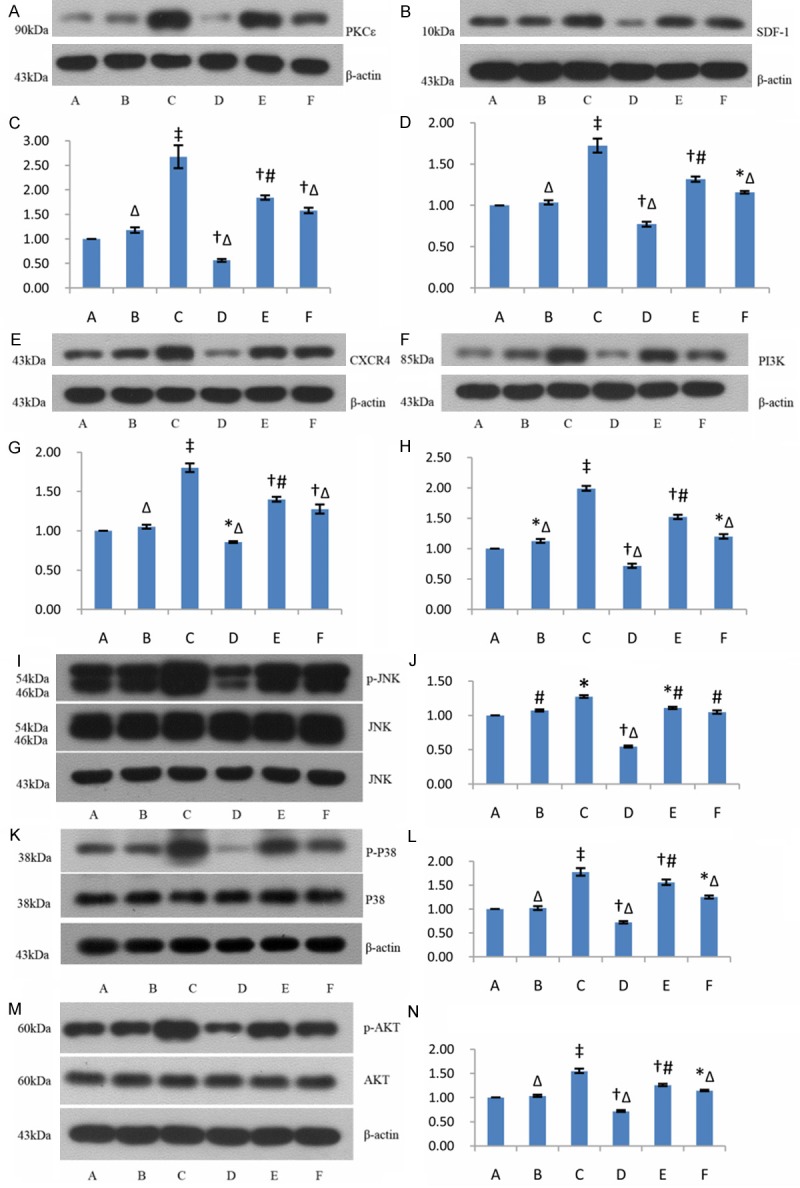
Expressions of capital signal proteins in PKCε, SDF-1/CXCR4 and PI3K/AKT pathway in different groups. A, B, E, F, I, K, M: Western blots of expressions of PKCε, SDF-1, CXCR4, PI3K, JNK (p-JNK), P38MAPK(p-P38) and AKT (p-AKT); C, D, G, H: Bar graphs showing relative expressions of PKCε, SDF-1, CXCR4, PI3K over β-actin after densitometric scanning for Western blots; J, L, N: Bar graphs showing relative expressions of p-JNK, p-P38 and p-AKT. Data were represented as means ± SD for three different experiments. A: control group; B: low-dose PMA group; C: high-dose PMA group; D: PKCε inhibiting group; E: CXCR4 antagonist group; F: PI3K inhibiting group. *P < 0.05 vs. the control; †P < 0.01 vs. the control; ‡P < 0.001 vs. the control; #P < 0.05 vs. group C; ΔP < 0.01 vs. group C.
Migration ability in vitro of MSCs in different groups
The number of migrated MSCs in PMA group was greater significantly than that in control, in contrast, migrated MSCs decreased significantly in PKCε inhibitor group. Inhibiting activity of CXCR4 or PI3K only moderately attenuated the effect of enhancement, but not abolished completely the effect (Figure 6), which indicated that activation of PKCε could enhance significantly ability of movement and PKCε signaling enhanced migration ability of MSCs partly at least independent of SDF-1/CXCR4 axis and PI3K/AKT pathway.
Figure 6.
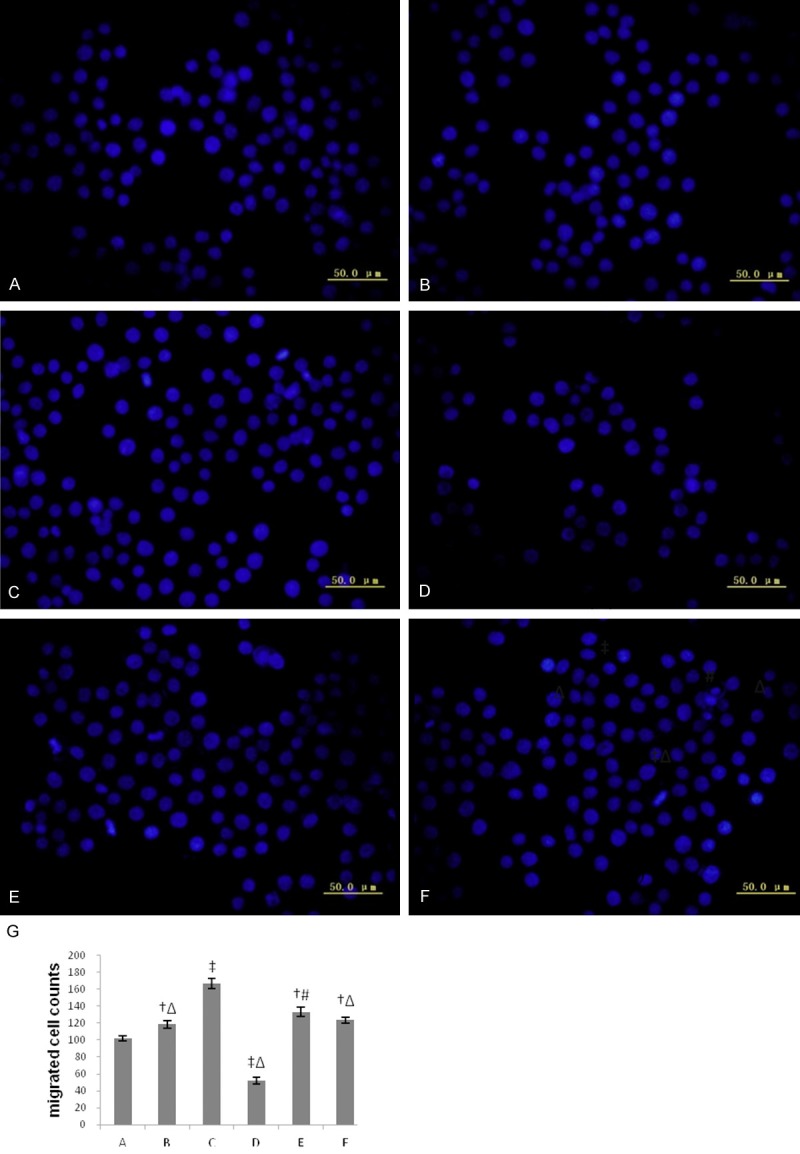
The migratory response to SDF-1 of MSCs in each group. A-F: Representative images observed and counted by inversion fluorescence microscope (200 ×) in each group; G: Bar graphs showing numbers of transmigrated MSCs in each group. Five non-overlapping visual fields in each sample were selected, and data were represented as means ± SD for five different experiments. A: control group; B: low-dose PMA group; C: high-dose PMA group; D: PKCε inhibiting group; E: CXCR4 antagonist group; F: PI3K inhibiting group. *P < 0.05 vs. the control; †P < 0.01 vs. the control; ‡P < 0.001 vs. the control; #P < 0.05 vs. group C; Δ P < 0.01 vs. group C.
Changes of paracrine factors of MSCs in different groups
There were 27 proteins with positive results and being identified in detected 90 proteins with the assay kit (Table 1). After MSCs were treated with different drugs, several chemokines, including MCP-1, MIP-1 alpha, IP-10, MIF, changes significantly, whereas CINC-2 alpha/beta increased in PKCε inhibitor group (P < 0.05 vs. the control). All the expressions of TGF-beta3, TLR4, Thrombospondin, IP-10, MIF, TIMP-2, insulin degrading enzyme increased significantly in PMA + PI3K inhibiting group. Integrin alpha M beta 2 increased in PMA + CXCR4 antagonist group and PMA + PI3K inhibiting group. TRAIL decreased in high-dose PMA group, and Activin A increased obviously in PMA group. So the factors related with migration, proliferation and differentiation increased in PMA group and decreased in PKCε inhibitor group. Inhibiting activity of CXCR4 or PI3K could affect the effects, but not abolished completely (Table 1), which suggested that activation of PKCε could enhance paracrine function of MSCs partly at least independent of SDF-1/CXCR4 axis and PI3K/AKT pathway.
Table 1.
The changes of paracrine cytokines in MSCs in each group
| Protein information | B/A (fold) | C/A (fold) | D/A (fold) | E/A (fold) | F/A (fold) |
|---|---|---|---|---|---|
| MCP-1 | 1.34 | 1.12 | 1.24 | 1.52* | 1.17 |
| TRAIL | 0.81 | 0.46*,#,Δ | 0.77 | 0.79 | 0.70 |
| MIP-2 | 1.52* | 1.36 | 1.41 | 0.99 | 1.07 |
| CINC-2 alpha/beta | 1.01 | 0.98 | 1.56* | 0.97 | 1.01 |
| CD106 | 1.12 | 0.79 | 1.06 | 0.88 | 1.07 |
| Activin A | 1.53*,Δ | 1.43*,Δ | 0.99 | 1.63*,Δ | 1.32*,Δ |
| MDC | 0.82 | 0.73 | 1.13 | 0.83 | 1.30 |
| Adiponectin/Acrp30 | 0.78 | 0.78 | 1.01 | 1.10 | 1.23 |
| IL-4 | 1.02 | 0.87 | 1.17 | 0.93 | 1.20 |
| RALT/MIG-6 | 1.05 | 0.87 | 0.91 | 0.94 | 1.19 |
| IL-10 | 1.12 | 1.01 | 1.29 | 1.07 | 1.31 |
| Integrin alpha M beta 2 | 1.15 | 1.00 | 1.14 | 1.85*,#,Δ | 3.62*,#,Δ |
| GM-CSF | 1.22 | 1.06 | 1.28 | 1.06 | 1.30 |
| TGF-beta3 | 1.02 | 1.01 | 1.11 | 1.08 | 1.44*,#,Δ |
| GFR alpha-2 | 1.28 | 1.03 | 1.24 | 1.09 | 1.32 |
| MIP-1 alpha | 1.73*,Δ | 1.83*,Δ | 0.89 | 1.42*,Δ | 1.48*,Δ |
| IL-6 | 1.34 | 1.18 | 1.37 | 1.09 | 1.32 |
| EG-VEGF/PK1 | 1.03 | 0.87 | 1.09 | 0.98 | 1.27 |
| TLR4 | 1.14 | 1.00 | 0.84 | 1.07 | 1.52*,#,Δ |
| Prolactin R | 0.81 | 0.72 | 1.08 | 0.86 | 1.12 |
| Thrombospondin | 1.03 | 1.09 | 1.23 | 1.39*,# | 1.70*,# |
| IL-1 beta | 1.07 | 1.12 | 1.50 | 1.25 | 1.48 |
| IL-5 | 1.02 | 0.77 | 0.94 | 0.86 | 1.13 |
| IP-10 | 1.20 | 1.16 | 1.38 | 1.27 | 1.75*,# |
| MIF | 1.60*,#,Δ | 1.08 | 1.04 | 1.27 | 1.72*,#,Δ |
| TIMP-2 | 1.17 | 0.94 | 0.97 | 1.07 | 2.06*,#,Δ |
| Insulin Degrading Enzyme | 1.13 | 1.19 | 1.19 | 1.32 | 4.27*,#,Δ |
A: control group; B: low-dose PMA group; C: high-dose PMA group; D: PKCε inhibiting group; E: CXCR4 antagonist group; F: PI3K inhibiting group.
P < 0.05 vs. the control;
P < 0.05 vs. group C;
P < 0.05 vs. group D.
Discussion
The study investigated the changes in mRNA and protein levels of PKCε signaling, SDF-1/CXCR4 axis and PI3K/AKT pathway in the MSCs in the conditions treating with PKC agonist, specific PKCε inhibitor, CXCR4 antagonist and PI3K inhibitor, and observed the changes of migration ability and paracrine factors in different treatments, and firstly demonstrated that there were cross-talking between PKCε signaling, SDF-1/CXCR4 axis and PI3K/AKT pathway during signal transduction of MSCs and activation of PKCε enhanced migration ability and paracrine function of MSCs partly at least independent of SDF-1/CXCR4 axis and PI3K/AKT pathway.
The role of SDF-1/CXCR4 axis, PI3K/AKT pathway in signal transduction of stem cells
It is known that SDF-1/CXCR4 axis play a critical role during homing, proliferation, survival and differentiation of stem cell and is most important pathway in immigration of stem cells [9-13]. Decreasing degradation of SDF-1 [9], pretreatment with SDF-1 [10,11] or increasing expression of SDF-1 and CXCR4 by gene modification [12,21] could increase homing and enhanced therapeutic effects. Pretreatment in hypo-oxygen condition could also increase level of CXCR4, thus increase recruitment to ischemic damage zone [13]. In addition, matching the time of expression of SDF-1 and CXCR4 also improved effects of stem cells [13]. Previous study [22] had indicated that SDF-1/CXCR4 mediated migration of BMMSCs through activation of PI3K/Akt pathway, a known cytoprotective pathway. Another study in vitro indicated that conditional medium without stem cells could attenuate myocardial reperfusion injury and the cardioprotection effect was mediated by activating PI3K pathway through paracrine factors [23]. These evidences suggested that PI3K/Akt pathway play an important role in migration and paracrine function of BMSCs.
Cross-talking between PKCε signaling and SDF-1/CXCR4 axis and PI3K/AKT pathway in signal transduction of MSCs
It is known that PKCε signaling play a crucial role in ischemic cardioprotection [16], and many dada had indicated that activation of PKCε could attenuate reperfusion injury, increase resistance to ischemia, diminish infarct size and improve cardiac function, which were accomplished by activating its serial downstream proteins: MAPK, ERK, Lck, src, etc [18,24,25], and inhibiting PKCε abolished the protective effect [17]. So activating PKCε is critical event and necessary and sufficient condition in ischemic cardioprotection. The role of PKCε in hypertrophy and heart failure and its signaling complexes had also been verified and identified in our previous study [26]. However, whether PKCε have an effect on migration and paracrine function of MSCs and potential molecular mechanisms were unclear at all. Our study indicated that activation of PKCε could enhance the activity of SDF-1/CXCR4 axis and PI3K/AKT pathway, and there were interactions between PKCε signaling and SDF-1/CXCR4 axis and PI3K/AKT pathway in MSCs.
Activation of PKCε could enhance migration ability of MSCs
One of two unresolved bottle-neck problems in stem cells therapy was poor homing. Substantial breakthrough hadn’t still been got on homing of stem cells though scientists had endeavored to explore related methods [9-15]. Our study firstly indicated that activating PKCε enhanced significantly migration ability of MSCs. Although there were cross-talking between PKCε signaling and SDF1/CXCR4 axis and PI3K/AKT pathway in MSCs, inhibiting activity of SDF-1/CXCR4 axis and PI3K/AKT pathway only partly attenuated but not abolished completely the effect of PKCε, which suggested that activation of PKCε improve migration of MSCs partly at least independent of the two pathways.
Activation of PKCε could enhance paracrine function of MSCs
In the study, several chemokines (including MCP-1, MIP-1 alpha, IP-10, MIF) increased significantly after treatment with PKC activator, which could greatly increase mobilization, homing of MSCs and angiogenesis [27]. Granulocyte could adhere and have direct toxic effects on cardiomyocytes. The increase of CINC-2 after inhibiting activity of PKCε might enhance the toxic effects and decrease survival by increasing recruitment of Granulocyte. TGF-beta3 and Activin A play a critical role in differentiation and proliferation of MSCs by affecting activity of FGFs, BMP7, Wnt3 [28]. Damage and loss of Extracellular Matrix (ECM) resulting from degradation of ECM due to MMPs is initiating and crucial factor of ventricle remodeling, and TIMP-2, specific inhibitor of MMPs, could decrease degradation of ECM and improve significantly remodeling and dysfunction after MI [29,30]. Integrin play an important role in adhesion and migration of stem cells. TNF-related apoptosis-inducing ligand (TRAIL) belongs to TNF superfamily and could activate TNF receptor and induce subsequent apoptosis [31]. So the factors related with migration, proliferation and differentiation of stem cells and anti-remodeling increased after treating with PMA, in contrast, these factors decreased and apoptosis related factor increased after treating with specific PKCε inhibitor, which indicated that PKCε activation could improve paracrine functions. Changes of activity of CXCR4 or PI3K pathway could affect the effects, but not abolished completely, which suggested that activation of PKCε enhance paracrine function of MSCs partly at least independent of SDF-1/CXCR4 axis and PI3K/AKT pathway.
Clinical implication
Previous studies had verified that activation of PKCε could significantly attenuate reperfusion injury, improve cardiac function probably by enhancing mitochondrial Oxidative Phosphorylation and maintaining level of ATP [32], accomplishing anti-apoptosis effects by inactivating pro-apoptotic protein, Bad, and inducing expression of bcl-2 [33], etc. However, whether PKCε signaling has effects on stem cells therapy and potential mechanisms are unclear. Our study verified that activating PKCε could improve migration ability and paracrine function of MSCs by direct and indirect pathways. So Intervention for PKCε might become a new effective potential approach to enhance effects of stem cells therapy.
Conclusion
There was cross-talking between PKCε signaling and SDF-1/CXCR4 axis and PI3K/AKT pathway in signal transduction of MSCs. Activating PKCε could improve migration ability and paracrine function of MSCs partly at least independent of SDF-1/CXCR4 axis and PI3K/AKT pathway.
Acknowledgements
This work was supported by the Beijing Anzhen Hospital, Capital University of Medical Sciences, and Beijing Institute of Heart Lung and Blood Vessel Diseases. We thank Dr YanBin Xu for his valuable suggestions regarding migration assay, Dr Xing Wang for his assistance for screening paracrine factors with protein clips. This work was supported by Chinese Natural Science Foundation Grants (81100142), Open Topic Funds Grants of Essential Laboratory in Cardiovascular Remodeling and Transforming Medicine, National Ministry of Education (2010XGCS02), Superintendent Cultivating Funds Grants of Beijing Anzhen Hospital (2010F03).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mendis S, Puska P, Norrving B, editors. Switzerland: World Health Organization; 2011. Global atlas on cardiovascular disease prevention and control. [Google Scholar]
- 3.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 4.Tang XL, Rokosh G, Sanganalmath SK, Yuan F, Sato H, Mu J, Dai S, Li C, Chen N, Peng Y, Dawn B, Hunt G, Leri A, Kajstura J, Tiwari S, Shirk G, Anversa P, Bolli R. Intracoronary administration of cardiac progenitor cells alleviates left ventricular dysfunction in rats with a 30-day-old infarction. Circulation. 2010;121:293–305. doi: 10.1161/CIRCULATIONAHA.109.871905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanz-Ruiz R, Gutiérrez Ibañes E, Arranz AV, Fernández Santos ME, Fernández PL, Fernández-Avilés F. Phases i-iii clinical trials using adult stem cells. Stem Cells Int. 2010;2010:579142–579144. doi: 10.4061/2010/579142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Traverse JH, McKenna DH, Harvey K, Jorgenso BC, Olson RE, Bostrom N, Kadidlo D, Lesser JR, Jagadeesan V, Garberich R, Henry TD. Results of a phase 1, randomized, double-blind, placebo-controlled trial of bone marrow mononuclear stem cell administration in patients following ST-elevation myocardial infarction. Am Heart J. 2010;160:428–434. doi: 10.1016/j.ahj.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez DL, Zambrano JP, Suncion VY, Tracy M, Ghersin E, Johnston PV, Brinker JA, Breton E, Davis-Sproul J, Schulman IH, Byrnes J, Mendizabal AM, Lowery MH, Rouy D, Altman P, Wong Po Foo C, Ruiz P, Amador A, Da Silva J, McNiece IK, Heldman AW. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA. 2012;308:2369–2379. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He H, Han FS. Clinical studies on stem cells therapy for ischemic heart diseases. Adv Cardiovasc Dis. 2012;33:181–184. [Google Scholar]
- 9.Zaruba MM, Franz WM. Role of the SDF-1-CXCR4 axis in stem cell-based therapies for ischemic cardiomyopathy. Expert Opin Biol Ther. 2010;10:321–335. doi: 10.1517/14712590903460286. [DOI] [PubMed] [Google Scholar]
- 10.Pasha Z, Wang Y, Sheikh R, Zhang D, Zhao T, Ashraf M. Preconditioning enhances cell survival and differentiation of stem cells during transplantation in infarcted myocardium. Cardiovasc Res. 2008;77:134–142. doi: 10.1093/cvr/cvm025. [DOI] [PubMed] [Google Scholar]
- 11.Frederick JR, Fitzpatrick JR 3rd, McCormick RC, Harris DA, Kim AY, Muenzer JR, Marotta N, Smith MJ, Cohen JE, Hiesinger W, Atluri P, Woo YJ. Stromal cell-derived factor-1alpha activation of tissue-engineered endothelial progenitor cell matrix enhances ventricular function after myocardial infarction by inducing neovasculogenesis. Circulation. 2010;122:S107–117. doi: 10.1161/CIRCULATIONAHA.109.930404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang D, Huang W, Dai B, Zhao T, Ashraf A, Millard RW, Ashraf M, Wang Y. Genetically manipulated progenitor cell sheet with diprotin A improves myocardial function and repair of infarcted hearts. Am J Physiol Heart Circ Physiol. 2010;299:H1339–1347. doi: 10.1152/ajpheart.00592.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang YL, Zhu W, Cheng M, Chen L, Zhang J, Sun T, Kishore R, Phillips MI, Losordo DW, Qin G. Hypoxic preconditioning enhances the benefit of cardiac progenitor cell therapy for treatment of myocardial infarction by inducing CXCR4 expression. Circ Res. 2009;104:1209–1216. doi: 10.1161/CIRCRESAHA.109.197723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, Luther K. Genetically manipulated progenitor/stem cells restore function to the infarcted heart via the SDF-1alpha/CXCR4 signaling pathway. Prog Mol Biol Transl Sci. 2012;111:265–284. doi: 10.1016/B978-0-12-398459-3.00012-5. [DOI] [PubMed] [Google Scholar]
- 15.He H, Han FS. Strategies for improving homing and paracrine function of stem cells. Cardiovasc Res. 2013;40:3–5. [Google Scholar]
- 16.Gregory KN, Hahn H, Haghighi K, Marreez Y, Odley A, Dorn GW 2nd, Kranias EG. Increased particulate partitioning of PKC epsilon reverses susceptibility of phospholamban knockout hearts to ischemic injury. J Mol Cell Cardiol. 2004;36:313–318. doi: 10.1016/j.yjmcc.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Shinmura K, Nagai M, Tamaki K, Bolli R. Loss of ischaemic preconditioning in ovariectomized rat hearts: possible involvement of impaired protein kinase C epsilon phosphorylation. Cardiovasc Res. 2008;79:387–394. doi: 10.1093/cvr/cvn086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyer KD, Zhang H, Zhang L. Prenatal cocaine exposure abolished ischemic preconditioning-induced protection in adult male rat hearts: role of PKCepsilon. Am J Physiol Heart Circ Physiol. 2009;296:H1566–1576. doi: 10.1152/ajpheart.00898.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deng W, Bivalacqua TJ, Chattergoon NN, Jeter JR Jr, Kadowitz PJ. Engineering ex vivo-expanded marrow stromal cells to secrete calcitonin gene-related peptide using adenoviral vector. Stem Cells. 2004;22:1279–1291. doi: 10.1634/stemcells.2004-0032. [DOI] [PubMed] [Google Scholar]
- 20.Pogwizd SM, Qi M, Yuan W, Samarel AM, Bers DM. Upregulation of Na(+)/Ca(2+) exchanger expression and function in an arrhythmogenic rabbit model of heart failure. Circ Res. 1999;85:1009–1019. doi: 10.1161/01.res.85.11.1009. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Ou LL, Cheng ZK, Jia XH, Gao NF, Kong DL. Migration assay in vitro on Cxcr4 gene-modified Bone Marrow mesenchymal stem cells. Journal of Biomedical Engineering. 2009;26:595–600. [PubMed] [Google Scholar]
- 22.Yu J, Li M, Qu Z, Yan D, Li D, Ruan Q. SDF-1/CXCR4-mediated migration of transplanted bone marrow stromal cells toward areas of heart myocardial infarction through activation of PI3K/Akt. J Cardiovasc Pharmacol. 2010;55:496–505. doi: 10.1097/FJC.0b013e3181d7a384. [DOI] [PubMed] [Google Scholar]
- 23.Angoulvant D, Ivanes F, Ferrera R, Matthews PG, Nataf S, Ovize M. Mesenchymal stem cell conditioned media attenuates in vitro and ex vivo myocardial reperfusion injury. J Heart Lung Transplant. 2011;30:95–102. doi: 10.1016/j.healun.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 24.Ping P, Song C, Zhang J, Guo Y, Cao X, Li RC, Wu W, Vondriska TM, Pass JM, Tang XL, Pierce WM, Bolli R. Formation of protein kinase c(epsilon)-lck signaling modules confers cardioprotection. J Clin Invest. 2002;109:499–507. doi: 10.1172/JCI13200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rafiee P, Shi Y, Kong X, Pritchard KA Jr, Tweddell JS, Litwin SB, Mussatto K, Jaquiss RD, Su J, Baker JE. Activation of protein kinases in chronically hypoxic infant human and rabbit hearts: role in cardioprotection. Circulation. 2002;106:239–245. doi: 10.1161/01.cir.0000022018.68965.6d. [DOI] [PubMed] [Google Scholar]
- 26.He H, Wang W, Zhang H, Ma L, Wu H, Wang P, Gao J. Fosinopril and carvedilol reverse hypertrophy and change the levels of protein kinase C epsilon and components of its signaling complex. Cardiovasc Drugs Ther. 2006;20:259–271. doi: 10.1007/s10557-006-0079-5. [DOI] [PubMed] [Google Scholar]
- 27.Amin MA, Volpert OV, Woods JM, Kumar P, Harlow LA, Koch AE. Migration inhibitory factor mediates angiogenesis via mitogen-activated protein kinase and phosphatidylinositol kinase. Circ Res. 2003;93:321–329. doi: 10.1161/01.RES.0000087641.56024.DA. [DOI] [PubMed] [Google Scholar]
- 28.Zhang W. The effects of TGF-β/Activin signaling on directional differentiation of stem cells. In: Yuan JD, Xiao L, editors. Academic Dissertations. Jinan,Shan Dong: ShanDong Normal University; 2007. pp. 34–35. [Google Scholar]
- 29.Ducharme A, Frantz S, Aikawa M, Rabkin E, Lindsey M, Rohde LE, Schoen FJ, Kelly RA, Werb Z, Libby P, Lee RT. Targeted deletion of matrix metalloproteinase-9 attenuates left ventricular enlargement and collagen accumulation after experimental myocardial infarction. J Clin Invest. 2000;106:55–62. doi: 10.1172/JCI8768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heymans S, Luttun A, Nuyens D, Theilmeier G, Creemers E, Moons L, Dyspersin GD, Cleutjens JP, Shipley M, Angellilo A, Levi M, Nube O, Baker A, Keshet E, Lupu F, Herbert JM, Smits JF, Shapiro SD, Baes M, Borgers M, Collen D, Daemen MJ, Carmeliet P. Inhibition of plasminogen activators or matrix metalloproteinases prevents cardiac rupture but impairs therapeutic angiogenesis and causes cardiac failure. Nat Med. 1999;5:1135–1142. doi: 10.1038/13459. [DOI] [PubMed] [Google Scholar]
- 31.Fulda S, Debatin KM. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene. 2006;25:4798–4811. doi: 10.1038/sj.onc.1209608. [DOI] [PubMed] [Google Scholar]
- 32.McCarthy J, McLeod CJ, Minners J, Essop MF, Ping P, Sack MN. PKCepsilon activation augments cardiac mitochondrial respiratory post-anoxic reserve--a putative mechanism in PKCepsilon cardioprotection. J Mol Cell Cardiol. 2005;38:697–700. doi: 10.1016/j.yjmcc.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 33.Chang Y, Ceacareanu B, Dixit M, Sreejayan N, Hassid A. Nitric oxide-induced motility in aortic smooth muscle cells: role of protein tyrosine phosphatase SHP-2 and GTP-binding protein Rho. Circ Res. 2002;91:390–397. doi: 10.1161/01.res.0000033524.92083.64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


