Abstract
Objective: To investigate osteogenesis of bone marrow mesenchymal stem cells (BMSCs) on strontium-substituted nano-hydroxyapatite (Sr-HA) coated roughened titanium surfaces. Methods: Sr-HA coating and HA coating were fabricated on roughened titanium surfaces by electrochemical deposition technique and characterized by field emission scanning electron microscope (FESM). BMSCs were cultured on Sr-HA coating, HA coating and roughened titanium surfaces respectively. Cell proliferation, alkaline phosphatase (ALP) activity, mineralized nodules formation and cell osteocalcin (OC) secretion were measured. Results: Electrochemically deposited Sr-HA coating and HA coating had no effect on the proliferation of BMSCs and demonstrated that the materials have a good biocompatibility. BMSCs cultured on Sr-HA coating showed increased alkaline phosphatase activity, mineralized nodules formation, and cell OC secretion compared with the other two groups. Cells cultured on HA coating also showed increased biological activity compared with the roughened group. Conclusion: Sr-HA coated titanium surfaces by electrochemical deposition can promote osteogenesis of BMSCs in vitro and have the potential to shorten bone healing period and enhance implant osseointegration.
Keywords: Titanium, strontium, hydroxyapatite, bone marrow mesenchymal stem cells, osteogenesis
Introduction
Oral implant denture is a recently booming novel restoration. Contributing to effective support and good masticatory function, it is increasingly recognized by patients who lost teeth. It is also widely used in clinical practice. But patients with implants still need longer time for healing, which causes incontinence to daily life. The titanium implant currently used in clinical practice is biologically inert material and its surface is insufficient in bioactivity. To shorten implant osseointegration period for immediate loading or early loading, more and more studies focus on modification of pure titanium surface of the implant to improve its biological property.
Some studies have demonstrated that calcium is a main component of skeleton, often in the form of calcium phosphate. Some microelements are also present in minerals of skeleton, with strontium mass accounting for about 0.01%. The strontium ions have signal pathways identical to the calcium ions. If calcium ions in the hydroxyapatite are substituted by strontium ions, the increase of strontium ionic radius causes increase of crystal lattice of hydroxyapatite and crystallinity, and greater change of structure [1-3]. The strontium in hydroxyapatite facilitates proliferation of osteoblasts and synthesis of collagen, and inhibits differentiation and function of osteoclasts in vitro [4-7]. It also facilitates bone formation, inhibits bone absorption, and improves fine structure of bone in vivo [8-11]. The fabrication of strontium on the surface of titanium implant, therefore, improves biological property of the implant surface and facilitates early implant osseointegration.
In recent years, there is a growing research related to fabrication of microelements on roughened implant surfaces by variety of techniques. No studies, however, reported fabrication of microelements on HA coating by electrochemical deposition technique. In the current study, strontium-substituted nano-hydroxyapatite (Sr-HA) coating and nano-hydroxyapatite (HA) coating were fabricated on roughened titanium surfaces by electrochemical deposition technique. The effects of microelement-strontium-on the proliferation of bone marrow mesenchymal stem cells (BMSCs) and osteogenesis were analyzed. The current study has the potential to help develop new technique to modify property of implant surfaces that shorten bone healing period and enhance implant osseointegration.
Materials and methods
Main reagents and instruments
The main reagents included Alizarin red S, sodium β-glycerophosphate, dexamethasone and ascorbic acid (Sigma, USA); low-sugar DMEM media, trypsin, penicillin/streptomycin and phosphate buffer (Hyclone, USA); fetal bovine serum (Gibco, USA); cell lysis buffer (Sigma, USA); ALP kit (Wako, Japan); AlamarBlue cell activity kit (Invitrogen, USA); rat osteocalcin (OC) ELISA kit (Biomedical Technologies, USA); anti-rat CD29, CD90, CD11b/c, and CD45 monoclonal antibodies (Abcam, UK). The main instruments included electrochemical workstation (Chenhua, Shanghai); field emission scanning electron microscope (FESM) (FEI, Netherlands); flow cytometer (BD, USA); microplate reader (TECAN, Switzerland); CO2 incubator (Thermo Fisher, USA).
Preparation of roughened titanium plates (sandblasting and acid-etching method)
Two types of titanium plates (d = 10, 30 mm) were polished, sandblasted using silicon carbide abrasive paper (320~4000 mesh), and ultrasonically washed with acetone, 75% ethanol and distilled water for 15 min, and then dried. The plates were added to the mixture of hydrofluoric acid and nitric acid (distilled water, 0.11 M hydrofluoric acid and 0.09 M nitric acid at a volume ratio of 1000:2:4) and treated for 10 min at room temperature. After being washed, ultrasonically washed and dried, the plates were added to mixture of hydrochloric acid and sulfuric acid (distilled water, 5.8 M hydrochloric acid and 8.96 M sulfuric acid at a volume ratio of 2:1:1) and treated for 30 min at 80°C. After being washed, ultrasonically washed and dried by N2, the plates were placed in a clean glass culture dish for use [12].
Preparation of Sr-HA coating on roughened titanium surfaces (electrochemical deposition method)
The electrolytes including 1.2×10-3 M calcium chloride, 7.2×10?-4 M ammonium dihydrogen phosphate, 1.33×10-4 M strontium chloride, 0.1 M sodium chloride for Sr-HA coatings were prepared. The electrolytes including 1.2×10-3 M calcium chloride, 7.2×10-4 M ammonium dihydrogen phosphate, 0.1 M sodium chloride for HA coatings were prepared. The roughened titanium plates were soaked in the above electrolytes and deposited for 30 min at 85°C with direct voltage of 3 V using electrochemical workstation. The Sr-HA in the electrolytes was crystallized on titanium surface and formed thin Sr-HA layer attached to titanium. The coatings for use were washed, dried and exposed to ultraviolet radiation for 2 h on both sides [12].
Observation of surface morphology of the coated titanium plates
The surface morphology of above coated titanium plates was observed using field emission scanning electron microscope (FSEM).
Isolation and incubation of rat BMSCs
Newborn SD rats were sacrificed by dislocation of cervical vertebra, soaked in 75% ethanol for 5 min. The tibia and femur were aseptically removed and placed in a culture dish containing PBS. After removal of attached soft tissues and end cartilages, the bones were placed in a culture dish containing low-sugar DMEM culture medium (containing 10% fetal bovine serum and 1% penicillin/streptomycin. The long bone was snipped in the middle using a scissor. The marrow cavity was washed repeatedly with medium using a 5-mL sterile syringe. The medium was replaced after 48 h, and then replaced every 2-3 days. The growth of cells was observed under inverted phase contrast microscope. When these cells grew adhering to the wall to a plating density close to 80%, the original medium was discarded. The cells were washed twice with PBS, digested with 0.25% trypsin, and then added to medium to terminate digestion. The single-cell suspension was obtained by gently pipetting up and down, and cultured for passages.
Identification of rat BMSCs surface markers
The BMSCs of the fourth passage that grew well in the logarithmic phase were collected, washed twice with PBS, routinely digested and centrifuged. The supernatant was removed. The pre-cooling PBS was added. The cells were resuspended. After washing twice, Single-cell suspension at a concentration 1×107/ml was prepared by adding PBS. Each 100 μL of suspension was transferred to a cytometry tube and successively added with fluorescently-labeled antibody CD29, D90, CD11b/c and CD45. The blank control was also prepared. The cells were incubated at 4°C for 30 min. The expressions of surface markers were determined using flow cytometer.
Determination of proliferation of BMSCs using AlamarBlue method
The BMSCs of the fourth passage in the logarithmic phase were collected and inoculated on 24-well titanium plates. Approximate l×104 cells per well were inoculated on each titanium plate. The standard wells of non- titanium plate were plated with 0, 0.625×104, 1.25×104, 2.5×104, 5×104, l×105 cells, respectively. Each well was replenished with medium to 500 μL. When cells were almost attached, 50 μl of AlamarBlue cell activity reagent was added to each well. The cells were incubated for 4 h at 37°C under protection from light. The fluorescence intensity was determined using fluorescence microplate (excitation wavelength of 540-570 nm, emission wavelength of 580-610 nm). The standard curve was prepared. The cells on titanium plate were determined using AlamarBlue method after incubation for 1 d, 3 d and 7 d. The medium was discarded. 500 μL of equivalent medium was added to each well, and then 50 μl of AlamarBlue cell activity reagent was added. The cells were incubated for 4 h at 37°C. The fluorescence intensity was determined using fluorescence microplate. The amount of cells was calculated based on standard curve.
Determination of activity of alkaline phosphatase
The BMSCs of the fourth passage in the logarithmic phase were collected and inoculated on 6-well titanium plates. Approximate l×105 cells per well were inoculated on each titanium plate. The cell plates were removed at D7 and D14. The medium was discarded. The titanium plates were washed twice with PBS. 200 μL of cell lysis buffer was added to each titanium plate. The cells were lysed at 4°C for 15 min. The lysate was collected and stored at -80°C. When all samples were collected, the activity of alkaline phosphatase (ALP) was determined using ELISA kit as per instruction. The activity of ALP was standardized using total protein level.
Determination of serum OC and calcium nodule staining
The BMSCs of the fourth passage in the logarithmic phase were collected and inoculated on 6-well titanium plates. Approximate l×105 cells per well were inoculated on each titanium plate. The medium was replaced by osteogenic induction medium (10% serum, 10 mM sodium β-glycerophosphate, 100 nM dexamethasone, 50 μg/ml ascorbic acid, 2 mM glutamine) after 24 h. The cell medium was collected at D7 and D14, and stored at -80°C. When all samples were collected, the level of OC secreted by cells in the medium was determined using rat OC ELISA kit. The level of OC was standardized using total protein level. After induction for 28 d, cells were washed twice with PBS, blocked for 5 min with 4% paraformaldehyde, stained for 1 h with alizarin red S (1%, pH4.2), and photographed after washing with PBS.
Statistical analysis
The above experiments were repeated three times or more. The quantitative data was expressed as mean ± SD. The statistical analysis was performed using SPSS 16.0 software. Statistical significance was indicated by P<0.05.
Results
Surface morphology of titanium plates under FSEM
The results of FSEM were shown in Figure 1. In the control group, the surface of sandblasted and acid-etched titanium plate was full of pores of 10-30 μm in diameter and even smaller pores of 0.5-3.0 μm in diameter on porous surface. The HA coating fabricated on roughened titanium by electrochemical method was 6-7 µm in thickness and appeared as rods with transverse section of 70-80 nm in diameter. The diameter of rods of Sr-HA coating was less compared with HA coating.
Figure 1.

FSEM micrograph of the roughened surfaces, HA surface and Sr-HA surface.
Identification of surface markers on cells
The results of flow cytometry showed that the positive rates of immunophenotype on BMSCs of the fourth passage were 100.0% for CD29, 98.0% for CD90, 0.1% for CD45 and 0.2% for CD11b, respectively (Figure 2).
Figure 2.
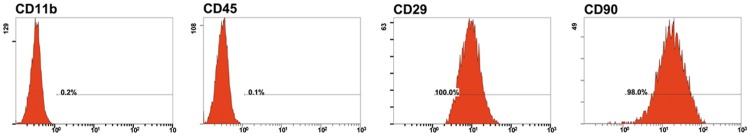
The expressions of surface antigens on rat BMSCs.
Effects of different coatings on rat BMSCs proliferation
The Figure 3 showed BMSCs proliferation on different titanium plates. The cells increased markedly over time of incubation. But there were no statistical difference between groups, demonstrating that Sr-HA coating and HA coating had no toxicity on BMSCs and had no marked impact on activity and proliferation. The results also indicated that Sr-HA coating and HA coating had biocompatibility comparable to roughened titanium implant surface.
Figure 3.
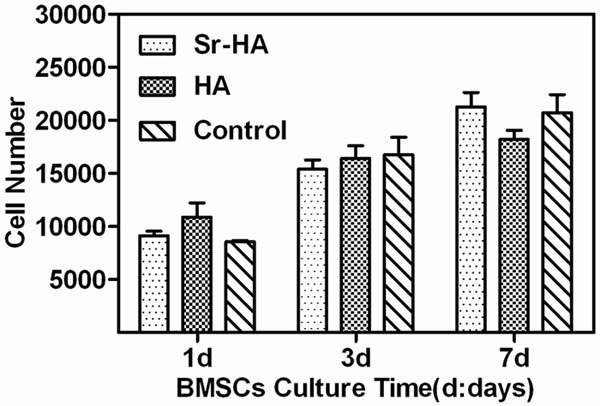
Effects of different coatings on rat BMSCs proliferation.
Effect of different coatings on cell ALP activity of rat BMSCs
The activity of ALP is an indicator for early differentiation of osteogenic precursor cells. As shown in Figure 4, the ALP activity of BMSCs increased over time. At D7, the Sr-HA group and HA group had markedly higher ALP activity than the roughened group (P<0.05); the Sr-HA group also had significant higher ALP activity than the HA group (P<0.05). At D14, the Sr-HA group and HA group had significant higher ALP activity than the roughened group (P<0.05); the Sr-HA group also had markedly higher ALP activity than the HA group (P<0.05), which were similar to those at D7.
Figure 4.
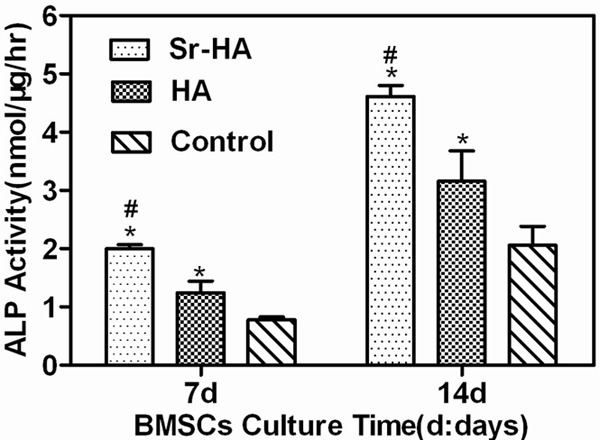
Effect of different coatings on cell ALP activity of rat BMSCs.
Effect of different coatings on mineralized nodules formation of rat BMSCs
The change of calcium deposition in cell matrix is best direct indicator of osteogenesis. The Figure 5 showed the appearances of BMSCs on different titanium surfaces after osteogenic induction for 28 days. The calcified areas in the Sr-HA group and HA group were markedly larger than that of the roughened group, revealing that Sr-HA coating substantially promoted mineralized nodules formation of BMSCs.
Figure 5.
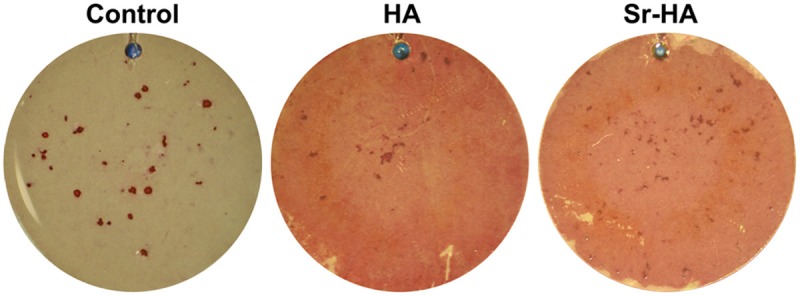
Effect of different coatings on mineralized nodules formation of rat BMSCs.
Effect of different coatings on OC secretion of rat BMSCs
The OC is a specific indicator for mature differentiation of osteogenic precursor cells and bone renewal. As shown in Figure 6, at D7, the Sr-HA group had substantially higher OC level compared with the HA group and the roughened group (P<0.05); at D14, the Sr-HA group and the HA group had significantly higher OC level compared with the HA group and the roughened group (P<0.05), suggesting that Sr-HA promoted OC secretion of BMSCs.
Figure 6.
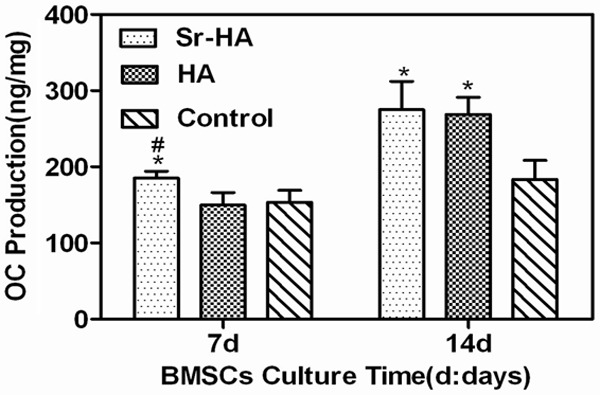
Effect of different coatings on extracellular OC secretion of rat BMSCs.
Discussion
There are a number of methods that can modify the property of titanium implant surfaces. In this study, Sr-HA coating and HA coating were successfully fabricated on roughened titanium surfaces by electrochemical deposition technique. Compared with conventional techniques such as spray coating of titanium paste, the technique is simple, effective, low in cost and able to control the thickness of coating in nanoscale. The structure of coating fabricated by the technique is organized and homogeneous, which has intact three-dimensional structure and good mechanical property.
The implant surfaces commonly used in clinical practice are often sandblasted and acid-etched. But the technique greatly increases area of implant surface and promotes osseointegration of the surrounding of implant. In the current study, the surfaces in the control group were sandblasted and acid-etched. The results showed that there were no statistical difference in proliferation of BMSCs on different titanium coatings, demonstrating that Sr-HA coating and HA coating had no toxicity on BMSCs and had good biocompatibility, which was similar to sandblasted and acid-etched titanium implant surface.
ALP is an enzyme necessary for bone formation. It is an indicator for assessment of early differentiation of osteoblasts. The expression of ALP increases as differentiation of osteoblasts develops. It catalyzes hydrolysis of organophosphorus to release phosphate anion and free hydroxide radical that promotes formation of hydroxyapatite. The OC is non-collagen containing γ-carboxyl glutamic acid, which is synthesized and secreted by osteoblasts. It is an indicator of late differentiation of osteoblasts. OC regulates bone metabolism and plays an important role in maintenance of normal bone mineralization, inhibition of cartilage calcification and irregular crystal deposition. The change of calcium deposition in cell matrix is directly associated with osteogenesis of cells. The alizarin red staining is a common and specific method used to determine calcium deposition in cell matrix. The results of ALP, OC and alizarin red in the current study showed that Sr-HA coating and HA coating fabricated on roughened titanium surfaces by electrochemical deposition technique significantly promoted differentiation of rat BMSCs and mineralization of extracellular matrix. Compared with the HA group and roughened group, the Sr-HA coating significantly enhanced ALP activity of BMSCs and secretion of OC, and promoted formation of mineralized nodules, which was consistent with results from Capuccini et al [4]. This may be explained by the following causes. Hydroxyapatite is a main component of human skeleton and mineral substance of teeth. It is also important substance of extracellular matrix and has good biocompatibility. In the current study, HA coating fabricated by electrochemical deposition method well mimicked three-dimensional structure of extracellular matrix and promoted differentiation of BMSCs and osteogenic precursor cells into osteoblasts. The high purity and crystallinity of HA, however, declines the rate of absorption and degradation, which impacts deposition of new bone in late osseointegration. When HA coating is substituted with strontium, the structure of hydroxyapatite alters and is more closed to that of natural hydroxyapatite in the body. The greater crystallinity and higher solubility of hydroxyapatite and elevated level of local calcium ions facilitate formation of new bone [13,14]. A great deal of research attempted to explain mechanism of strontium. But the defined molecular mechanism remains unclear. Saidak Z et al has proposed that strontium activates Wnt and ERK1/2-MAPK signal pathway to increase expression of Runx2 and reduce expression of PPARγ2, thereby promoting differentiation of BMSCs into osteoblasts [15]. Furthermore, it activates calcium sensitive receptor (CASR) to promote proliferation and differentiation of osteoblasts, inhibits differentiation and function of osteoclasts, thereby increasing osteogenesis.
In conclusion, Sr-HA coating fabricated by electrochemical deposition technique facilitates osteogenesis of BMSCs. If it is used to modify the property of implant surfaces, it has potential to promote early formation of new bone surrounding the implant and implant osseointegration. But the molecular mechanism through which the microelements act on BMSCs remains to be further studied.
Disclosure of conflict of interest
None.
References
- 1.Wang X, Ye J. Variation of crystal structure of hydroxyapatite in calcium phosphate cement by the substitution of strontium ions. J Mater Sci Mater Med. 2008;19:1183–1186. doi: 10.1007/s10856-007-3209-0. [DOI] [PubMed] [Google Scholar]
- 2.Li ZY, Lam WM, Yang C, Xu B, Ni GX, Abbah SA, Cheung KM, Luk KD, Lu WW. Chemical composition, crystal size and lattice structural changes after incorporation of strontium into biomimetic apatite. Biomaterials. 2007;28:1452–1460. doi: 10.1016/j.biomaterials.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 3.O’Donnell MD, Fredholm Y, de Rouffignac A, Hill RG. Structural analysis of a series of strontium-substituted apatites. Acta Biomater. 2008;4:1455–1464. doi: 10.1016/j.actbio.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 4.Capuccini C, Torricelli P, Sima F, Boanini E, Ristoscu C, Bracci B, Socol G, Fini M, Mihailescu IN, Bigi A. Strontium-substituted hydroxyapatite coatings synthesized by pulsed-laser deposition: in vitro osteoblast and osteoclast response. Acta Biomater. 2008;4:1885–1893. doi: 10.1016/j.actbio.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Capuccini C, Torricelli P, Boanini E, Gazzano M, Giardino R, Bigi A. Interaction of Sr-doped hydroxyapatite nanocrystals with osteoclast and osteoblast-like cells. J Biomed Mater Res A. 2009;89:594–600. doi: 10.1002/jbm.a.31975. [DOI] [PubMed] [Google Scholar]
- 6.Ni GX, Yao ZP, Huang GT, Liu WG, Lu WW. The effect of strontium incorporation in hydroxyapatite on osteoblasts in vitro. J Mater Sci Mater Med. 2011;22:961–967. doi: 10.1007/s10856-011-4264-0. [DOI] [PubMed] [Google Scholar]
- 7.Boanini E, Torricelli P, Fini M, Bigi A. Osteopenic bone cell response to strontium-substituted hydroxyapatite. J Mater Sci Mater Med. 2011;22:2079–2088. doi: 10.1007/s10856-011-4379-3. [DOI] [PubMed] [Google Scholar]
- 8.Li Y, Li Q, Zhu S, Luo E, Li J, Feng G, Liao Y, Hu J. The effect of strontium-substituted hydroxyapatite coating on implant fixation in ovariectomized rats. Biomaterials. 2010;31:9006–9014. doi: 10.1016/j.biomaterials.2010.07.112. [DOI] [PubMed] [Google Scholar]
- 9.Yan J, Zhang YM, Han Y, Zhao YT, Sun JF, Yan H. [Improvement of osseointegration of titanium dental implant surfaces modified with strontium-substituted hydroxyapatite] . Zhonghua Kou Qiang Yi Xue Za Zhi. 2010;45:89–93. [PubMed] [Google Scholar]
- 10.Yang GL, Song LN, Jiang QH, Wang XX, Zhao SF, He FM. Effect of strontium-substituted nanohydroxyapatite coating of porous implant surfaces on implant osseointegration in a rabbit model. Int J Oral Maxillofac Implants. 2012;27:1332–1339. [PubMed] [Google Scholar]
- 11.Fu DL, Jiang QH, He FM, Yang GL, Liu L. Fluorescence microscopic analysis of bone osseointegration of strontium-substituted hydroxyapatite implants. J Zhejiang Univ Sci B. 2012;13:364–371. doi: 10.1631/jzus.B1100381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang GL, He FM, Hu JA, Wang XX, Zhao SF. Effects of biomimetically and electrochemically deposited nano-hydroxyapatite coatings on osseointegration of porous titanium implants. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:782–789. doi: 10.1016/j.tripleo.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 13.Verberckmoes SC, De Broe ME, D’Haese PC. Dose-dependent effects of strontium on osteoblast function and mineralization. Kidney Int. 2003;64:534–543. doi: 10.1046/j.1523-1755.2003.00123.x. [DOI] [PubMed] [Google Scholar]
- 14.Bonnelye E, Chabadel A, Saltel F, Jurdic P. Dual effect of strontium ranelate: stimulation of osteoblast differentiation and inhibition of osteoclast formation and resorption in vitro. Bone. 2008;42:129–138. doi: 10.1016/j.bone.2007.08.043. [DOI] [PubMed] [Google Scholar]
- 15.Saidak Z, Marie PJ. Strontium signaling: molecular mechanisms and therapeutic implications in osteoporosis. Pharmacol Ther. 2012;136:216–226. doi: 10.1016/j.pharmthera.2012.07.009. [DOI] [PubMed] [Google Scholar]


