Abstract
Background and Aims: Several existing studies indicated that nonalcoholic fatty liver disease (NAFLD) may be associated with colorectal adenoma, but the results and risk factors are controversial. A systematic review of studies was conducted to explore these issues by meta-analysis. Methods: We searched the Pubmed, Embase, Cochrane library, Medline and Web of Science databases for studies published before May 30th, 2014. A statistical analysis was performed using RevMan 5.2 software. Results: Seven studies involving 11,905 participants from different regions were included. Among them, five trials carried out subgroup of NAFLD patients in colorectal adenoma population. The result showed NAFLD was significantly correlated with adenoma of colon (Odds ratio [OR] = 1.56, 95% confidence interval [CI]: 1.22-1.99, P = 0.0003). It could be found in stratified analysis that patients had more chance to get multiple adenomas when they suffered NAFLD (Rate ratio [RR]: 1.52, 95% CI: 1.08-2.13, P = 0.02). Such risk factors of NAFLD as age, waist circumference, body mass index (BMI), disorder of lipid metabolism, hyperglycemia and high blood pressure (HBP) increased risk of colorectal adenoma (Age: mean difference [MD]: 2.81, 95% CI: 0.33-5.28; Waist: MD: 2.84, 95% CI: 2.14-3.54; BMI: MD: 0.85, 95% CI: 0.69-1.01; High-density lipoprotein: MD: -2.46, 95% CI: -3.68 to -1.24; Triglyceride: MD: 16.12, 95% CI: 8.89-23.36; Low-density lipoprotein: MD: 6.04, 95% CI: 3.60-8.48; Cholesterol: MD: 4.25, 95% CI: 0.87-7.63; Fasting glucose: MD: 2.27, 95% CI: 1.24-3.30; HBP: OR = 1.51, 95% CI: 1.22-1.88), while diabetes had no significant association with it (OR = 1.43, 95% CI: 0.94-2.17, P = 0.09). Besides, NAFLD didn’t affect the location, size and advanced type of colorectal adenoma (P > 0.05). Conclusion: The present systematic review and meta-analysis demonstrated NAFLD was closely associated with great risk of colorectal adenoma and its number, but not with its location, size and advanced type. Waist, obesity, lipid profiles, glucose, hypertension played roles in the process of colorectal adenoma.
Keywords: Nonalcoholic fatty liver disease, colorectal adenoma, risk factors, meta-analysis
Introduction
Nonalcoholic fatty liver disease (NAFLD) is the commonest chronic liver disease in Western population and becomes a burgeoning health problem of developing countries due to high prevalence [1]. It represents a spectrum of diseases associated with excessive fat accumulation in the liver in the absence of excessive alcohol consumption. NAFLD ranges from simple steatosis to nonalcoholic steatohepatitis (NASH), through to advanced fibrosis, cirrhosis and even hepatocellular carcinoma [2]. The underlying mechanisms of disease progression are poorly understood. The classical supporting theory is the “multi-hit hypothesis”, in which insulin resistance (IR) and oxidative stress play an important role. And then, mitochondrial fatty acids oxidation, nuclear-factor-kappaB (NFκB)-dependent inflammatory cytokine expression as well as adipocytokines are lead to dysregultion, resulting hepatocellular damage, inflammation, fibrosis and progressive liver disease [3]. Generally, obesity, diabetes (DM), and hyperlipidemia are regarded as common risk factors for acquiring NAFLD [4]. Besides, the gradual shift of high blood pressure (HBP) is likely to increase the prevalence of NAFLD [5]. NAFLD is also associated with greater waist circumference, mainly dependent on dietary and exercise [6].
Liver has close relationship with intestine for the same origin in embryology. Colorectal cancer (CRC) is one of the commonest cancers worldwide and the second of cancer deaths [7]. It established risk factors include increased age, black race, smoking and low-fiber diet. Given the shared features between NAFLD and CRC, it becomes hot research interest whether NAFLD is an independent risk factor for increased colon events. In fact, recent clinical studies have already found NAFLD patients had a higher prevalence of colorectal adenoma and advanced neoplasm, and then developing into colorectal cancer (adenoma-carcinoma sequence) if untreated [8,9]. Suggestions from data now point out NAFLD might be a potential risk factor. However, this point is still controversial [10].
Therefore, the aim of this study was to conduct a meta-analysis of the pooled data from the existing clinical studies to assess the relationship between NAFLD and colorectal adenoma.
Materials and methods
Search strategy
A comprehensive, computerized literature search was conducted in Pubmed, Embase, Cochrane library, Medline and Web of Science from the beginning of indexing for each database to May 30th, 2014, by two independent investigators (D.W.J. and Q.J.J.). The conference proceedings and reference lists of reviews were searched manually for additional relevant studies. Search items included “NAFLD” or “NASH” or “nonalcoholic steatohepatitis” or “nonalcoholic fatty liver disease” or “fatty liver” and “colorectal adenoma” or “colonic adenoma” or “colorectal neoplasia” or “colorectal neoplasm” or “colorectal malignant neoplasm” or “colorectal cancer” or “CRC” or “adenoma of colon”. No language restrictions were imposed.
Inclusion and exclusion criteria
Three investigators (D.W.J., F.J.G. and Q.J.J.) determined the inclusion and exclusion criteria, and reviewed the titles and abstracts of the studies identified. Inclusion criteria were: (a) published as an original article; (b) used cohort or cross-sectional design; (c) Random controlled trials (RCTs) with participants of any sex or ethnic origin with colorectal adenoma on the basis of histological evidence, and with NAFLD/NASH diagnosed by imaging examination or histology; (d) had objective outcome measures, at least one of the following items: body mass index (BMI), waist circumference, HBP, fasting plasma glucose (FPG), alanine aminotransferase (ALT), aspartate transaminase (AST), cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglycerides and DM; or included characteristics of colorectal adenoma, like location, size, number and histological type. Exclusion criteria were as follows: (a) non-human studies or non-randomized trials; (b) other causes of fatty liver disease such as viral, alcoholic, drug-induced, autoimmune and genetic liver injury; (c) patients suffered colorectal cancer before trials. Discrepancies between three reviewers were solved by discussion.
Definition
Locations of colorectal adenoma were categorized as proximal colon (including the cecum, ascending colon, or transverse colon) and/or distal colon (including the splenic flexure, descending colon, sigmoid colon, or rectum). Histopathologically, colorectal adenoma referred to an adenoma in the colorectum regardless of grading or amount of villous component. Hyperplastic and inflammatory polyps were excluded. Advanced adenoma was defined as adenoma with high-grade dysplasia or containing > 25% villous features.
Data extraction and methodological quality
Data were abstracted independently by two reviewers and included: author, publication year, country, participants and subgroup, study design and outcomes. The quality of the studies was assessed by Newcastle-Ottawa scale (NOS) score, of which 1-3 for low-quality, 4-6 for intermediate and 7-9 for high-quality. All included studies scored ≥ 7.
Statistical analysis
The analyses were conducted using the Review Manager (RevMan Version 5.2). Some outcomes (HBP, NAFLD, DM, BMI etc.) were assessed as a dichotomous variable (presented as odds ratio [OR] with 95% confidence interval [CI]). Other outcomes like ALT, AST, HDL and LDL etc. were presented as continuous variables (mean difference [MD] with 95% CI). Subgroup analyses of association of colorectal adenoma with NAFLD were calculated by RR (rate ratio) on lesion location, size, number and type. The preferred method of data presentation was the calculated RR compared with the general population. Mantel-Haenszel chi-square tests were used to determine significant level of difference. If the chi-square test was significant below P = 0.05, the amount of heterogeneity using I2 statistics was quantified. If there was obvious heterogeneity (over 50%), the random effects model was chosen; Otherwise, the fixed effects model was adopted.
Results
Search results
The literature search yielded a total of 219 potentially publications (Figure 1). Full text articles were retrieved only for 66 publications and assessed for eligibility. Among these 66 publications, 59 were excluded because they did not address people with NAFLD, or not assess the association between colorectal adenoma and NAFLD, or duplicated. Overall, we identified and included 7 publications that met the inclusion criteria in the systematic review [8,9,11-15]. Among them, 6 articles showed basic data and risk factors related to NAFLD of individuals between colorectal adenoma and non-adenoma group. 5 of 7 studies recorded characteristics of colorectal adenoma among NAFLD patients.
Figure 1.
Flow diagram of study identification.
Characteristics of the studies
The main adjustments of the studies included in this analysis are provided in Table 1. Among them, one study was originated from Austria, three from China (different regions) and three from Korea (conducted by different research groups), with a total of 11,905 participants. According to the NOS score, all seven studies were of high quality.
Table 1.
Characteristics of Studies in Meta-analysis
| Study author | Region/Country | Participants and subgroup (number of cases) | Study design | Diagnostic method of fatty liver | Adjustments | NOS score | ||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Adenoma/nonadenoma group | NAFLD/non-NAFLD group of colorectal adenoma population | Basic data | Characteristics of colorectal adenoma | |||||
| Huang et al, 2013 | Taipei/China | 1522 (216/1306) | 216 (120/96) | Cohort | Ultrasonography | Gender, age, BMI, waist, FPG, ALT, AST, cholesterol, HDL, LDL, triglycerides, HBP, DM, smoking, NAFLD | Location, size, number, histological type | 8 |
| Hwang et al, 2010 | Seoul/Korea | 2917 (556/2361) | 556 (231/325) | Cross-sectional | Ultrasonography | Gender, age, BMI, waist, HBP, FPG, ALT, AST, cholesterol, HDL, LDL, triglycerides, DM, smoking, NAFLD | Location, size, number, histological type | 8 |
| Kang et al, 2010 | Seoul/Korea | 2244 (1122/1122) | NA | Cross-sectional | NA | Age, gender, smoking, DM, HBP, BMI, waist, cholesterol, triglycerides, HDL, FPG | NA | 7 |
| Kim et al, 2010 | Seoul/Korea | 1316 (263/1053) | NA | Cross-sectional | NA | Gender, age, smoking, DM, BMI, HBP, FPG, cholesterol, triglycerides, HDL, LDL | NA | 8 |
| Lin et al, 2014 | Wenzhou/China | 2315 (1946/369) | 1946 (216/1730) | Cohort | Ultrasonography | FPG, BMI, HDL, HBP, TC | Histological type | 7 |
| Stadlmayr et al, 2011 | Oberndorf/Austria | 1211 (341/870) | 331 (215/126) | Cross-sectional | Ultrasonography | Age, BMI, waist, cholesterol, triglycerides, HDL, FPG, AST, ALT | Location, size, histological type | 8 |
| Wong et al, 2011 | Hongkong/China | NA | 380 (199/181) | Cohort | Histology | Age, gender, smoking, BMI, waist, FPG, cholesterol, triglycerides, HDL, LDL, AST, ALT, DM, HBP | Location, histological type | 7 |
NAFLD and colorectal adenoma
Five studies recorded on NAFLD with 9281 participants totally in trials. Random effects model was used because of high heterogeneity (I2 = 76%). A statistically significant association was observed between NAFLD and colorectal adenoma. OR was 1.56 (95% CI: 1.22-1.99, P = 0.0003) (Figure 2A). Only two studies showed the activities of liver enzymes (ALT and AST) in the included analysis. Heterogeneity was low (I2: 0% and 2%, respectively). Modest but statistically significant elevation was observed in colorectal adenoma group (ALT: MD: 3.48, 95% CI: 2.07-4.88, P < 0.00001; AST: MD: 1.30, 95% CI: 0.55-2.05, P = 0.0007) (Figure 2B).
Figure 2.
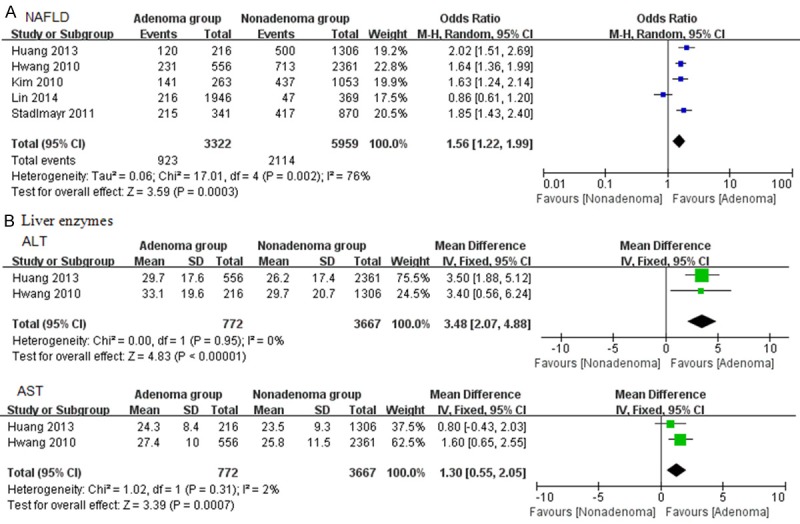
Forrest plot of NAFLD and colorectal adenoma. A. NAFLD; B. Liver enzymes.
Risk factors of NAFLD and colorectal adenoma
Basic data
Three RCTs were analyzed the effect of age on colorectal adenoma, and showed difference in the experiments group compared with control group. (MD: 2.81, 95% CI: 0.33-5.28, I2: 96%, P: 0.03) (Figure 3A). Three RCTs provided sufficient data of waist circumference. As shown in Figure 3B, the length of waist had a significant elevation between these two groups (MD: 2.84, 95% CI: 2.14-3.54, I2: 53%, P < 0.00001). Five studies provided gender and BMI information of the participants. Significant difference was found on gender with high heterogeneity (OR: 1.85, 95% CI: 1.17-2.35, I2: 90%, P = 0.005) (Figure 3C). Three research papers recorded BMI as mean ± SD, while others were in the forms of “BMI ≥ 25 kg/m2”. Therefore, they were analyzed in the subgroup, and found high BMI had an obvious effect during the process of colorectal adenoma (MD: 0.85, 95% CI: 0.69-1.01, I2: 26, P < 0.00001; OR = 1.34, 95% CI: 1.07-1.66, I2: 0%, P = 0.009) (Figure 3D).
Figure 3.
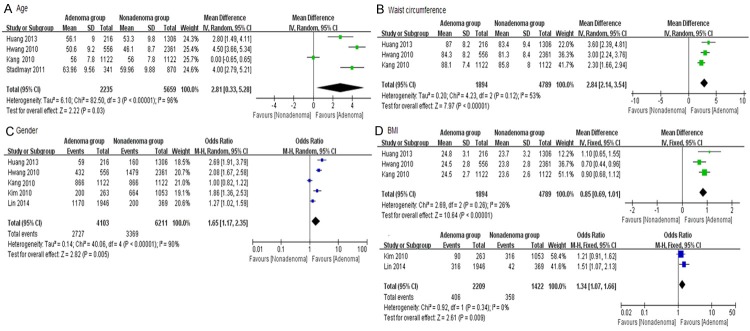
Forrest plot of basic data of colorectal adenoma patients. A. Age; B. Waist circumference; C. Gender; D. BMI.
Lipid profiles
HDL and triglyceride were reported in six studies. However, they were assessed by different forms (“mean ± SD” in three articles and “Yes/No” in others). In order to get accurate results, we analysis them in subgroup by different methods. Overall, colorectal adenoma patients showed obvious reduction in HDL (MD: -2.46, 95% CI: -3.68 to -1.24, I2: 64%, P < 0.0001) and increase in serum triglyceride (MD: 16.12, 95% CI: 8.89-23.36, I2: 63%, P < 0.0001), compared with the control group. The patients with low plasma HDL (≤ 1.03 mmol/L for men or ≤ 1.29 mmol/L for women) or hypertriglyceridemia (≥ 1.7 mmol/L) seemed to get more chance of colorectal adenoma than others. OR was 1.33 of HDL (95% CI: 1.13-1.58, I2: 0%, P = 0.0008) and 1.67 of triglyceride (95% CI: 1.67-2.55, I2: 83%, P = 0.02) (Figure 4A, 4B). Two RCTs provided LDL data and three recorded serum cholesterol. Colorectal adenoma was significantly related to increasing LDL and cholesterol (MD: 6.04, 95% CI: 3.60-8.48, I2: 0%, P < 0.00001; MD: 4.25, 95% CI: 0.87-7.63, I2: 64%, P = 0.01; respectively) (Figure 4C, 4D).
Figure 4.
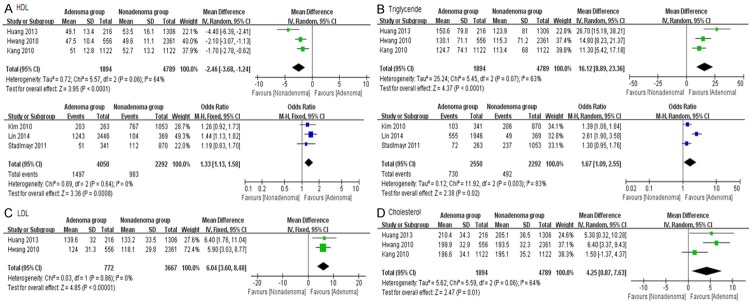
Forrest plot of lipid profiles of colorectal adenoma patients. A. HDL; B. Triglyceride; C. LDL; D. Cholesterol.
Glucose and HBP
FPG was reported in six studies in different ways (three in “mean ± SD” [8,9,11], two in “FPG ≥ 5.6 mmol/l” [12,13] and one “FPG ≥ 6.1 mmol/l” [14]). The one in “FPG ≥ 6.1 mmol/l” was excluded and subgroup analysis showed significant increased FPG in the experimental group (MD: 2.27, 95% CI: 1.24-3.30, I2: 49%, P < 0.0001). OR was 1.31 (95% CI: 1.13-1.61, I2: 0%, P = 0.0009). The included studies were homogeneous (Figure 5A). However, diabetes reported in four trials had no significant relation to colorectal adenoma (OR = 1.43, 95% CI: 0.94-2.17, P = 0.09) with high heterogeneity (I2: 77%) (Figure 5B). Among the seven studies, six provided the number of HBP patients, which showed a great difference in experiment group compared to control one. OR was 1.51 (95% CI: 1.22-1.88, I2: 78%, P = 0.0002) (Figure 5C).
Figure 5.
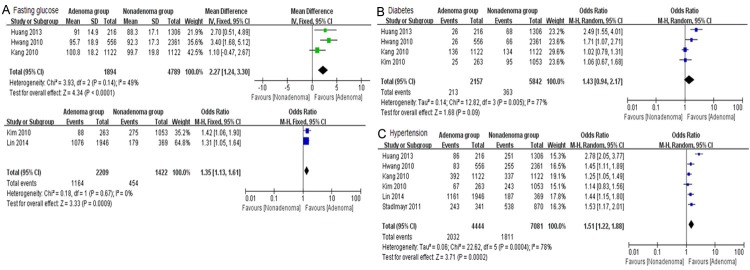
Forrest plot of glucose and hypertension of colorectal adenoma patients. A. Fasting glucose; B. Diabetes; C. Hypertension.
Characteristics of colorectal adenoma and NAFLD
When combining the results on studies of the population with colorectal adenoma, the RR of adenoma number was much stronger in NAFLD patients than in non-NAFLD population (I2: 0%, P = 0.02). Further analysis found that NAFLD patients had a higher risk to get multiple adenomas of colon (n ≥ 3) (RR: 1.52, 95% CI: 1.08-2.13, P = 0.02). However, the results showed no significant association between NAFLD and location/size of colorectal adenoma (Location: distal: RR: 0.90, 95% CI: 0.75-1.07, P = 0.24, proximal: RR: 1.10, 95% CI: 0.92-1.31, P = 0.29; Size: < 10 mm: RR: 1.0, 95% CI: 0.97-1.04, P = 0.98, ≥ 10 mm: RR: 0.99, 95% CI: 0.56-1.75, P = 0.98). Besides, NAFLD patients had a similar chance to get advanced adenoma in colorectal adenoma population (RR: 0.99, 95% CI: 0.92-1.06, I2: 81%, P = 0.83) (Table 2).
Table 2.
Stratified Analysis of Characteristics of Colorectal Adenoma in NAFLD Patients
| Characteristic | n of studies | References | RR (95% CI) | Heterogeneity | P difference | |
|---|---|---|---|---|---|---|
|
| ||||||
| P value | I2 (%) | |||||
| Location | 4 | Huang, Hwang | ||||
| Distal | St, Wong | 0.90 [0.75, 1.07] | 0.05 | 61 | 0.24 | |
| Proximal | 1.10 [0.92, 1.31] | 0.07 | 58 | 0.29 | ||
| Size | 2 | Huang, Hwang | ||||
| < 10 mm | 1.0 [0.97, 1.04] | 0.19 | 41 | 0.98 | ||
| ≥ 10 mm | 0.99 [0.56,1.75] | 0.23 | 32 | 0.98 | ||
| Number | 3 | Huang, Hwang | ||||
| < 3 | St | 0.95 [0.91, 0.99] | 0.91 | 0 | 0.02 | |
| ≥ 3 | 1.52 [1.08, 2.13] | 0.54 | 0 | 0.02 | ||
| Advanced adenoma | 5 | Huang, Hwang | ||||
| No | St, Wong, | 1.12 [0.71, 1.76] | 0.003 | 75 | 0.64 | |
| Yes | Lin | 0.99 [0.92, 1.06] | 0.0004 | 81 | 0.83 | |
Discussion
NAFLD is a popular issue in public health due to its epidemiologic burden. It is now recognized to represent the hepatic manifestation of the metabolism syndromes, which is closely associated with obesity, hyperlipidemia, hyperglycemia, and lifestyle such as dietary and exercises. It is well known there is strong relationship between the intestine and liver [16]. Not only they have the same origin in embryology the foregut, but also the liver continuously receives intestinal blood through the portal system. Several existing studies have demonstrated that the patients with NAFLD have higher rates of prevalent colonic diseases than their counterparts without NAFLD [8,9,11-15,17], though NAFLD has no influence on the prognosis in CRC patients [18]. In addition, modulation of gut microbiota could reduce clinical symptoms of NAFLD [16,19,20]. As mentioned above, the well-known risk factors of CRC, a common cancer in the world, are high-fat, low-fiber intake, less physical activity, alcoholic drinking and a family history of CRC21. Interestingly, it shared several aforementioned risks of NAFLD. Colorectal adenoma is recognized as a precursor of CRC through the adenoma-carcinoma sequence [22,23]. It is necessary to detect and treat colorectal adenoma, and then CRC could be prevented as early as possible.
To provide a objective basis for clinical recommendations, a meta-analysis was conducted, which recruited a total of 11,905 individuals from four cross-sectional and three cohort studies. To our knowledge, this is the first meta-analysis on this topic to assess the association between NAFLD and colorectal adenoma. Using the NOS, it could be found that seven studies included in this meta-analysis were of high quality. NAFLD was a predictor of colorectal adenoma with OR of 1.56 (95% CI: 1.22-1.99, P = 0.0003). Its relevant conditions (overweight, impaired fasting glucose, hyperlipidemia and hypertension) increased the risk of colorectal adenoma (P < 0.05). Besides, elevated ALT and AST reflecting the severity of liver injury were found to be associated with colorectal adenoma in NAFLD patients (P < 0.05).
In our meta-analysis, some clinical studies in this field were excluded due to the different way of group division. Actually, most of them shared similar opinions. Kim et al. [24] detected fasting serum insulin and homeostasis model assessment (HOMA)-IR of 3,606 participants with histologically confirmed colorectal adenoma and 6,019 controls with no abnormal findings on colonoscopy. They confirmed fasting serum insulin and HOMA-IR were significantly higher in colorectal adenoma population compared with controls. Multivariate regression analysis was used and revealed the experimental participants with higher quartiles of fasting serum insulin levels (P < 0.05) as well as HOMA-IR (P < 0.05). A retrospective cohort study of 375 patients undergoing index colonoscopy was conducted in the United States to determine the association between DM and colorectal adenoma [25]. The result showed colorectal adenoma was higher in those ages 40-49 years with DM than that of the participants at the same age but without DM (OR = 3.1; 95% CI: 1.5-6.4; P = 0.002). Besides, obesity-related disorders were also ascertained as a direct and independent risk for colorectal events [26]. In contrast, Touzin’s publication has yielded diverse result [10]. After performing a retrospective cohort observational study on 233 patients, they found no significant increase in incidence of colorectal adenomas in NASH patients.
The underlying mechanism of “NAFLD-colorectal adenoma relationship” was complex and still unclear. One of the possibilities is considered to be the growth promoting effects of adipokines [27]. Leptin expression, decreased in liver tissues of NAFLD individual [28], was more frequently observed in colonic adenomas, especially in larger adenocarcinoma in situ, which might affect colonic tumorigenesis and progression, especially to obese patients.
However, the present meta-analysis has several limitations. First, only one study diagnosed NAFLD by histology, the others were based on ultrasonography and the exclusion of known causes of chronic liver disease. Although the gold standard for NAFLD evaluation remains liver biopsy, it is difficult to carry out invasive operation in large populations. Ultrasound and computed tomography are the commonest ways in clinical practice due to certain sensitivity and specificity in detecting steatosis [29,30]. Second, NAFLD ranges from simple steatosis to NASH. The latter is related to fibrosis, cirrhosis and even hepatocellular carcinoma [31,32]. In the present meta-analysis, NAFLD histological subtypes were not taken into account. Despite these limitations, the present meta-analysis also has notable strengths. Firstly, pooling data from a number of clinical trials were obtained. To some indexes recorded by different ways (“mean ± SD” or “Yes/No”), we assessed both by Revman 5.2. Therefore, statistical power of the analysis was more accurate compared with a single study. Secondly, we not only analyzed the association among NAFLD, its risk factors and colorectal adenoma, but also studied the effect of NAFLD on the characteristics of colorectal adenoma. Thirdly, all studies in this meta-analysis scored 7 or more by NOS, which meant they were of high quality. Last but not least, seven studies originated from seven different research groups in five regions and a variety of ethnic background was included.
In conclusion, the present systematic review and meta-analysis revealed that NAFLD is significantly associated with increased risk of colorectal adenoma, especially with its number. These two diseases shared the common risks like obesity (BMI ≥ 25 kg/m2), dysfunction of lipid profiles and HBP. Although there is no significant association between DM and colorectal adenoma in the present meta-analysis, it could be found hyperglycemia (FPG ≥ 5.6 mmol/l) patients got more chance of colorectal adenoma. Therefore, once NAFLD is diagnosed, the individual colorectal risk factor profile should be reviewed and modified appropriately.
Acknowledgements
Supported by National Natural Science Foundation of China (No. 81400610, No. 51408438); The Project-sponsored by SRF for ROCS, SEM; Shanghai Pujiang Program (No. 14PJ1408300); Program of Shanghai Municipal Commission of Health and Family Planning for Youth (No. 20134Y043) and Li Jieshou Academician Foundation (No. LJS_201319).
Disclosure of conflict of interest
N/A.
Abbreviations
- ALT
alanine aminotransferase
- AST
aspartate transaminase
- BMI
body mass index
- CI
confidence interval
- DM
diabetes
- FPG
fasting plasma glucose
- HBP
high blood pressure or hypertension
- HDL
high-density lipoprotein
- LDL
low-density lipoprotein
- MD
mean difference
- NAFLD
nonalcoholic fatty liver disease
- NOS
Newcastle-Ottawa Scale
- OR
odds ratio
- RR
rate ratio
References
- 1.Chalasani N, Younossi Z, Lavine JE, Dieh AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ American Association for the Study of Liver Diseases; American College of Gastroenterology; American Gastroenterological Association. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142:1592–609. doi: 10.1053/j.gastro.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Pan JJ, Fallon MB. Gender and racial differences in nonalcoholic fatty liver disease. World J Hepatol. 2014;6:274–283. doi: 10.4254/wjh.v6.i5.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polyzos SA, Kountouras J, Zavos C. Nonalcoholic fatty liver disease: the pathogenetic roles of insulin resistance and adipocytokines. Curr Mol Med. 2009;9:299–314. doi: 10.2174/156652409787847191. [DOI] [PubMed] [Google Scholar]
- 4.Hui E, Xu A, Bo Yang H, Lam KS. Obesity as the common soil of non-alcoholic fatty liver disease and diabetes: Role of adipokines. J Diabetes Investig. 2013;4:413–425. doi: 10.1111/jdi.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryoo JH, Suh YJ, Shin HC, Cho YK, Choi JM, Park SK. Clinical association between non-alcoholic fatty Liver disease and the development of hypertension. J Gastroenterol Hepatol. 2014 doi: 10.1111/jgh.12643. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 6.Kargulewicz A, Stankowiak-Kulpa H, Grzymisławski M. Dietary recommendations for patients with nonalcoholic fatty liver disease. Prz Gastroenterol. 2014;9:18–23. doi: 10.5114/pg.2014.40845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sawbridge D, Probert C. Population-based screening in colorectal cancer - current practice and future developments: faecal biomarkers review. J Gastrointestin Liver Dis. 2014;23:195–202. doi: 10.15403/jgld.2014.1121.232.dsw1. [DOI] [PubMed] [Google Scholar]
- 8.Huang KW, Leu HB, Wang YJ, Luo JC, Lin HC, Lee FY, Chan WL, Lin JK, Chang FY. Patients with nonalcoholic fatty liver disease have higher risk of colorectal adenoma after negative baseline colonoscopy. Colorectal Dis. 2013;15:830–835. doi: 10.1111/codi.12172. [DOI] [PubMed] [Google Scholar]
- 9.Hwang ST, Cho YK, Park JH, Kim HJ, Park DI, Sohn CI, Jeon WK, Kim BI, Won KH, Jin W. Relationship of non-alcoholic fatty liver disease to colorectal adenomatous polyps. J Gastroenterol Hepatol. 2010;25:562–567. doi: 10.1111/j.1440-1746.2009.06117.x. [DOI] [PubMed] [Google Scholar]
- 10.Touzin NT, Bush KN, Williams CD, Harrsion SA. Prevalence of colonic adenomas in patients with nonalcoholic fatty liver disease. Therap Adv Gastroenterol. 2011;4:169–176. doi: 10.1177/1756283X11402118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kang HW, Kim D, Kim HJ, Kim CH, Kim YS, Park MJ, Kim JS, Cho SH, Sung MW, Jung HC, Lee HS, Song IS. Visceral obesity and insulin resistance as risk factors for colorectal adenoma: a cross-sectional, case-control study. Am J Gastroenterol. 2010;105:178–187. doi: 10.1038/ajg.2009.541. [DOI] [PubMed] [Google Scholar]
- 12.Kim KS, Moon HJ, Choi CH, Baek EK, Lee SY, Cha BK, Lee HW, Kim HJ, Do JH, Chang SK. The Frequency and Risk Factors of Colorectal Adenoma in Health-Check-up Subjects in South Korea: Relationship to Abdominal Obesity and Age. Gut Liver. 2010;4:36–42. doi: 10.5009/gnl.2010.4.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin XF, Shi KQ, You J, Liu WY, Luo YW, Wu FL, Chen YP, Wong DK, Yuen MF, Zheng MH. Increased risk of colorectal malignant neoplasm in patients with nonalcoholic fatty liver disease: a large study. Mol Biol Rep. 2014;41:2989–2997. doi: 10.1007/s11033-014-3157-y. [DOI] [PubMed] [Google Scholar]
- 14.Stadlmayr A, Aigner E, Steger B, Scharinger L, Lederer D, Mayr A, Strasser M, Brunner E, Heuberger A, Hohla F, Steinwendner J, Patsch W, Datz C. Nonalcoholic fatty liver disease: an indepent risk factor for colorectal neoplasia. J Intern Med. 2011;270:41–49. doi: 10.1111/j.1365-2796.2011.02377.x. [DOI] [PubMed] [Google Scholar]
- 15.Wong VW, Wong GL, Tsang SW, Fan T, Chu WC, Woo J, Chan AW, Choi PC, Chim AM, Lau JY, Chan FK, Sung JJ, Chan HL. High prevalence of colorectal neoplasm in patients with non-alcoholic steatohepatitis. Gut. 2011;60:829–836. doi: 10.1136/gut.2011.237974. [DOI] [PubMed] [Google Scholar]
- 16.Miele L, Marrone G, Lauritano C, Cefalo C, Gasbarrini A, Day C, Grieco A. Gut-liver axis and microbiota in NAFLD: insight pathophysiology for novel therapeutic target. Curr Pharm Des. 2013;19:5314–5324. [PubMed] [Google Scholar]
- 17.Muhidin SO, MAgan AA, Osman KA, Syed S, Ahmed MH. The relationship between nonalcoholic fatty liver disease and colorectal cancer: the future challenges and outcomes of the metabolic syndrome. J Obes. 2012;2012:637538. doi: 10.1155/2012/637538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Min YW, Yun HS, Chang WI, Kim JY, Kim YH, Son HJ, Kim JJ, Rhee JC, Chang DK. Influence of non-alcoholic fatty liver disease on the prognosis in patients with colorectal cancer. Clin Res Hepatol Gastroenterol. 2012;36:78–83. doi: 10.1016/j.clinre.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 19.Eslamparast T, Eghtesad S, Hekmatdoost A, Poustchi H. Probiotics and Nonalcoholic Fatty liver Disease. Middle East J Dig Dis. 2013;5:129–136. [PMC free article] [PubMed] [Google Scholar]
- 20.Mouzaki M, Comelli EM, Arendt BM, Bonengel J, Fung SK, Fischer SE, McGilvray ID, Allard JP. Intestinal microbiota in patients with nonalcoholic fatty liver disease. Hepatology. 2013;58:120–127. doi: 10.1002/hep.26319. [DOI] [PubMed] [Google Scholar]
- 21.Zhu JZ, Wang YM, Zhou QY, Zhu KF, Yu CH, Li YM. Systematic review with meta-analysis: alcohol consumption and the risk of colorectal adenoma. Aliment Pharmacol Ther. 2014 doi: 10.1111/apt.12841. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.Yamada A, Minamiguchi S, Sakai Y, Horimatsu T, Muto M, Chiba T, Boland CR, Goel A. Colorectal Advanced Neoplasms Occur through Dual Carcinogenesis Pathways in Individuals with Coexisting Serrated Polyps. PLoS One. 2014;9:e98059. doi: 10.1371/journal.pone.0098059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alecu M, Simion L, Straja N, Brătucu E. Multiple Polyps and Colorectal Cancer. Chirurgia (Bucur) 2014;109:342–346. [PubMed] [Google Scholar]
- 24.Kim EH, Kim HK, Bae SJ, Chang HS, Park HW, Do MY, Kim KJ, Jung CH, Lee WJ, Park JY, Choe J. Fasting serum insulin levels and insulin resistance are associated with colorectal adenoma in Koreans. J Diabetes Investig. 2014;5:297–304. doi: 10.1111/jdi.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vu HT, Ufere N, Yan Y, Wang JS, Early DS, Elwing JE. Diabetes mellitus increases risk for colorectal adenomas in younger patients. World J Gastroenterol. 2014;20:6946–6952. doi: 10.3748/wjg.v20.i22.6946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riondino S, Roselli M, Palmirotta R, Della-Morte D, Ferroni P, Guadagni F. Obesity and colorectal cancer: role of adipokines in tumor initiation and progression. World J Gastroenterol. 2014;20:5177–5190. doi: 10.3748/wjg.v20.i18.5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oh JS, Kim HH, Hwang HS, Yun DY, Kim BS, Lee CH, Han J, Kim HG, Jung JT, Kwon JG, Kim EY. Comparison of blood leptin concentration and colonic mucosa leptin expression in colon adenoma patients and healthy control. Korean J Gastroenterol. 2014;63:354–360. doi: 10.4166/kjg.2014.63.6.354. [DOI] [PubMed] [Google Scholar]
- 28.Ding WJ, Wang Y, Fan JG. Regulation of adipokines by polyunsaturated fatty acids in a rat model of non-alcoholic steatohepatitis. Arch Iran Med. 2014;17:563–567. [PubMed] [Google Scholar]
- 29.Bora A, Alptekin C, Yavuz A, Batur A, Akdemir Z, Berköz M. Assessment of liver volume with computed tomography and comparison of findings with ultrasonography. Abdom Imaging. 2014 doi: 10.1007/s00261-014-0146-5. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 30.Khov N, Sharma A, Riley TR. Bedside ultrasound in the diagnosis of nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20:6821–6825. doi: 10.3748/wjg.v20.i22.6821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amarapurkar DN, Dharod M, Gautam S, Patel N. Risk of development of hepatocellular carcinoma in patients with NASH-related cirrhosis. Trop Gastroenterol. 2013;34:159–63. doi: 10.7869/tg.120. [DOI] [PubMed] [Google Scholar]
- 32.Shibahara J, Ando S, Sakamoto Y, Kokudo N, Fukayama M. Hepatocellular carcinoma with steatohepatitic features: a clinicopathological study of Japanese patients. Histopathology. 2014;64:951–962. doi: 10.1111/his.12343. [DOI] [PubMed] [Google Scholar]


