Abstract
Studies have shown that the development of breast cancer (BC) is a multi-step process that occurs sequentially from normal to usual hyperplasia, atypical hyperplasia, carcinoma in situ, and finally the invasive stages of carcinoma. Our study investigated the histopathological alterations in breast tissue in a Sprague-Dawley (SD) rat model induced by 7,12-Dimethylbenz (a) anthracene (DMBA) and estrogen-progestogen (E-P). Fifty rats were randomly divided into five groups (n = 10 each) and administered the E-P/DMBA combination. After the induction of BC, breast tissue samples were obtained from the rats and stained with hematoxylin-eosin (HE). Breast tissues from 10 rats and 10 human patients were obtained for comparison. The expression of P63, CK5/6 and CK34βE12 was observed and analyzed using the SPSS 17.0 software. The HE results showed ductal epithelial hyperplasia with forming a second lumen or papillary structure, atypical hyperplasia with atypical proliferative cells, forming a cross-bridge or cribriform structure in breast tissues from the rats samples. The IHC results showed that the expression of P63 was not significantly different between rat and human breast tissue (P > 0.05), but its expression in rat and human tissue was significantly different between UDH, ADH, DCIS and IDC (P < 0.01). A similar trend was observed for the expression of CK5/6 and CK34βE12 too. Thus, the findings in this model may reflect the histopathological changes that occur during the progression of human BC. Therefore, this model could be used for the establishment of BC models to investigate the prevention and treatment of BC.
Keywords: Breast cancer, rat model, 7, 12-Dimethylbenz (a) anthracene, estrogen, progestogen
Introduction
Breast cancer (BC) is one of the most common malignancies that affect women worldwide, and its morbidity has been increasing every year. Recently, more attention has been directed towards breast cancer prevention. There are several well-established experimental animal models of BC that provide a wide range of options for investigating the environmental and genetic factors associated with BC and the therapeutic methods. These models can be separated into induced and transgenic models, and they vary with regard to their suitability for investigating each of these factors. Using a rat BC model induced by chemicals, Singh et al. [1] concluded that the histopathological changes that occur in BC in rats are similar to those in human beings.
Numerous factors and molecular and cellular pathways are considered to be involved in the development and carcinogenesis of the mammary gland [2,3]. 7,12-Dimethylbenz (a) anthracene (DMBA), a highly toxic chemical, is one of these ecological factors, and it is frequently used to induce tumors in animal experiments. Researchers have concluded that it is a potential carcinogen and could act at numerous sites, including the skin [4], mammary gland [5], oral cavity [6] and also the pancreas [7]. It is a precarcinogen that unlike other carcinogens such as N-nitrosomethylurea [8] can be converted into the ultimate carcinogenic metabolite DMBA-3,4-dihydrodiol-1,2-epoxide [9]. Similar to the structure of the human breast, the rat mammary gland is also believed to have a terminal duct-lobular unit (TDLU) and is comprised of a terminal duct and lobules. The model induced by DMBA closely resembles human BC originating from TDLU, and the histopathological changes and hyperplastic progression to form premalignant and then malignant lesions are alike in many respects to those of human BC [10]. Thus, DMBA-induced mammary gland tumors in rats are a useful tool for investigating the mechanisms of pathogenesis and development of human BC and its prevention.
The mammary gland is a hormone-dependent organ that is affected by steroid hormones secreted by the ovaries. Ohi [11] found that BC is induced by DMBA under hormonal conditions in which suitable levels of estrogen are present. They also proposed that the growth of DMBA-induced BC in rats is accelerated by additional administration of progestogen. Numerous studies have shown that estrogen could stimulate ductal growth, epithelial hyperplasia, and the development of milk ducts and lobules surrounded by connective tissue, and increase the number of progestogen receptors in breast cells to promote lobular acinar development. The principal role of progestogen is to facilitate the complete development of breast lobules and acini, based on estrogen stimulation of breast ductal tissue [12]. During puberty, estrogen directly stimulates duct development, while progestogen promotes epithelial cell DNA synthesis and acinar development [13].
Although there have been various BC models induced by DMBA in rats, so far, the histomorphological changes in these models induced by DMBA have not been studied too well. Herein, we studied the histomorphological changes in rat breast tissue induced by DMBA and estrogen-progestogen combinations, in order to understand whether the development of BC in rats is similar to that in human beings, and provide a pathological standard for the establishment of BC models.
Materials and methods
Animals and treatments
Fifty Sprague-Dawley (SD) non-inbred female rats (weight, 40-50 g; age, 21 days) with a 5-day menstrual cycle were bought from the Shandong University Experimental Animal Center. To prepare the DMBA (Sigma Chemical Co., St. Louis, MO) solution, 1 g DMBA was dissolved in 100 ml of olive oil. The end concentration was 10 mg/ml (dissolved in olive oil at a dosing concentration of 12 ± 20 mg in 1 ml). All the procedures were in accordance with the guidelines of the Bioethics Committee of Shandong University. Paraffin-embedded breast tissue from 10 female patients was collected from the Department of Pathology, Affiliated Hospital of Shandong Academy of Medical Sciences; samples of normal breast ductal tissue (ND), usual ductal hyperplasia (UDH), atypical ductal hyperplasia (ADH), ductal carcinoma in situ (DCIS) and invasive ductal carcinoma (IDC) lesions were obtained. The samples were obtained from patients who had not undergone chemoradiotherapy before radical mastectomy for the BC. Ten rat tissue samples of the same lesions as collected for the human tissue were obtained.
All the animals were maintained in an environmental-controlled room with clean air at 24°C with a 12-h light/12-h dark cycle. They were fed standard fodder and tap water. The rats were divided into five groups of 10 rats each after 7 days of acclimatization: G0, blank control group in which the rats were treated with saline; GI, the rats were treated with 0.15 mg/kg of intra-gastric DMBA on the 1st, 55th, and 96th day of the experiment; GII, the rats were treated with 0.2 mg/kg of intra-gastric DMBA on the 1st, 55th, and 96th day; GIII, the rats were treated with 0.25 mg/kg of intragastric DMBA on the 1st, 55th, and 96th day; GIV, the rats were treated with 0.3 mg/kg of intragastric DMBA on the 1st, 55th, and 96th day. The rats in all four groups were administered an intraperitoneal injection of estradiol benzoate at a dose of 0.5 mg/kg a day from the first to third days. An intraperitoneal injection of progestin was administered at a dose of 4 mg/kg a day on the 4th day, and the rats were observed on the 5th day (each cycle lasted 5 days) till the endpoint.
Rat sample collection and processing
After DMBA or hormone administration, all the rats were palpated every week and were examined by ultrasound examination twice a week. When palpable masses were found or when the ultrasound examination revealed abnormal breast images, the rats were immediately treated with an intraperitoneal injection of 10% chloral hydrate to induce anesthesia, and the breast masses and adjacent tissues were resected. All the rats were sacrificed after 200 days.
Hematoxylin-eosin and immunohistochemistry staining
All the rat samples were fixed in 10% neutral buffered formalin; after 24 h, the tissues were dehydrated in a graded ethanol series. The tissues were then paraffin-embedded, cut into 4-μm serial sections, stained with hematoxylin-eosin, and observed under a light microscope (BX51, Olympus company, Japanese). Changes in UDH, ADH, DCIS and IDC tissues were observed in each animal.
The PV-9000 two-step plus Poly-HRP Anti-Mouse/Rabbit IgG Detection System (Beijing Zhongshan Golden Bridge Biotechnology Co. Ltd., China) was used to detect the expression of P63 (Abcam, 1:200 dilution), CK5/6 and CK34βE12 (Beijing Zhongshan Golden Bridge Biotechnology Co. Ltd., China). The experiment was conducted according to the manual of the PV-9000 kit, and the slides were processed using the 3, 3’-diaminobenzidine tetrahydrochloride concentrated kit (Beijing Zhongshan Golden Bridge Biotechnology Co. Ltd., China) to obtain a colored reaction. Finally, the slides were re-dyed with hematoxylin and enveloped with neutral balsam. The slides were observed under a light microscope (BX51, Olympus company, Japanese).
Histopathological evaluation
Histopathological evaluation of rat breast tissue was conducted according to the pathological diagnostic criteria for human breast tissue. UDH was characterized by dilation of the terminal ducts, budding and extension of the ducts to the surrounding fat, irregular curvature of the lumen, the presence of occasional shedding cells and phagocytic histiocytes, clustering of proliferative lumen epithelial cells, papillary or forming a second lumen, morphological diversity of the cells (with regard to size) and absence of atypia. ADH was characterized by enlargement of normal epithelial cells, presence of cells of similar size and morphology, increase in the cytoplasm-nucleus ratio and increase in basophilicity. DCIS was characterized by the presence of atypical epithelia cells in the duct, solid or cribriform morphology, and comedo necrosis in the center. IDC was characterized by infiltration of the cancer cells into the interstitial tissues and through the basement membrane of the duct.
Immunohistochemistry
P63 expression, indicated by dark brown staining, was observed in the nucleus of myoepithelial cells of the peri-duct. P63 expression was divided into three categories [14]: negative expression, none of the cells stained positive; diffuse positive, ≥ 10% of the cells stained positive and the myoepithelial cells also showed continuous expression; focal positive, < 10% of the cells stained positive and the myoepithelial cells showed focal or discontinuous expression.
Positive expression of CK5/6 and CK34βE12 was found in the cell cytoplasm. The expression was evaluated using a semiquantitative method, according to which the staining intensity (0, 1, 2 or 3+) was multiplied by the percentage of positive cells, and the final score was called the intensity score.
Statistical analysis
The quantitative data were recorded as X̅ ± SD and analyzed by one-way ANOVA and t-test, but the counting data were analyzed with the χ2-test or Fisher’s exact probability method using the SPSS 17.0 software. Multiple comparisons were carried out with the LSD test and Bonferroni correction to adjust the inspection level.
Results
Establishment of the SD rat model
Since the initial carcinogen administration, all the rats in the five groups had poor appetite and matted hair. Further, yellow hair; mild diarrhea; and orbital, canal, and nasal bleeding were observed in the experiment groups. After the primary carcinogen intervention, 2, 1, 1, 0, and 5 rats in G0, GI, GII, GIII, and GIV died respectively, during the 5th to 24th day after the primary carcinogen intervention. Torpor, poor appetite and matted hair were observed after the second and third administration of the carcinogen, but diarrhea and bleeding were not observed.
At 121 days after the carcinogen intervention, lumps and/or abnormal ultrasound image was discovered by palpation and ultrasound examination in each group. Further, the HE pathological examination confirmed the presence of premalignant lesions (UDH, ADH, and DCIS) and IDC (Table 1).
Table 1.
Establishment of the SD rat model of breast cancer
| Group | Number of deaths | Survival | Days after premalignant lesions were detected | Days after breast cancer was detected |
|---|---|---|---|---|
| GI | 2 | 8 | 121-181 | 163-200 |
| GII | 1 | 9 | 156-200 | 157-200 |
| GIII | 0 | 10 | 126-177 | 144-200 |
| GIV | 5 | 5 | 169 | 141-200 |
Normal histopathological characteristics of breast tissue in the model rats
The normal breast tissue samples obtained from non-inbred adult SD rats contained parenchyma and interstitial tissue. Analogous to the TDLU in the human breast, the parenchyma was composed of terminal ducts and acini. The interstitial tissues were mostly composed of fatty tissues (Figure 1A). Normal ducts had a three-layer structure that comprised of ductal epithelial cells, myoepithelial cells and basement membrane. The ductal epithelial cells were comprised of two kinds of cells-dark cells and intermediate cells [15]; the cells formed a slender club-shaped structure composed of a single layer of cells. The acini were lined by a single layer of cells that were mainly dark cells with occasional myoepithelial cells (Figure 1B).
Figure 1.
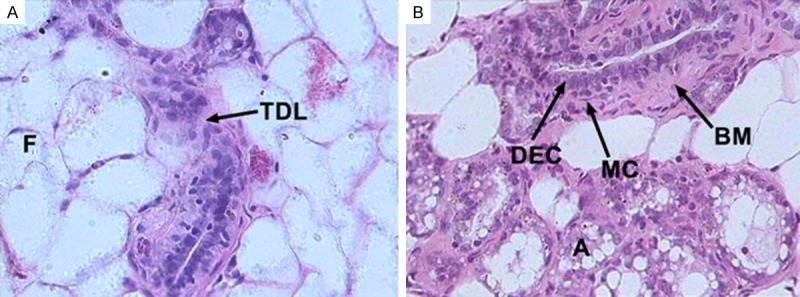
Normal histopathological characteristics of breast tissue in the rats. (A) The rat breast tissues were composed of terminal duct-lobule (TDL) units and fatty tissue (F); each TDL unit included terminal ducts and acini. (B) The terminal duct was composed of ductal epithelial cells (DEC) and myoepithelial cells (MC) surrounded by thick basement membrane (BM). The acini (A) were lined by a single layer of cells that were mainly dark cells with occasional myoepithelial cells (HE, original magnification ×400).
Histopathological changes in breast tissue in the SD rat model
Gross observation: The lump was discovered by palpation. It was easy to observe the lump after hair removal. The lump was removed using an aseptic procedure with the rats under general anesthesia. Tumor size ranged from 0.1 cm to 1 cm, and the lump was observed in the neck, abdomen and armpit. The cross-sectional view showed a solid or cystic tumor that was gray in color. The cystic lumps contained residue that resembled bean curd, which was assumed to be necrotic tissue.
Microscopic observation: The UDH sample showed proliferative cells that were arranged in a mixed and disorderly pattern; further, the size of the proliferative cells was similar to that of normal ductal epithelial cells (Figure 2A). Some of the UDH samples also showed a second lumen (Figure 2B) and papillary structure (Figure 2C). In the ADH samples, the ductal epithelial cells were bigger than normal cells and had an increased cytoplasm-nucleus ratio. Nuclear basophilicity was enhanced, and eosinophilic substances were observed in the lumen (Figure 2D). The ADH showed a cross-bridge structure (Figure 2E) and was cribriform (Figure 2F). In the DCIS sample, the lumen of the duct was filled with atypical proliferative cells that formed a cribriform (Figure 2G) and dense structure with comedo necrosis in the center (Figure 2H). Further, proliferative tumor cells were observed to damage the basement membrane and then infiltrate into the fibrous connective tissue, namely IDC (Figure 2I).
Figure 2.
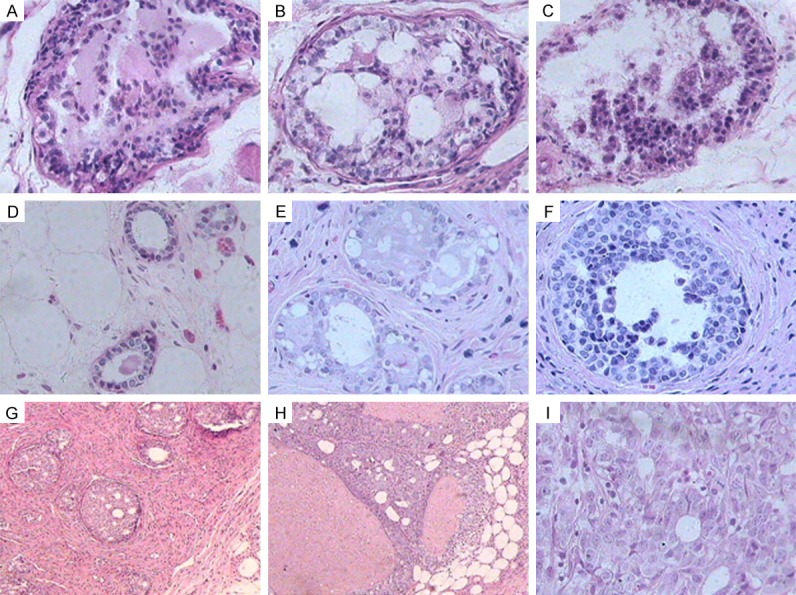
Histopathological changes in breast tissue in the SD rat model. (A-C) UDH tissue showed proliferative cells in a mixed and disorderly pattern; the size of the proliferative cells was similar to that of the normal ductal epithelial cells (A), with a second lumen (B) and papillary structure (C). (D-F) ADH tissue showed that the ductal epithelial cells were larger than normal cells and had an increased cytoplasm-nucleus ratio. Nuclear basophilicity was enhanced and eosinophilic substances were found in the lumen (D), forming a cross-bridge (E) and cribriform (F) structure. (G, H) DCIS tissue showed that the lumen of the duct was filled with atypical proliferative cells, which formed a cribriform (G) and solid structure with comedo necrosis in the center (H) (HE, original magnification ×100). (I) IDC tissue showed that the proliferative cells in the DCIS tissue had damaged the basement membrane and then infiltrated into the fibrous connective tissue (HE, original magnification ×400).
Expression of P63 in human and rat breast lesion tissue
P63 expression was observed in the nuclei of myoepithelial cells of the ducts. In the normal duct, the myoepithelial cells around ductal epithelial cells were poloidal and neatly arranged, but they appeared disorganized in UDH tissue. Positive staining for P63 was observed in the ND and UDH tissues, but only focal or discontinuous expression was observed in the ADH and DCIS tissues; further, no myoepithelial cells were found in the IDC tissue samples (Figure 3).
Figure 3.
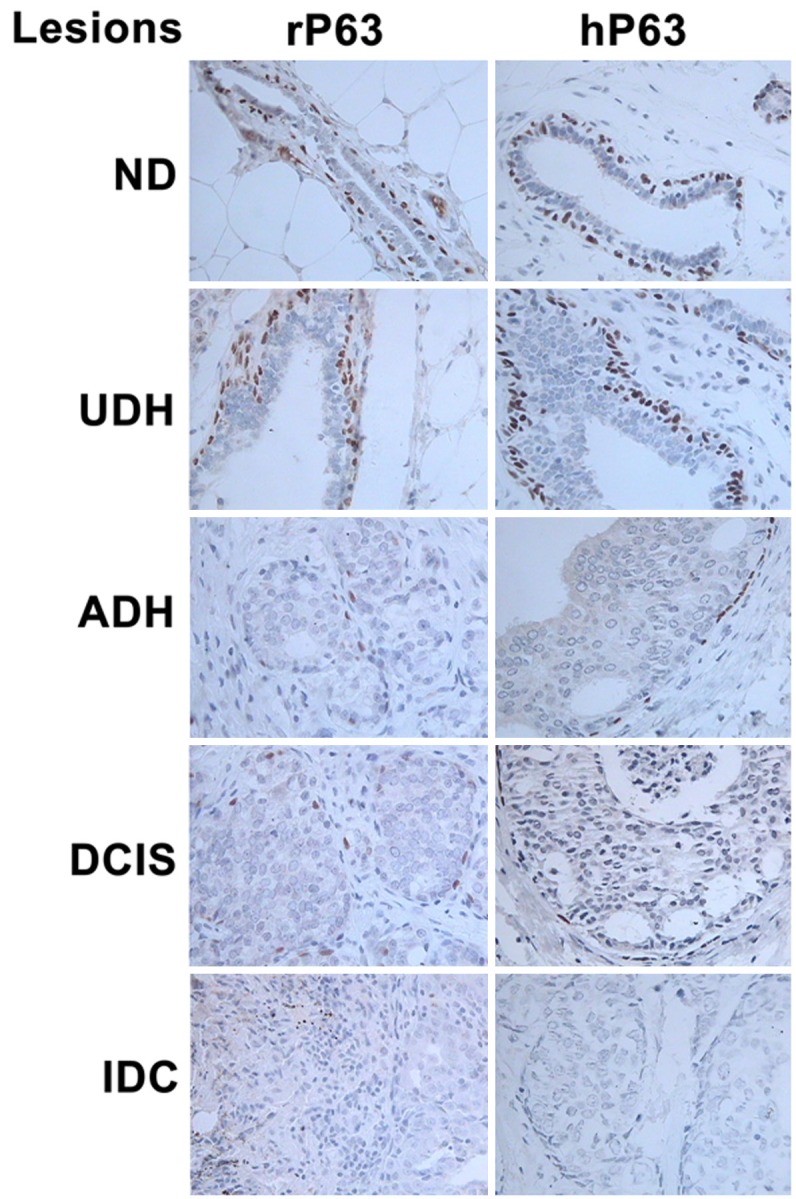
Expression of P63 in the breast tissues from SD rats (left column) and human samples (right column). Positive expression was found in the nuclei of myoepithelial cells of the ducts. Further, the myoepithelial cells around the ductal epithelial cells were poloidal and neatly arranged and showed intense staining for P63 in the normal duct (ND), but they became disorganized in the UDH tissue. The amount of myoepithelial cells in ADH and DCIS was less than that in ND and UDH, and the cells showed focal or discontinuous expression; however, no myoepithelial cells were found in IDC (IHC, original magnification ×400) (r = rat, h = human).
P63 expression was not significantly different in the rat and human tissues (P > 0.05), but significant differences were observed among UDH, ADH, DCIS and IDC tissues in the rat and human samples (P < 0.01). Multiple comparison showed that there was no difference between ADH and DCIS in the rats (P > 0.01, α = 0.05/5), but a significant difference was observed among UDH, ADH, DCIS and IDC in the human tissue (P < 0.01, α = 0.05/5). Moreover, P63 expression was not observed in human or rat IDC tissue (Table 2).
Table 2.
P63, CK5/6 and CK34βE12 expression in the breast lesions (ND, UDH, ADH, DCIS and IDC) of SD rat and human tissue (X̅ ± SD)
| Marker | ND | UDH | ADH | DCIS | IDC | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
||||||
| R | H | R | H | R | H | R | H | R | H | |
| P63 | ||||||||||
| Negative (%) | 0 (0) | 0 (0) | 0 (10) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 10 (100) | 10 (100) |
| Diffuse (%) | 10 (100) | 10 (100) | 10 (100) | 10 (100) | 5 (50) | 8 (80) | 1 (10) | 0 (0) | 0 (0) | 0 (0) |
| Focal (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 5 (50) | 2 (20) | 9 (90) | 10 (100) | 0 (0) | 0 (0) |
| CK5/6 | ||||||||||
| Percentage of positive cells (%) | 99 ± 3.16 | 88 ± 16.19 | 94 ± 6.99 | 84 ± 15.78 | 66 ± 29.14 | 47 ± 29.08 | 62 ± 28.21 | 57 ± 27.10 | 0 | 0 |
| Intensity score | 297 ± 9.49 | 264 ± 48.60 | 262 ± 37.65 | 228 ± 66.13 | 167 ± 84.60 | 124 ± 85.01 | 143 ± 70.25 | 152 ± 83.90 | 0 | 0 |
| CK34βE12 | ||||||||||
| Percentage of positive cells (%) | 97 ± 6.75 | 84 ± 20.66 | 89 ± 7.38 | 83 ± 21.63 | 51 ± 36.95 | 40 ± 36.82 | 58 ± 30.84 | 31 ± 31.84 | 0 | 0 |
| Intensity score | 271 ± 42.28 | 215 ± 81.55 | 220 ± 36.21 | 201 ± 75.20 | 113 ± 86.29 | 81 ± 72.79 | 118 ± 58.27 | 62 ± 63.03 | 0 | 0 |
Expression of CK5/6 and CK34βE12 in the breast tissues of SD rats and humans
In rat ND and UDH tissue, the expression of CK5/6 and CK34βE12 was observed in the cell cytoplasm of myoepithelial and basal cells. The expression of CK5/6 and CK34βE12 decreased gradually till it was almost absent in the order of ADH, DCIS and IDC (Figure 4). In human ND and UDH tissue, CK5/6 and CK34βE12 expression was observed in the cytoplasm of ductal epithelial cells, myoepithelial cells and basal cells. As observed in the rat tissues, their expression decreased gradually in the order of ADH, DCIS and IDC, till it was absent in the ductal epithelial cells and almost absent in the myoepithelial cells and basal cells (Figure 5). In both rat and human samples, CK5/6 and CK34βE12 expression showed a significant difference among ND, UDH, ADH, DCIS and IDC (P = 0.000). Nonetheless, their expression was not significantly different between rats and humans (P > 0.05) (Table 2).
Figure 4.

Expression of CK5/6 (left column) and CK34βE12 (right column) in the breast tissues of SD rats. Both markers were expressed in the cell cytoplasm of myoepithelial cells and basal cells in ND and UDH. The expression of CK5/6 and CK34βE12 decreased gradually till it was absent in the order of ADH, DCIS and IDC (IHC, original magnification ×400) (r = rat).
Figure 5.
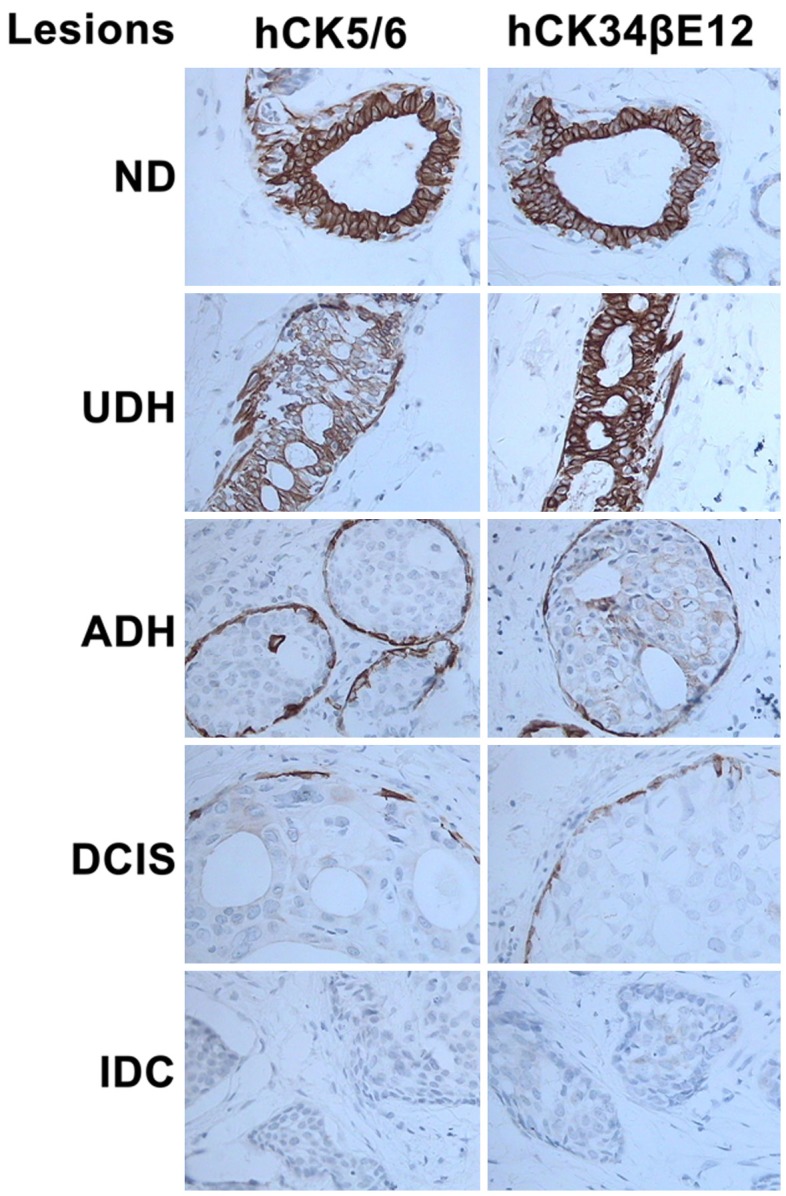
Expression of CK5/6 (left column) and CK34βE12 (right column) in human breast tissue. Expression of these two markers was observed in the cytoplasm of ductal epithelial cells, myoepithelial cells and basal cells in ND and UDH. Their expression gradually decreased in the order of ADH, DCIS and IDC till it was absent in ductal epithelial cells and almost absent in myoepithelial cells and basal cells (IHC, original magnification ×400) (h = human).
Discussion
Histomorphological analysis plays a major role in the diagnosis and study of tumors; the histomorphological features of the human breast are well known, but details about the mechanisms involved in BC and the associated histomorphological changes are not clear yet. Russo revealed that the morphological characteristics of rat breast are similar to those of the human breast [10]; therefore, the rat model has advantages over other models for studying human BC and its mechanisms. Our study confirmed the similarities between human and rat breast tissue: normal breast tissue in rats was found to have parenchyma and interstitial tissue, of which the former was composed of TDLUs, including terminal ducts and acini, while the latter was mainly composed of fatty tissue. However, differences in lobule units between rat and human breast tissue have also been reported: each lobule unit is surrounded by thick and dense fibrous connective tissue in the human breast, while the lobule units are surrounded by thin connective tissue in the rat breast [16]. Despite these small differences, the histomorphological structure of the rat breast is nearly the same as that of the human breast.
Myoepithelial cells play a considerable role in the development of the mammary glands [17]. Usual hyperplasia, atypical hyperplasia and ductal cancer are commonly diagnosed in situ in the human breast, while invasive cancer is diagnosed by staining of myoepithelial cells. The myoepithelial cell markers that are typically used in the clinical setting are P63, CK5/6, and CK34βE12. In canine mammary tissues, P63 is a sensitive and highly specific marker of myoepithelial cells [18]. In nonhuman primates, P63 is highly useful in studying the invasive nature of mammary tumors [19]. Herein, we used P63 for the diagnosis of lesions in SD rats: positive expression of P63 was observed in myoepithelial cells, and P63 expression was not significantly different between rats and humans. However, there were significant differences among UDH, ADH, DCIS and IDC tissues in rat and human samples. Specifically, no difference was found between ADH and DCIS in rats, while significant differences were found between UDH, ADH, DCIS and IDC tissues in human samples. Moreover, P63 expression was not observed in human and rat tissue. Therefore, the findings of the present study also indicate that P63 is a sensitive marker that can be used for the diagnosis of lesions in SD rats as well as human breast lesions. Moreover, in rats, CK5/6 and CK34βE12 were expressed in the cell cytoplasm of myoepithelial cells and basal cells in ND and UDH. Although they were not as sensitive as P63 as myoepithelial cell markers, they play a similar role to P63 in myoepithelial cells. Our study showed that in the rat samples, the expression of CK5/6 and CK34βE12 decreased gradually until it was almost absent in the order of ADH, DCIS and IDC. However, the cytoplasm of human ductal epithelial cells, myoepithelial cells and basal cells showed expression of CK5/6 and CK34βE12 in ND and UDH; their expression gradually decreased in the order of ADH, DCIS and IDC till it was absent in the ductal cells and almost absent in the myoepithelial cells and basal cells. They not only played the role of myoepithelial cell markers in human tissue, but also played the role of markers for distinguishing UDH from ADH in rat and human tissue. Similar to P63 expression, CK5/6 and CK34βE12 expression was also significantly different between ND, UDH, ADH, DCIS and IDC but not significantly different between rat and human tissue. Therefore, rats and humans have the same molecular markers for myoepithelial cells, and these markers may also play an important part in the differential diagnosis of breast lesions in rats.
DMBA, as a proven toxic tumor-inducing agent in animal models, has been reported to induce BC too [20]. It is reported to mainly induce the formation of lesions of the terminal ducts and induce hyperplasticity of the ductal epithelial cells, atypical hyperplasticity, and subsequently canceration of the ducts [21]. Studies have also reported that estrogen-progestogen could induce lobular hyperplasia and canceration of lobules [22]. Further, hormone replacement therapy in postmenopausal women is associated with BC [23], which means that estrogen-progestogen plays a definite role in the occurrence of BC. It is speculated that a combination of DMBA and estrogen-progestogen would work synergistically to induce the development of BC. This combination can be used to produce the SD rat model for premalignant breast lesions [24]. In our study, we found that many types of breast tissue lesions that occurred in the rat model were induced by the DMBA and estrogen-progestogen combinations, including benign lesions, precancerous lesions and malignant lesions of the duct and lobule, which are similar to lesions in the human breast. In our study, we used ductal lesions of human and rat breast tissues, including UDH, ADH, DCIS and IDC. The changes in the ductal lesions indicated that the development of BC was a sequential process that progressed from benign lesions to precancerous lesions and then malignant lesions. Such changes in the lobule lesions of rat breast tissues need to be investigated further in the future. These findings indicate that this SD model can be used for studying the development and progression of human BC and may be more suitable than the currently available models for investigating BC.
Considering the complex pathways involved in the development of BC, different kinds of models will be needed to explore its mechanism. Our model specifically provides a histomorphological basis for probing the molecular mechanisms of BC. In the future, this model can be used for molecular typing and studying the effects of endocrine therapy in drug-resistant cases.
Acknowledgements
This study was supported by grants from the National Science Foundation of Shandong Province, China (No. ZR2010HM106).
Disclosure of conflict of interest
None.
References
- 1.Singh M, McGinley JN, Thompson HJ. A comparison of the histopathology of premalignant and malignant mammary gland lesions induced in sexually immature rats with those occurring in the human. Lab Invest. 2000;80:221–231. doi: 10.1038/labinvest.3780025. [DOI] [PubMed] [Google Scholar]
- 2.Cocola C, Sanzone S, Astigiano S, Pelucchi P, Piscitelli E, Vilardo L, Barbieri O, Bertoli G, Reinbold RA, Zucchi I. A rat mammary gland cancer cell with stem cell properties of selfrenewal and muti-lineage differentiation. Cytotechnology. 2008;58:25–32. doi: 10.1007/s10616-008-9173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kariagina A, Xie J, Leipprandt JR, Haslam SZ. Amphiregulin mediates estrogen, progesterone, and EGFR signaling in the normal rat mammary gland and in hormone-dependent rat mammary cancers. Horm Cancer. 2010;1:229–244. doi: 10.1007/s12672-010-0048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manoharan S, Selvan MV. Chemopreventive potential of geraniol in 7,12-dimethylbenz (a) anthracene (DMBA) induced skincarcinogenesis in Swiss albino mice. J Environ Biol. 2012;33:255–260. [PubMed] [Google Scholar]
- 5.Pugalendhi P, Manoharan S, Suresh K, Baskaran N. Genistein and daidzein, in combination, protect cellular integrity during 7,12-dimethylbenz [a] anthracene (DMBA) induced mammary carcinogenesis in Sprague-Dawley rats. Afr J Tradit Complement Altern Med. 2011;8:91–97. doi: 10.4314/ajtcam.v8i2.63196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vidya Priyadarsini R, Kumar N, Khan I, Thiyagarajan P, Kondaiah P, Nagini S. Gene expression signature of DMBA-induced hamster buccal pouch carcinomas: modulation by chlorophyllin and ellagic acid. PLoS One. 2012;7:e34628. doi: 10.1371/journal.pone.0034628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kimura K, Satoh K, Kanno A, Hamada S, Hirota M, Endoh M, Masamune A, Shimosegawa T. Activation of Notch signaling in tumorigenesis of experimental pancreatic cancer induced by dimethylbenzanthracene in mice. Cancer Sci. 2007;98:155–162. doi: 10.1111/j.1349-7006.2006.00369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aras U, Gandhi YA, Masso-Welch PA, Morris ME. Chemopreventive and anti-angiogenic effects of dietary phenethyl isothiocyanate in an N-methyl nitrosourea-induced breast cancer animal model. Biopharm Drug Dispos. 2013;34:98–106. doi: 10.1002/bdd.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vinothkumar V, Manoharan S. Chemopreventive efficacy of geraniol against 7,12-dimethylbenz [a] anthracene-induced hamster buccal pouch carcinogenesis. Redox Rep. 2011;16:91–100. doi: 10.1179/174329211X13020951739839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russo J, Saby J, Isenberg WM, Russo IH. Pathogenesis of mammary carcinomas induced in rats by 7,12-dimethylbenz [a] anthracene. Natl Cancer Inst. 1977;59:435–445. [Google Scholar]
- 11.Ohi Y, Yoshida H. Influence of estrogen and progesterone on the induction of mammary carcinomas by 7,12-dimethylbenz (a) anthracene in ovariectomized rats. Virchows Arch B Cell Pathol Incl Mol Pathol. 1992;62:365–370. doi: 10.1007/BF02899705. [DOI] [PubMed] [Google Scholar]
- 12.Stingl J. Estrogen and progesterone in normal mammary gland development and in cancer. Horm Cancer. 2011;2:85–90. doi: 10.1007/s12672-010-0055-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graham JD, Mote PA, Salagame U, van Dijk JH, Balleine RL, Huschtscha LI, Reddel RR, Clarke CL. DNA replication licensing and progenitor numbers are increased by progesterone in normal human breast. Endocrinology. 2009;150:3318–3326. doi: 10.1210/en.2008-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reisenbichler ES, Balmer NN, Adams AL, Pfeifer JD, Hameed O. Luminal cytokeratin expression profiles of breast papillomas and papillary carcinomas and the utility of a cytokeratin 5/p63/cytokeratin 8/18 antibody cocktail in their distinction. Mod Pathol. 2011;24:185–93. doi: 10.1038/modpathol.2010.197. [DOI] [PubMed] [Google Scholar]
- 15.Russo J, Tait L, Russo IH. Susceptibility of the mammary gland to carcinogenesis. III. The cell of origin of rat mammary carcinoma. Am J Pathol. 1983;113:50–66. [PMC free article] [PubMed] [Google Scholar]
- 16.McGinley JN, Zhu Z, Jiang W, Thompson HJ. Collection of epithelial cells from rodent mammary gland via laser capture microdissection yielding high-quality RNA suitable for microarray analysis. Biol Proced Online. 2010;12:31–43. doi: 10.1007/s12575-010-9026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faraldo MM, Taddei-De La Hosseraye I, Teulière J, Deugnier MA, Moumen M, Thiery JP, Glukhova MA. [Mammary gland development: Role of basal myoepithelial cells] . J Soc Biol. 2006;200:193–198. doi: 10.1051/jbio:2006021. [DOI] [PubMed] [Google Scholar]
- 18.Gama A, Alves A, Gartner F, Schmitt F. p63: a novel myoepithelial cell marker in canine mammary tissues. Vet Pathol. 2003;40:412–20. doi: 10.1354/vp.40-4-412. [DOI] [PubMed] [Google Scholar]
- 19.Williams-Fritze MJ, Carlson Scholz JA, Bossuyt V, Booth CJ. Use of p63, a myoepithelial cell marker, in determining the invasiveness of spontaneous mammary neoplasia in a rhesus macaque (Macaca mulatta) J Am Assoc Lab Anim Sci. 2011;50:252–7. [PMC free article] [PubMed] [Google Scholar]
- 20.Benakanakere I, Besch-Williford C, Carroll CE, Hyder SM. Synthetic progestins differentially promote or prevent DMBA-induced mammary tumors in sprague-dawley rats. Cancer Prev Res (Phila) 2010;3:1157–1167. doi: 10.1158/1940-6207.CAPR-10-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ethier SP, Ullrich RL. Detection of ductal dysplasia in mammary outgrowths derived from carcinogen-treated virgin female BALB/c mice. Cancer Res. 1982;42:1753–1760. [PubMed] [Google Scholar]
- 22.Torres SM, Simões RS, Baracat MC, Gomes RC, Soares Júnior JM, Carbonel AA, Baracat EC. Breast histomorphometry of rats with estrogen and/or progestogen. Rev Assoc Med Bras. 2011;57:177–181. doi: 10.1590/s0104-42302011000200015. [DOI] [PubMed] [Google Scholar]
- 23.Würtz AM, Tjønneland A, Christensen J, Dragsted LO, Aarestrup J, Kyrø C, Overvad K, Olsen A. Serum estrogen and SHBG levels and breast cancer incidence among users and never users ofhormone replacement therapy. Cancer Causes Control. 2012;23:1711–1720. doi: 10.1007/s10552-012-0050-7. [DOI] [PubMed] [Google Scholar]
- 24.Wang F, Ma Z, Wang F, Fu Q, Fang Y, Zhang Q, Gao D, Li Y, Li L, Yu L, Yu Z. Establishment of novel rat models for premalignant breast disease. Chin Med J (Engl) 2014;127:2147–52. [PubMed] [Google Scholar]


