Abstract
Numerous papers have reported ABCB1 polymorphisms are associated with the response to chemotherapy of cancers. The inconclusive results call for a comprehensive analysis, for the sample size of the published studies is comparatively small. Therefore, a comprehensive meta-analysis was performed on the basis of the published studies for an accurate estimation of such association. Altogether 8 comparative studies including 2463 cancer patients were involved in our meta-analyses. ABCB1 C1236T (rs1128503) polymorphism was shown to be associated with tumor response to chemotherapy in cancer patients under the dominant model (OR=1.72, 95% CI=1.09-2.73, P=0.177, I2=36.60%) and additive model (OR=1.99, 95% CI=1.39-2.85, P=0.222, I2=25.90%). A subgroup meta-analysis indicated a significant association under dominant model between ABCB1 C1236T (rs1128503) polymorphism and breast cancer in the Asians (OR=2.15, 95% CI=1.22-3.77, P=0.210, I2=33.70%). These results suggest that ABCB1 C1236T (rs1128503) might contribute to the tumor response to chemotherapy in cancers from the Asians, especially in the osteosarcoma and breast cancer.
Keywords: Polymorphism, chemotherapy, cancer, tumor response
Introduction
Cancers caused about 14.6% of all human deaths, and is one of the leading causes of death [1]. Most of the patients with malignant tumors received neoadjuvant therapy before surgical resection of the primary tumor and received postoperative chemotherapy. And, as one of the major cancer treatments, chemotherapy is the treatment of cancer with one or more cytotoxic anti-neoplastic drugs as part of a standardized regimen. In combination with surgery, chemotherapy has proven useful in a number of different cancer types. The efficacy of chemotherapy depends on the type of cancer and the stage. Generally, it is well known that personalized chemotherapy could improve response and survival of cancer patients. However, chemotherapy face the intricate chemotherapy-resistant which continues to be a major clinical obstacle to a successful treatment [2,3].
When a normal cell transforms into a cancer cell, the genes which regulate cell growth and differentiation must be altered. And, genetic changes can occur at different levels and by different mechanisms. Both genetic and environmental factors have an impact on clinical response to chemotherapy. A higher concentration of anticancer drugs can cause inevitable toxicity and a lower concentration decreases the therapeutic efficacy. Meanwhile, most anticancer drugs present a narrow therapeutic range. Differences in response and toxicity of each chemotherapeutic drug varied from individual patients in pharmacokinetics and pharmacodynamics [4] together with the genetic factors may account for the personal susceptibility to anticancer therapy, for the genes have a role in controlling drug absorption, distribution, metabolism, and excretion [5].
ABCB1 (MDR1) gene encodes for P glycoprotein (P-gp), working as an energy-dependent efflux pump, plays a vital role in the bioavailability and cell-toxicity limitation of multifarious substances and xenobiotics, including cancer-fighting drugs, antibiotic medicines, immunosuppressant, anti-HIV protease inhibitors, among others [6]. Three single nucleotide polymorphisms (SNPs), namely C1236T (rs1128503), C3435T (rs1045642) and G2677T/A (rs2032582), are the most widely studied SNPs in ABCB1 and exhibit higher frequencies in Asian and Caucasian populations, but much lower in the African populations [7]. Moreover, the three SNPs were associated with the substrate and inhibitor-dependent functional modifications in vitro studies and reduced expressions were found in the tissue samples. In addition to cancers, ABCB1 SNPs have been investigated in a wide range of diseases including inflammatory bowel disease (IBD) [8], Parkinson disease [9], HIV-1 [10], and rheumatoid arthritis [11], among others.
Chen et al. confirmed the lack of association between ABCB1 C3435T polymorphism and chemotherapy response in advanced breast cancer patients, while Wei et al. detected a significant association between platinum-based chemotherapies in advanced non-small cell lung cancer patients with objective response for CC genotype of ABCB1 C3435T in overall and Asian populations stratified [12,13]. Nevertheless, few studies in regard to the 1236T allele of ABCB1 have been performed, let alone a comprehensive meta-analysis. The inconsistent conclusions of polymorphisms of ABCC1 (rs1128503) in the chemotherapy response of cancer patients enlightened us to conduct a meta-analysis on the evidence from all published studies to provide a more precise estimation of the association.
Materials and methods
Publication search
The literature was searched using PubMed, Embase and China National Knowledge Internet (CNKI) to identify relevant and available published articles. The following terms were applied in search of the forementioned databases: ‘ABCB1’ or ‘MDR1’, ‘Polymorphism’ or ‘mutation’, in combination with ‘cancer’ or ‘sarcoma’. The last search was up to September 15th, 2014. In order to achieve a comprehensive literature, we also identified additional studies by screening reference lists of key studies or reviews. No restriction was set on time period, sample size, population, and type of report, and only papers written in English were included. When a study reported the results on different ethnicities, we treated it as separate studies in this meta-analysis. Two independent reviewers were employed when the literature retrieval was performed in duplication. Conformed to the provisions of the Declaration of Helsinki, this study was approved by the Ethics Committee of our institution.
Inclusion and exclusion criteria
The inclusion criteria of this meta-analysis were as follows: (1) studies for humans only; (2) information on the relationship between ABCB1 (rs1128503) polymorphism and chemotherapy response of malignant tumors (evaluated by RECIST, response evaluation criteria in solid tumor); (3) the papers providing the sample size, alleles distribution, genotypes or other relevant data that can indirectly help us calculate the results; (4) of the studies published by the same author or institute, we only chose the largest of the available published data sets. Thus, studies had to be excluded once one of the following criteria existed: (1) studies that contained overlapping data; (2) incomplete data, and (3) studies not based on the RECIST.
Data extraction
All the data was carefully extracted from all eligible studies independently by two investigators according to the aforementioned inclusion criteria. The following data of the studies were extracted: first author, published year, ethnicity, numbers of patients, gene locus, cancer type, odd ratio (OR) and 95% confidence interval (95% CI) in cases. Two reviewers were in charge of the data extraction and reached consensus on all of the data extracted. Once there is a discrepancy in data extraction, problems were resolved by repeating the study review and discussion. A third reviewer was invited to check the extracted data, the analysis results, and solve the disagreement if it still existed after two reviewers’ discussion.
Methodological quality assessment
The methodological quality of each study was assessed by total score of quality assessment (TSQA) and a star system of TSQA has been employed for the evaluation, ranging from zero to nine stars [14]. The study with six or more stars are considered as a study with a high quality.
Statistical analysis
Crude OR with 95% CI in each study was used to assess the strength of association between ABCB1 (rs1128503) polymorphism and clinical outcomes in cancer patients who received chemotherapy. The heterogeneity among the studies was assessed by the χ2-test-based Q-statistic and I2 (I2=(Q-df)/Q×100%) [15]. The overall estimate of risk was calculated by a fixed effects model or a random effects model according to the absence (P > 0.10 or I2 < 50%) or presence (P < 0.10 or I2 > 50%) of heterogeneity, respectively. Subgroup analyses were applied once there are enough studies (≥ 3 studies) to extract and/or calculate the relevant data. Sensitivity analyses were conducted by sequential omission of individual studies respectively to detect the potential influence of each study set to the pooled ORs. The publication bias was tested by Begg’s and Egger’s tests and P value < 0.05 was considered as a statistically significant publication bias [16,17]. And the symmetryof the plot distribution on the funnel plot also indicated the absence of publication bias. We performed all the calculation by STATA, version 12.0 (Stata Corporation, College Station, TX, USA), and the two-sided statistical tests were used.
Results
Literature search process
After a complete research based on the criteria above, 68 literatures were found. After screening the titles or abstracts, 35 studies were excluded because they were reviews, meta-analysis, non-English studies, or not relevant to the role of ABCB1 (rs1128503) polymorphism in cancer patients. Therefore, 17 papers written in English were preliminarily identified for further evaluation. Moreover, on the basis of the exclusion criteria, 9 publications were excluded: 6 papers provided insufficient genetic information and 3 were inconformity to RECIST. Additional articles were not found in the references cited in the published studies by manual search. Finally, a total of 8 relevant literatures met the inclusion criteria (Figure 1) [2,4,5,18-22]. The main characteristics were presented in Table 1: all the studies were conducted in Asian populations, namely China [5,19-22], India [2], Korea [18], and Saudi Arabia [4]; the sum of the patients equaled to 2463; four studies were conducted in the breast cancer [2,4,21,22], three in the osteosarcoma [5,19,20], and one in the gastric cancer; the mean TSQA value of the included studies was 8, ranging from 7 to 9.
Figure 1.
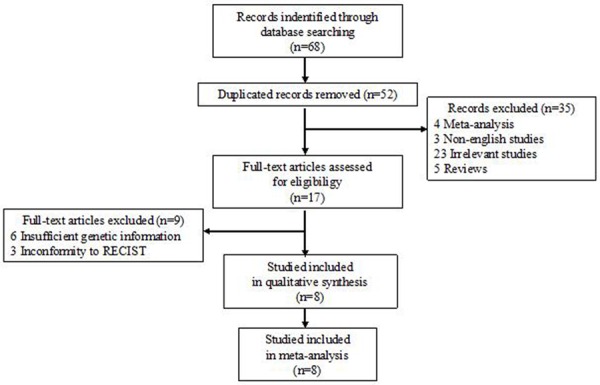
Flow diagram of the trial selection process RECIST, Response Evaluation Criteria in Solid Tumor.
Table 1.
The studies summary of ABCB1 (rs1128503) polymorphisms with cancer
| Investigator (Year) | Country | Patients | Method | Tumors | TSQA |
|---|---|---|---|---|---|
| Li et al. (2014) | China | 162 | PCR | Osteosarcoma | 8 |
| Liu et al. (2014) | China | 186 | PCR | Osteosarcoma | 8 |
| Yang et al. (2013) | China | 208 | PCR | Osteosarcoma | 7 |
| Chaturvedi et al. (2013) | India | 207 | PCR-RFLP | Breast cancer | 7 |
| Alsaif et al. (2013) | Saudi Arabia | 100 | PCR | Breast cancer | 8 |
| Ji et al. (2012) | China | 153 | PCR | Breast cancer | 9 |
| Wu et al. (2012) | China | 1240 | PCR-RFLP | Breast cancer | 8 |
| Shim et al. (2010) | Korea | 207 | PCR-RFLP | Gastric cancer | 9 |
PCR, polymerase chain reaction; PCR-RFLP, Polymerase chain reaction-Restriction fragment length polymorphism; TSQA, Total score of quality assessment.
Quantitative data synthesis
All the pooled analysis and subgroups analysis adopted the fixed model due to the absence of heterogeneity. The pooled OR in the TT vs. CC model was 1.99 (95% CI: 1.39-2.85, P=0.222, I2=25.9%) while OR equaled to 1.63 (95% CI: 1.18-2.27, P0.785, I2=0.00%) in CT vs. CC model (Table 2; Figure 2). Without doubt, strong association was observed in the TT+CT vs. CC model (OR=1.72, 95% CI: 1.09-2.73, P0.177, I2=36.60%) between ABCB1 (rs1128503) polymorphism and tumor response to chemotherapy (Figure 3). Meanwhile, As shown in Figures 4 and 5, strong association were detected in the breast cancer subgroup (TT vs. CC, OR=1.94, 95% CI: 1.00-3.79, P0.123, I2=48.10%; CT vs. CC, OR=2.22, 95% CI=1.19-4.12, P0.476, I2=0.00%) and osteosarcoma subgroup (TT vs. CC, OR=2.53, 95% CI=1.55-4.13, P0.994, I2=0.00%). All the ORs discussed above showed the strong association between the ABCB1 (rs1128503) polymorphism and tumor response to chemotherapy. No between-study heterogeneity was observed (all P > 0.05)
Table 2.
Meta-analysis of ABCB1 (rs1128503) polymorphism with tumor response to chemotherapy
| Allele/genotype | Model | OR | 95% CI | p | I2 | |
|---|---|---|---|---|---|---|
| TT vs. CC | Pooled | Fixed | 1.99 | 1.39-2.85 | 0.222 | 25.90% |
| Subgroup (Breast Cancer) | Fixed | 1.94 | 1.00-3.79 | 0.123 | 48.10% | |
| Subgroup (Osteosarcoma) | Fixed | 2.53 | 1.55-4.13 | 0.994 | 0.00% | |
| CT vs. CC | Pooled | Fixed | 1.63 | 1.18-2.27 | 0.785 | 0.00% |
| Subgroup (Breast Cancer) | Fixed | 2.22 | 1.19-4.12 | 0.476 | 0.00% | |
| Subgroup (Osteosarcoma) | Fixed | 1.51 | 0.98-2.31 | 0.985 | 0.00% | |
| TT+CT vs. CC | Pooled | Fixed | 1.72 | 1.09-2.73 | 0.177 | 36.60% |
| Subgroup (Breast Cancer) | Fixed | 2.15 | 1.22-3.77 | 0.210 | 33.70% |
Only the subgroups with enough data (≥ 3 papers) were analyzed in this table. OR, odds ratio; CI, confidence interval; P value and I2 value are used to test the heterogeneity.
Figure 2.
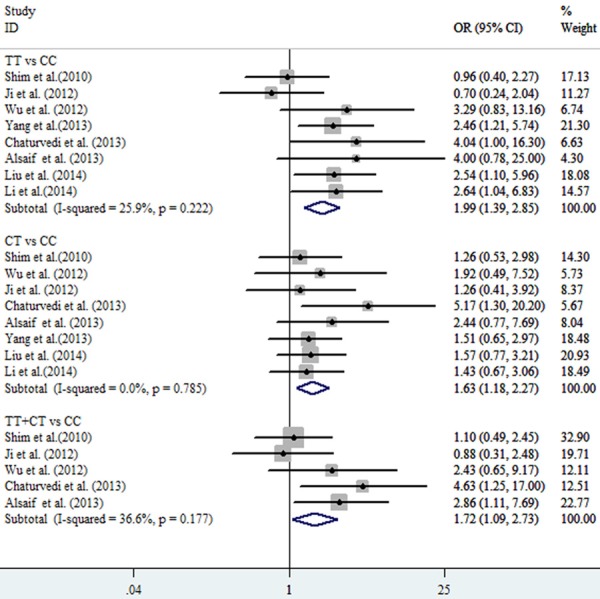
Pooled fixed-effects-based odds ratio of chemotherapy response in tumors associated with ABCB1 rs1128503 polymorphism. Comparison: TT vs. CC; CT vs CC; TT+TC vs. CC.
Figure 3.
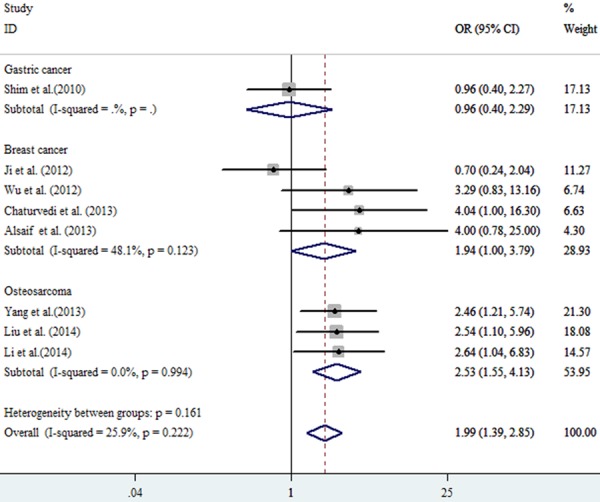
Forest plot of chemotherapy response in malignant tumors associated with ABCB1 rs1128503 polymorphism under the tumor types subgroup. Comparison: TT vs. CC.
Figure 4.
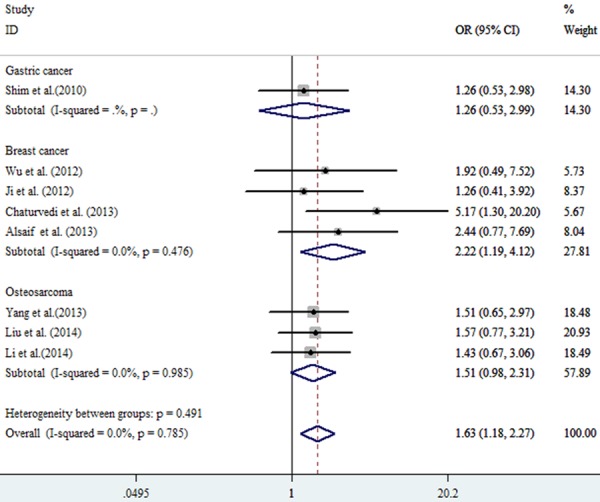
Forest plot of chemotherapy response in malignant tumors associated with ABCB1 rs1128503 polymorphism under the tumor types subgroup. Comparison: CT vs. CC.
Figure 5.
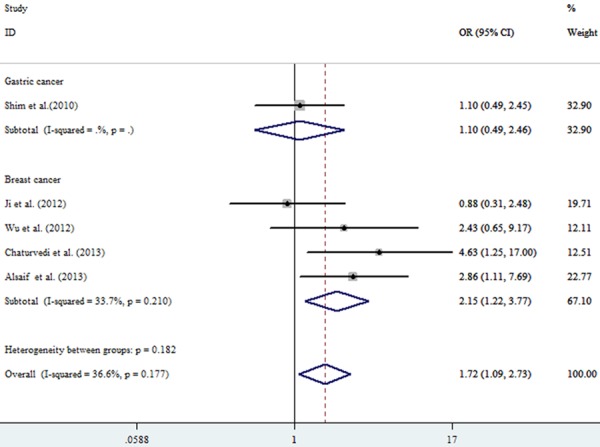
Forest plot of chemotherapy response in malignant tumors associated with ABCB1 rs1128503 polymorphism ]under the tumor types subgroup. Comparison: CT+TT vs. CC.
Publication bias
Funnel plot as well as Begg’s and Egger’s tests were carried out to access the publication bias of studies (Table 3). There was no evidence of publication bias in comparison of CT vs. CC of ABCB1 (rs1128503) (Figure 6). The comparison analyzed in this funnel is of the utmost numbers of 8 studies.
Table 3.
Evaluation of the bias for this meta-analysis of ABCB1 (rs1128503) polymorphisms with tumor response to chemotherapy
| Allele/genotype | Model | Article Numbers | p |
|---|---|---|---|
| TT vs. CC | Pooled | 8 | 0.466 |
| CT vs. CC | Pooled | 8 | 0.091 |
| TT+CT vs. CC | Pooled | 5 | 0.264 |
P value is employed to test whether there is bias or not.
Figure 6.
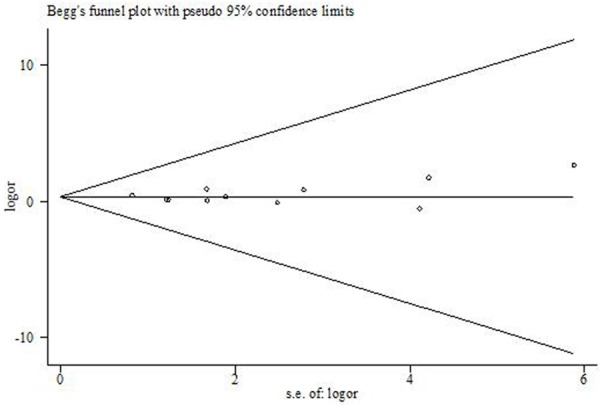
Funnel plots of the meta-analysis of ABCB1 rs1128503 polymorphism associated with tumor response to chemotherapy. (Comparison: CT vs. CC).
Discussion
ABCB1 is expressed in both normal human tissues and multidrug-resistant cancer cells, and such protein acts as an energy-dependent drug efflux pump for the absorption and distribution of xenobiotics, toxins, and drugs [5,20]. The synonymous ABCB1 C1236T SNP, located in exon 12, has been shown to have an effect on protein folding, for the use of a rare codon when combined with the ABCB1 C3435T SNP, and has shown a diminished influence on some inhibitors and other probable significant agents [16,23]. Studies in vitro failed to confirm such association with protein expression, and detected a relatively subdued function and a difference in substrate specificity and protein conformation when combined with either C1236T or G2677T/A SNPs [18,23].
In the present study, we found that (rs1128503) polymorphism of ABCB1 was associated with chemotherapy response in cancer patients, and the people with and CT and TT genotypes of (rs1128503) have a good response to chemotherapy compared to those patients with CC genotype. In addition, no significant heterogeneity was observed between studies, suggested the uniform of the subjects and methods of the included studies. In the subgroup analyzed by and tumor types found a relative stronger association breast cancer, while a weaker association was detected in osteosarcoma. Thus, nothing in this world could deny the strong association between the T allele in the (rs1128503) polymorhism of ABCB1 with the good response to chemotherapy, which confirmed by the pooled and subgroup analysis. Different from other conflicting or even contrary results in different types of cancers or diseases, in this meta-analysis, the breast and osteosarcoma subgroup analysis reached a consistent conclusion with the pooled analysis. Wu et al. [22] suggest that ABCB1 gene haplotype 1236T relate to the risk and clinical outcomes of breast carcinoma, while Yang et al. [19] found that ABCB1 (rs1128503) TT SNP is associated with response to chemotherapy and overall survival in osteosarcoma patients . Therefore, ABCB1 gene may function as candidate molecular markers of chemo-sensitivity and may be a potential prognostic biomarker for cancer patients, which could help in the design of individualized therapy [19,21]. Due to the few numbers of studies could provide the treatment outcomes, especially overall survival or progression free survival, we failed to predict the prognosis of cancer patients with ABCB1 (rs1128503) polymorphism. Additional studies are required in order to have a better approximation of their effect.
Wu et al. [22] suggest that ABCB1 gene haplotype 1236T relate to the risk and clinical outcomes of breast carcinoma, while Yang et al. [19] found that ABCB1 (rs1128503) TT SNP is associated with response to chemotherapy and overall survival in osteosarcoma patients. Therefore, ABCB1 gene may function as candidate molecular markers of chemo-sensitivity and may be a potential prognostic biomarker for cancer patients, which could help in the design of individualized therapy [19,21]. Due to the few numbers of studies could provide the treatment outcomes, especially overall survival or progression free survival, we failed to predict the prognosis of cancer patients with ABCB1 (rs1128503) polymorphism.
Although a comprehensive analysis was performed to identify the possible association between ABCB1 (rs1128503) polymorphism with response to chemotherapy in cancer patients, several potential limitations, that are inherent to any meta-analysis, are supposed to be declared. Firstly, the total sample size was still small and subgroup analysis could be performed only in the ethnicity classification, breast cancer, and osteosarcoma. Secondly, our study was lack of information to investigate the adverse effect of chemotherapy, and mixed regimens of chemotherapy may be also a factor to the results of this study. Thirdly, only these studies written in English and Chinese are included in this meta-analysis. Thus, a large meta-analysis including more studies, both other ethnicities and cancers, should be investigated in the future to probe the possibility of other specific associations.
Despite of shortages above, this is the first meta-analysis investigating the association of ABCB1 (rs1128503) SNP with the chemotherapy response in human cancers. And, the rigorous searching strategy coupled with computer-aided search and manual search made the maximum eligible studies to be included. And the well designed process of literature selection, data extraction and analysis made the final conclusion convincible and meaningful.
In conclusion, the present results of the meta-analysis showed a convincing significant association between ABCB1 gene (rs1128503) haplotype 1236T and chemotherapy response in cancer patients. Patients carrying ABCB1 rs1128503 TT genotype and T allele were more likely to have a good response to chemotherapy. And, therefore, it would encourage us to carry out large-scale studies for ABCB1 polymorphisms and prognosis of cancer patients. Further well-designed studies with large samples may allow accessing the specific gene functions of the ABCB1 and the genetic mechanisms linked to tumor sensitivity to chemotherapy, thus providing a big step in the design of specific therapeutic strategies to control cancer progression.
Acknowledgements
The financial support from the National Natural Science Foundation of China (31370986, 81202115), and the Key Project of Basic Research of Shanghai (11JC1410101) is gratefully acknowledged.
Disclosure of conflict of interest
None.
References
- 1.Stewart BW, Wild CP, editors. World Cancer Report 2014, 2012.
- 2.Alsaif AA, Hasan TN, Shafi G, Syed NA, Alsaif MA, Al-Assaf AH, Alshatwi AA. Association of multiple drug resistance-1 gene polymorphism with multiple drug resistance in breast cancer patients from an ethnic Saudi Arabian population. Cancer Epidemiol. 2013;37:762. doi: 10.1016/j.canep.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 3.Pan JH, Han JX, Wu JM, Sheng LJ, Huang HN, Yu QZ. MDR1 single nucleotide polymorphisms predict response to vinorelbine-based chemotherapy in patients with non-small cell lung cancer. Respiration. 2008;75:380. doi: 10.1159/000108407. [DOI] [PubMed] [Google Scholar]
- 4.Chaturvedi P, Tulsyan S, Agarwal G, Lal P, Agarwal S, Mittal RD, Mittal B. Influence of ABCB1 genetic variants in breast cancer treatment outcomes. Cancer Epidemiol. 2013;37:754. doi: 10.1016/j.canep.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 5.Liu S, Yi Z, Ling M, Shi J, Qiu Y, Yang S. Predictive potential of ABCB1, ABCC3, and GSTP1 gene polymorphisms on osteosarcoma survival after chemotherapy. Tumour Biol. 2014;35:9897–904. doi: 10.1007/s13277-014-1917-x. [DOI] [PubMed] [Google Scholar]
- 6.Pauli-Magnus C, Kroetz DL. Functional implications of genetic polymorphisms in the multidrug resistance gene MDR1 (ABCB1) Pharm Res. 2004;21:904. doi: 10.1023/b:pham.0000029276.21063.0b. [DOI] [PubMed] [Google Scholar]
- 7.Schaeffeler E, Eichelbaum M, Brinkmann U, Penger A, Asante-Poku S, Zanger UM, Schwab M. Frequency of C3435T polymorphism of MDR1 gene in African people. Lancet. 2001;358:383. doi: 10.1016/S0140-6736(01)05579-9. [DOI] [PubMed] [Google Scholar]
- 8.Onnie CM, Fisher SA, Pattni R, Sanderson J, Forbes A, Lewis CM, Mathew CG. Associations of allelic variants of the multidrug resistance gene (ABCB1 or MDR1) and inflammatory bowel disease and their effects on disease behavior: a case-control and meta-analysis study. Inflamm Bowel Dis. 2006;12:263. doi: 10.1097/01.MIB.0000209791.98866.ba. [DOI] [PubMed] [Google Scholar]
- 9.Lee CG, Tang K, Cheung YB, Wong LP, Tan C, Shen H, Zhao Y, Pavanni R, Lee EJ, Wong MC, Chong SS, Tan EK. MDR1, the blood-brain barrier transporter, is associated with Parkinson’s disease in ethnic Chinese. J Med Genet. 2004;41:e60. doi: 10.1136/jmg.2003.013003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kedmi M, Maayan S, Cohen SB, Hauzi M, Rund D. MDR1 and CYP3A4 polymorphisms are associated with HIV seropositivity in Israeli patients but do not influence the course of HIV disease. AIDS Patient Care STDS. 2007;21:653. doi: 10.1089/apc.2006.0148. [DOI] [PubMed] [Google Scholar]
- 11.Pawlik A, Wrzesniewska J, Fiedorowicz-Fabrycy I, Gawronska-Szklarz B. The MDR1 3435 polymorphism in patients with rheumatoid arthritis. Int J Clin Pharmacol Ther. 2004;42:496. doi: 10.5414/cpp42496. [DOI] [PubMed] [Google Scholar]
- 12.Chen G, Quan S, Hu Q, Wang L, Xia X, Wu J. Lack of association between MDR1 C3435T polymorphism and chemotherapy response in advanced breast cancer patients: evidence from current studies. Mol Biol Rep. 2012;39:5161. doi: 10.1007/s11033-011-1312-2. [DOI] [PubMed] [Google Scholar]
- 13.Wei HB, Lu XS, Shang LH, Xu G, Hu J, Che DH, Liu F, Wu Y, Zhang GM, Yu Y. Polymorphisms of ERCC1 C118T/C8092A and MDR1 C3435T predict outcome of platinum-based chemotherapies in advanced non-small cell lung cancer: a meta-analysis. Arch Med Res. 2011;42:412. doi: 10.1016/j.arcmed.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 15.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 16.Fung KL, Gottesman MM. A synonymous polymorphism in a common MDR1 (ABCB1) haplotype shapes protein function. Biochim Biophys Acta. 2009;1794:860. doi: 10.1016/j.bbapap.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egger M, Davey SG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shim HJ, Yun JY, Hwang JE, Bae WK, Cho SH, Lee JH, Kim HN, Shin MH, Kweon SS, Lee JH, Kim HJ, Chung IJ. BRCA1 and XRCC1 polymorphisms associated with survival in advanced gastric cancer treated with taxane and cisplatin. Cancer Sci. 2010;101:1247. doi: 10.1111/j.1349-7006.2010.01514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang J, Wang ZG, Cai HQ, Li YC, Xu YL. Effect of variation of ABCB1 and ABCC3 genotypes on the survival of bone tumor cases after chemotherapy. Asian Pac J Cancer Prev. 2013;14:4595. doi: 10.7314/apjcp.2013.14.8.4595. [DOI] [PubMed] [Google Scholar]
- 20.Li JZ, Tian ZQ, Jiang SN, Feng T. Effect of variation of ABCB1 and GSTP1 on osteosarcoma survival after chemotherapy. Genet Mol Res. 2014;13:3186. doi: 10.4238/2014.April.25.3. [DOI] [PubMed] [Google Scholar]
- 21.Ji M, Tang J, Zhao J, Xu B, Qin J, Lu J. Polymorphisms in genes involved in drug detoxification and clinical outcomes of anthracycline-based neoadjuvant chemotherapy in Chinese Han breast cancer patients. Cancer Biol Ther. 2012;13:264. doi: 10.4161/cbt.13.5.18920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu H, Kang H, Liu Y, Tong W, Liu D, Yang X, Lian M, Yao W, Zhao H, Huang D, Sha X, Wang E, Wei M. Roles of ABCB1 gene polymorphisms and haplotype in susceptibility to breast carcinoma risk and clinical outcomes. J Cancer Res Clin Oncol. 2012;138:1449. doi: 10.1007/s00432-012-1209-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimchi-Sarfaty C, Oh JM, Kim IW, Sauna ZE, Calcagno AM, Ambudkar SV, Gottesman MM. A “silent” polymorphism in the MDR1 gene changes substrate specificity. Science. 2007;315:525. doi: 10.1126/science.1135308. [DOI] [PubMed] [Google Scholar]


