Abstract
In the present study, we described an easily reproducable experimental pancreatits model induced by biliopancreatic duct injection of ethyl alcohol. Seventy Wistar albino rats were divided equally into seven groups randomly: the control group (group 1), acute pancreatitis groups; induced by 20% ethanol (group 2), 48% ethanol (group 3), 80% ethanol (group 4), chronic pancreatitis groups; induced by 20% ethanol (group 5), 48% ethanol (group 6) and by 80% ethanol (group 7). Acute pancreatitis groups were sacrified on postoperative day 3, while the control group and chronic pancreatitis groups were killed on postoperative day 7. Histopathologic evaluation was done, and P < 0.05 was accepted as statistically significant. All rats in group 3 developed acute pancreatitis (100%). Inflammatory infiltration of neutrophils and mononuclear cells, interstitial edema, and focal necrotic areas were seen in the pancreatic tissues. Similarly, all rats in group 6 developed chronic pancreatitis (100%). Interstitial fibrosis, lymphotic infiltration, ductal dilatation, acinar cell atrophy, periductal hyperplasia were seen in the pancreatic tissues. Mortality was seen only in group 7. The biliopancreatic ductal injection of 48% ethanol induced acute and chronic pancreatitis has 100% success rate.
Keywords: Experimental, acute pancreatitis, chronic pancreatitis, ethanol, animal model
Introduction
Acute pancreatitiswith an incidence of 5 to 80 cases per 100 000 population is a common disease in the Western world. Most common etiological factors are gallstones or alcohol-related acute pancreatitis [1,2]. Biliary pancreatitis associated with significant morbidity and mortality; is the most common cause of acute pancreatitis that refers to pancreatitis caused by biliary calculous diseases [3]. Most of the patients (85% to 90%) has a rather benign self limiting clinical course and responds well to conservative treatment. The remaining 10% to 15% of cases will end with necrotizing pancreatitis that has a grave clinical course mostly accompanied by local/systemic complications and multi-organ failure [4]. Severity of the disease varies from patient to patient.
The underlying mechanisms involved in the pathogenesis of acute pancreatitis are ill understood. A better understanding of the underlying pathophysiology of severe acute pancreatitis may lead to more targeted therapeutic options, potentially leading to improved survival. Animal models of acute pancreatitis are therefore an essential investigative tool for these aims to be achieved. An ideal model of experimental pancreatitis should be easily reproducible, should be able to vary the severity of acute and/or chronicpancreatitis. The morphology and pathophysiology should be just like the one in human. There has been a lot of non-invasive and invasive models described in the literaturesuch as hormone-induced, alcohol-induced, immune-mediated, diet-induced, and closed duodenal loop, antegrade pancreatic duct perfusion, combination of secretory hyperstimulation with minimal intraductal bile acid exposure, vascular-induced, ischaemia/reperfusion and duct ligation [5-8].
Existing alcohol-induced models are relatively simple and cheap to perform. Reproducibility, however, has not been successfully achieved. Furthermore, there is a lack of correlation with the clinical setting. In the present study, we described an alternativeeasily reproducible model of experimental pancreatitis induced by intrabiliary injection of ethanol on the bases of our own experiences that has been previously published in the literature [9-14]. We also discussed the advantages and disadvantages of our model, and compared the results with other experimental pancreatitis models described in the current literature.
Materials and methods
The present study was approved by the Cerrahpasa Medical Faculty Laboratory Animals Ethics Committee, and all procedures with animals were performed in accordance with the guide of the Committee on Care and Use of Laboratory Animals (CCULA) [15]. The animals were fed on standard laboratory diet and water ad libitum before and after surgery. All animals were anesthetized with i.m. 100 mg/kg ketamine injection and ether inhalation anesthesia to undergo a midline laparotomy, and all the experimental procedures were performed as described in our previous publications [9-14]. Acute pancreatitis groups were sacrified on postoperative day 3, while the control group and chronic pancreatitis groups were killed on postoperative day 7. Seventy male Wistar albino rats, weighing 300-400 g, were divided into seven groups randomly: the control group (group 1; n = 10), acute pancreatitis groups; induced by 20% ethyl alcohol (group 2, n = 10), by 48 % ethyl alcohol (group 3, n = 10), by 80% ethyl alcohol (group 4, n = 10), chronic pancreatitis groups; induced by 20% ethyl alcohol (group 5, n = 10), by 48 % ethyl alcohol (group 6, n = 10) and by 80% ethyl alcohol (group 7, n = 10).
Sham laparotomy was performed in group 1 without cannulation of the biliopancreatic duct. Other groups were injected 20, 48 or 80% ethanol, in a volume of 1 cm3, into the common biliary duct using an insulin injector (Figure 1). The duct was transiently tied with a 1/0 silk suture to prevent retrograde leakage of alcohol. Groups 1-4 were killed 3 days later. Gorups 5-7 were killed on postoperative day 7 under ether anesthesia, and 4 cm3 (3-7 cm3) blood was taken by cardiac punctureafter exploration of the thorax (Figure 2). Pancreatic tissue was removed upholding the stomach (Figure 3).
Figure 1.
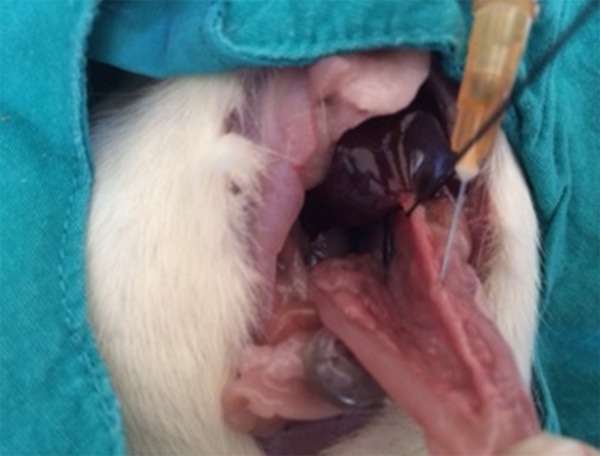
Common duct injection of ethanol by help of insulin injector.
Figure 2.
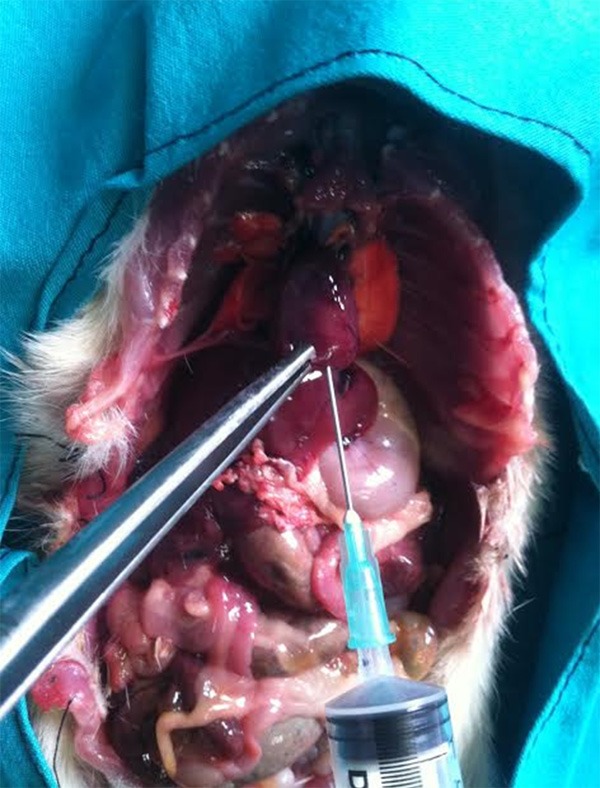
Cardiac puncture after midline laparotomy including thorax (sacrification).
Figure 3.
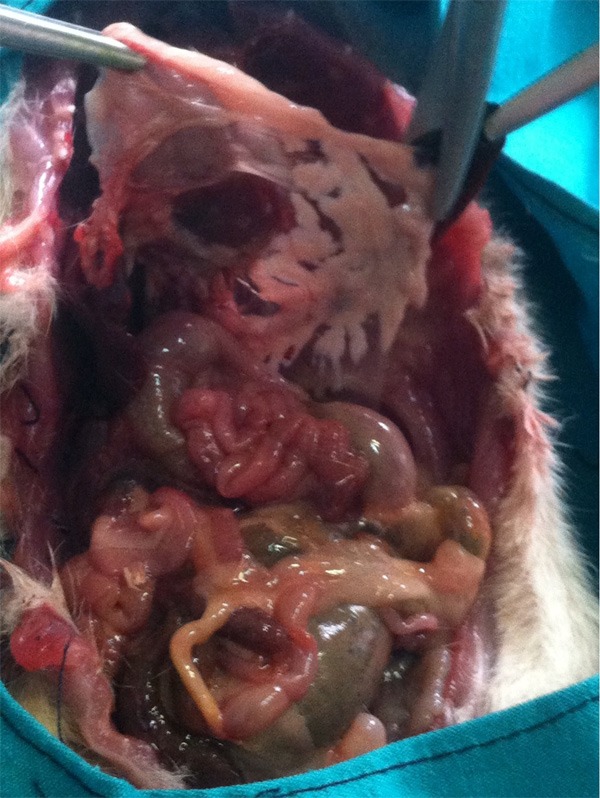
Dissection of pancreatic tissue for histological examination.
Tissue samples removed were fixed in 10% formalin solution for 24 h, paraffin embedded, and stained with hematoxylin and eosin (H & E). Light microscopy slides were examined and graded by a pathologist with experience in experimental pancreatitis who was unaware of the previous treatment.
Statistical analyses were carried out with the SPSS 11.5 version program (SPSS, Chicago, IL, US). Values were reported as the means ± SD. The Mann-Whitney’s U test, one-way analysis of varience, and Tukey’s honestly significant differences test were used to evaluate the significance of differences in characteristics between control and pancreatitis groups. Correlation analysis was performed by using Spearman’s rank correlation coefficient. Differences were considered significant at P < 0.05.
Results
During the early and late phases of alcohol-induced pancreatitis, which was studied on days 3 and 7 after injection, there was neither mortality nor morbidity in groups 1-6. However, three rats were found to be died (30% in group 7) and exploration showed multiorgan necrosis showing the systemic effects of high percentage of alhocol for prolonged period (two of them died on postoperative day 5, and one on day 6). There was no sign of acute pancreatitis in group 1. The pancreatic tissues of the rats in the control group were all normal, even on gross view (Figure 4).
Figure 4.
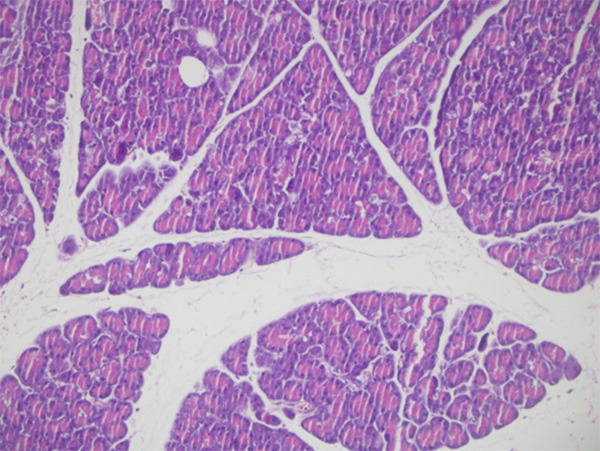
Histologic section showing normal pancreatic tissue (control group, ×100, H & E).
All rats in group 3 developed acute pancreatitis (100%). Inflammatory infiltration of neutrophils and mononuclear cells, interstitial edema, and focal necrotic areas were seen in the pancreatic tissues. Grossly, all pancreatic tissues showed edematous swelling and necrotic areas in this group (Figure 5). Similarly, all rats in group 6 developed chronic pancreatitis (100%). Interstitial fibrosis, lymphotic infiltration, ductal and ductular dilation, acinar cell atrophy, periductal ductular hyperplasia were seen in the pancreatic tissues (Figure 6).
Figure 5.
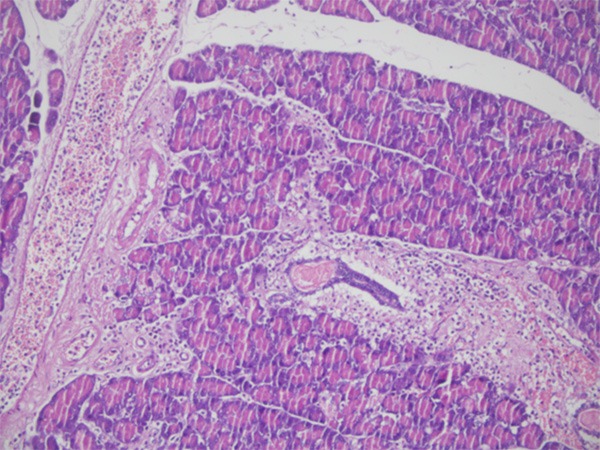
Interstitial edema, neutophil, and mononuclear cell infiltration showing acute pancreatitis (×100, H & E).
Figure 6.
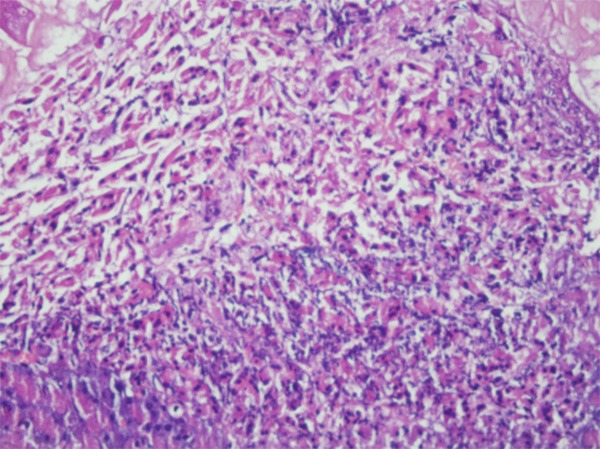
Interstitial fibrosis, lymphocyte infiltration, ductal dilatation and acinar cell atrophy showing chronic pancreatitis (×200, H & E).
Success rates in inducing acute and chronic pancreatitis in all groups were documented in Table 1. In summary, the most successful rates were held with 48% ethanol in both acute and chronic pancreatitis groups (groups 3 & 6, 100%, either).
Table 1.
Overall pancreatitis induction ratios of all groups
| All groups | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 | Group 7 |
|---|---|---|---|---|---|---|---|
| (n = 70) | (n = 10) | (n = 10) | (n = 10) | (n = 10) | (n = 10) | (n = 10) | (n = 10) |
| Normal pancreas | 10* (100%) | 8* (80%) | 0 | 0 | 2(20%) | 0 | 0 |
| Acute pancreatitis | 0 | 2 (20%) | 10* (100%) | 7* (70%) | 5* (50%) | 0 | 0 |
| Chronic pancreatitis | 0 | 0 | 0 | 3 (30%) | 3 (30%) | 10* (100%) | 7* (70%) |
Mortality (n = 3) was only seen in group 7;
P < 0.05.
As summarized in Table 1, success rate of 80% ethanol in producing acute pancreatitis (group 4) was 70%, however, there is a high risk of chronic pancreatitis induction (30%). On the other hand, 80% ethanol caused deaths in chronic pancreatic group (group 7). 20% ethanol caused acute pancreatitis in only 20% of rats (group 2), remaining 80% did not developed pancreatitis at all. On postoperative day 7, 20% ethyl alcohol was shown to produce acute pancreatitis 50%, and30% was seen to develop chronic pancreatitis. However, in 20% in this group, the pancreas tissues were shown to be unaffected.
Discussion
Because of the anatomical location of the pancreas, and the difficulty in procuring tissue at different stages of the inflammatory process in humans, our understanding of the pathogenesis of pancreatitis mainly relies on data from experimental animal models.The pathophysiology of experimental acute pancreatitis is tought to be consisting of the activation of pancreatic enzymes within the pancreatic acinar cells [4]. By the release of these activated enzymes in the interstitium, the autodigestion of the pancreas starts. After the release of these activated pancreatic enzymes into the circulation, the development of multiple organ dysfunction occurs [6]. The breaking down of stability between anti- and pro-inflammatory cytokines/chemokines, and the imbalance between necrosis and apoptosis, as a response to acinar cell injury have also been reported [5,6]. Our previous studies have also showed the role of reactive oxygen species and free radical scavengers in the etiophysiopathogenesis of pancreatitis in animal models [9,12]. These studies clearly detected rapid increases in the concentration of peroxidation products in pancreatic tissue and serum in acute pancreatitis.
There has been a lot of experimental pancreatitis models described in the literature. Cerulein, a cholecystokinin-pancreozymin analogue, -induced acute pancreatitis model is the most widely used noninvasive experimental animal model which is highly reproducible and economical [5,16,17]. As an advantage, this method allows investigation of healing of damaged tissue after cerulein has been discontinued. However, it has two major disadvantages. Even with maximum doses, only mild acute pancreatitis develops; also the course and severity of the acute pancreatitis is highly variable, making this technique not suitable for controlled studies. Immune-mediated models have been used as well, but the cost and high early mortality rates limited its usage [2]. On the other hand, diet-induced models were shown to induce pancreatitis in different stages [8]. As for the invasive pancreatitis models used in animals, ligation of the common biliopancreatic duct or closure of duodenal loop in the rat causes chronic lesions in the pancreas characterized by atrophy and apoptosis of acinar and ductal tissue, but not significant necrosis or inflammation [5,8].
Existing alcohol-induced models are relatively simple and cheap to perform. Acute ethanol administration selectively lessened pancreatic blood flow and microcirculation, suggesting that the effect of alcohol might increase ischaemia damage during the evolvement of acute pancreatitis with or without underlying chronic disease [6,16]. Another advantage of this model is that it allows alcohol to directly damage the pancreas by the influence of toxic ethanol metabolites, and perhaps by limitations of pancreatic regeneration [12,18]. Reproducibility, however, has not been successfully achieved. Furthermore, there is a lack of correlation with the clinical settings. Animal models of acute pancreatitis, induced by acute ethanol oral application alone, have been difficult to produce [2,5].
In this study, we aimed to describe an easily reproducible model of experimental pancreatitis induced by intrabiliary injection of ethanol on the bases of our own experiences that has been previously published in the literature [9-14]. Pancreatitis is an inflammatory process in the pancreas, and its diagnosis is made on the basis of the clinical presentation combined with appropriate laboratory determinations, such as serum amylase and lipase levels, and radiologic findings. However, regarding as experimental study, histopathological confirmation is the most important criteria for precise diagnosis of both acute anc chronic pancreatitis. Therefore, in the present study, histopathological evaluation of pancreatic tissues made it unnecessary to determine the serum amylase and lipase levels. All experimental rats were injected 20, 48 or 80% ethanol into the common biliary duct, and the groups were compared to see the success rate of desired pancreatitis, either acute or chronic.
In the current study, the most successful rates were held with 48% ethanol in both acute and chronic pancreatitis groups (100%, each) and there was no morbidity or mortality. Our previous studies had the similar success rates in inducing acute and/or chronic pancreatitis, therefore we recommend 48-50% ethanol in the pancreatitis model [11,13]. According to our model, the sacrification day has also vitalimportance as the postinduction day 3 is ideal for acute pancreatitis while day 7 is best to obtain the experimental chronic pancreatitis. As for the 20% ethanol induced groups, the insufficiency of alcohol rate in producing both acute and chronic pancreatitis were the outstanding finding (80% of rats had still normal pancreatic tissue in acute pancreatitis group, and only 30% of rats became chronic pancreatitis). On the other hand, 80% ethanol induced rats in acute pancreatitis group showed a 30% of chronic pancreatitis findings that could be regarded as an unwanted overdose affect. Furthermore, three rats were found to be died (30%) and exploration showed multiorgan necrosis showing the systemic effects of high percentage of alhocol for prolonged period. Therefore, 80% ethyl alcohol was seen to be systemically toxic and might cause death in experimental models.
Our model is an invasive procedure as all rats undergo laparotomy under general anesthesia. However, direct cannulation of the biliopancreatic duct and direct view of swelling in the pancreatic tissue guarantees the effective experimental result. The watchlist in this point is the precise dissection while cannulating common duct without harming the continuity of the duct itself, and the avoidance of unnecessary palpation of other organs and stretching the duct causing bleeding especially from the liver. In our previous studies, intraabdominal bleeding from the liver by applying high tension on the common canal resulted in intraoperative or early postoperative deaths [10,14]. However, there was no such mortality in the current study, as we gained experience in the laparotomy and cannulation techniques in rats. Pancreatic tissue lies behind the stomach and in the form of mucous layer, therefore even the touch/palpation of pancreas may cause pancreatitis, as an additional affect. In our opinion, to overcome the difficulty of ductal cannulation, mature rats weighing at least 300 g should be used as they have thicker ducts. On the other hand, the thinnest dental needles might help to cannulate the ducts of lower weighed animals. Furthermore, according to our own experience, small extravasations from the duct does not produce major problems, as pancreas tissue is situated just in neighbourhood of the distal end of common duct and the iatrogenic edema produced by this affect may only contribute to the induction of pancreatitis.
The most important advantage of alcohol induced experimental models of pancreatitis is its effects on morphology and pathophysiology like the one in human. Its direct cytotoxic and oxidative stress producing effects are well known, similar to those shown in the human studies [12]. Other ethanol-induced alterations and mechanisms are the reduction of pancreatic blood-flow and microcirculation, damaging effects of ethanol metabolites, increased pancreatic acinar cell expression of digestive and lysosomal enzymes, increased glandular enzyme content, additional nutritional factors, pancreatic duct obstruction, and limitations of pancreatic regeneration [5,11,13]. Although no satisfactory animal model for alcoholic pancreatitis has been developed, these animal models have provided insights in several factors that predispose the pancreas to development of pancreatic injury and contribute to alcoholic pancreatitis. In our previous studies, we also showed these effects of alcohol in pancreatic tissues of rats [10,14]. Also the effect on pancreatic acinar damage by alcohol-related free oxygen radicals, metabolites and the pancreatic regeneration [11]. Direct cytotoxicity in pancreatic acinar cells including interstitial edema and inflammatory cell infiltration indicating acute damage, and chronic fibrotic changes including lymphocytic infiltration and acinar cell atrophy are also shown in the current study.
In conclusion, the biliopancreatic ductal injection of 48% ethanol induced acute pancreatitis on postoperative day 3, and chronic pancreatitis on day 7, with 100% success rate. There was no morbidity and mortality. This presented model uses easily reproducable and reliable technique without any additional cost. Therefore, we highly recommend this simple technique in inducation of both acute and chronic pancreatitis in rats.
Disclosure of conflict of interest
None.
References
- 1.Mayerle J, Dummer A, Sendler M, Malla SR, van den Brandt C, Teller S, Aghdassi A, Nitsche C, Lerch MM. Differential roles of inflammatory cells in pancreatitis. J Gastroenterol Hepatol. 2012;2:47–51. doi: 10.1111/j.1440-1746.2011.07011.x. [DOI] [PubMed] [Google Scholar]
- 2.Foster JR. A review of animal models of nonneoplastic pancreatic diseases. Toxicol Pathol. 2014;42:243–259. doi: 10.1177/0192623313508479. [DOI] [PubMed] [Google Scholar]
- 3.Pandol SJ, Saluja AK, Imrie CW, Banks PA. Acute pancreatitis: bench to the bedside. Gastroenterology. 2007;132:1127–1151. doi: 10.1053/j.gastro.2007.01.055. [DOI] [PubMed] [Google Scholar]
- 4.Go VL, DiMagno EP, Gardner JD, Lebenthal E, Reber HA, Scheele GA, editors. The Pancreas: Biology, Pathobiology, and Disease. 2nd edition. New York: 1993. Steer ML: Etiology and pathophysiology of acute pancreatitis; pp. 581–592. [Google Scholar]
- 5.Schneider A, Whitcomb DC, Singer MV. Animal models in alcoholic pancreatitis: what can we learn? Pancreatology. 2002;2:189–203. doi: 10.1159/000058033. [DOI] [PubMed] [Google Scholar]
- 6.Lerch MM, Albrecht E, Ruthenburger M, Mayerle J, Halangk W, Krüger B. Pathophysiology of alcohol induced pancreatitis. Pancreas. 2003;27:291–296. doi: 10.1097/00006676-200311000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Poxleitner PJ, Seifert G, Richter SC, Hopt UT, Wittel UA. Infected pancreatic necrosis increases the severity of experimental necrotizing pancreatitis in mice. Pancreas. 2013;42:1150–1156. doi: 10.1097/MPA.0b013e318291c251. [DOI] [PubMed] [Google Scholar]
- 8.Hyun JJ, Lee HS. Experimental models of pancreatitis. Clin Endosc. 2014;47:212–216. doi: 10.5946/ce.2014.47.3.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kiziler AR, Aydemir B, Gulyasar T, Unal E, Gunes P. Relationships among iron, protein oxidation and lipid peroxidation levels in rats with alcohol-induced acute pancreatitis. Biol Trace Elem Res. 2008;124:135–143. doi: 10.1007/s12011-008-8127-6. [DOI] [PubMed] [Google Scholar]
- 10.Yavuz N, Unal E, Dogan M, Kiziler AR, Aydemir B, Titiz I. Serum free prostate-specific antigen and zinc levels in experimental acute pancreatitis. Biol Trace Elem Res. 2005;106:205–209. doi: 10.1385/BTER:106:3:205. [DOI] [PubMed] [Google Scholar]
- 11.Unal E, Uzun H, Kusaslan R, Dogan M, Genc H, Gunes P, Titiz I. Serum paraoxonase (a high-density lipoprotein-associated lipophilic antioxidant) activity and lipid profile in experimental acute pancreatitis. Pancreas. 2005;31:84–87. doi: 10.1097/01.mpa.0000168227.74203.e4. [DOI] [PubMed] [Google Scholar]
- 12.Andican G, Gelisgen R, Unal E, Tortum OB, Dervisoglu S, Karahasanoglu T, Burçak G. Oxidative stress and nitric oxide in rats with alcohol-induced acute pancreatitis. World J Gastroenterol. 2005;11:2340–2345. doi: 10.3748/wjg.v11.i15.2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yavuz N, Unal E, Memisoglu K, Gunes P, Krand O, Kiziler AR, Aydemir B, Kusaslan R, Dogan M, Titiz I. Plasma leptin levels in rats with pancreatitis. Tohoku J Exp Med. 2004;204:243–248. doi: 10.1620/tjem.204.243. [DOI] [PubMed] [Google Scholar]
- 14.Ferahman M, Unal E, Sakoglu N, Ersoy YE, As A, Ozdemir S. Zinc and copper status in acute pancreatitis: an experimental study. Biol Trace Elem Res. 2003;91:89–94. doi: 10.1385/BTER:91:1:89. [DOI] [PubMed] [Google Scholar]
- 15.Committee on Care and Use of Laboratory Animals (CCLA) Institute of Laboratory Animal Resources. Washington DC: National Research Council; 1985. Guide for the care and use of laboratory animal; p. 83. [Google Scholar]
- 16.Schneider L, Dieckmann R, Hackert T, Gebhard MM, Werner J. Acute alcohol-induced pancreatic injury is similar with intravenous and intragastric routes of alcohol administration. Pancreas. 2014;43:69–74. doi: 10.1097/MPA.0b013e3182a85ad7. [DOI] [PubMed] [Google Scholar]
- 17.Sledzinski M, Borkowska A, Sielicka-Dudzin A, Halon M, Wozniak M, Spodnik JH, Antosiewicz AH, Antosiewicz J. Cerulein-induced acute pancreatitis is associated with c-Jun NH(2)-terminal kinase 1-dependent ferritin degradation and iron-dependent free radicals formation. Pancreas. 2013;42:1070–1077. doi: 10.1097/MPA.0b013e318287d097. [DOI] [PubMed] [Google Scholar]
- 18.Dolai S, Liang T, Lam PP, Fernandez NA, Chidambaram S, Gaisano HY. Effects of ethanol metabolites on exocytosis of pancreatic acinar cells in rats. Gastroenterology. 2012;143:832–843. doi: 10.1053/j.gastro.2012.06.011. [DOI] [PubMed] [Google Scholar]


