Abstract
Ulcerative colitis (UC), characterized by inflammation, oxidative stress, and increased intestinal epithelial cell apoptosis, is an immunologically mediated chronic intestinal disorder. The present study was aimed at investigating the protective effects of alpha-lipoic acid (ALA) against trinitrobenzene sulfonic acid (TNBS)-induced UC and the underlying mechanism. ALA of 80 mg/kg bw/day was administered by gastric gavage to mice for 7 days after TNBS-induced UC. Our data indicated that ALA effectively facilitated recovery of pathologic changes in the colon, as evidenced by a significant increase of body weight, decrease of colon mass index and histopathological score. Furthermore, ALA significantly inhibited TNBS-induced apoptosis, which partly due to up-regulation of Bcl-2 expression, reduction of Bax expression and caspase-3, caspase-9 activity. ALA reduced malondialdehyde (MDA), nitric oxide (NO), and inducible nitric oxide synthase (iNOS) levels, and restored superoxide dismutase (SOD) activity and glutathione (GSH) content in colon tissues from TNBS-challenged mice. Additionally, phosphorylation of extracellular signal regulated kinase (ERK), c-Jun N-terminal kinase (JNK) and p38 kinase (p38) in colon tissues were significantly inhibited by ALA treatment. In summary, we demonstrate that ALA has protective properties against TNBS-induced UC through anti-apoptosis, anti-oxidant actions, and mitogen-activated protein kinase (MAPK) signaling pathway. Our present findings suggest a therapeutic potential of ALA in UC.
Keywords: Ulcerative colitis, alpha-lipoic acid, apoptosis, oxidative stress, mitogen-activated protein kinase
Introduction
Ulcerative colitis (UC) is a chronic intestinal inflammatory disorder which starts from the rectum, extends proximally in a continuous fashion, and has a variable clinical course, including abdominal pain, diarrhea, purulent stools and unpredictable relapses [1]. The impact of UC on quality of life is staggering, which includes increased susceptibility to infection, side effects of therapeutic drugs, and a higher risk of developing bowel cancer [2-4]. Many UC patients still have not better curative effect under current drug treatment [5]. Since UC seriously threatens human health, it is urgent to find an appropriate agent to ameliorate the severity of the disease.
Alpha-lipoic acid (ALA) is a naturally occurring sulfhydryl compound and it is a potent anti-inflammatory and anti-oxidant agent [6]. Current studies demonstrate that ALA had therapeutic potential in a variety of disease, such as hepatic fibrosis, diabetes mellitus, and cancers [7-9]. In addition, ALA attenuates liver cells damages induced by excess intake of polyunsaturated fatty acids [10]. Current researches have illustrated that the effects of ALA are associated with mediation of inducible nitric oxide synthase (iNOS) and mitogen-activated protein kinase (MAPK) signaling pathway [11,12]. A recent study has shown the modulatory effect of ALA against inflammation, oxidative stress, DNA damage and fibrosis, suggesting a therapeutic role of ALA in UC [13]. We therefore hypothesized that ALA exerted protective effects in UC via anti-apoptosis, anti-oxidant actions and further explored the underlying regulatory mechanism.
In this study, to address this hypothesis, ALA was considered to elucidate its possible beneficial effect against TNBS-induced UC in mice. Furthermore, expression levels of related molecules were detected to investigate the possible mechanism underlying these protective effects.
Materials and methods
Animals
Male BALB/c mice (22-25 g) were purchased from the Animal Center of China Medical University. The animals were maintained in an environmentally controlled at constant temperature of 23 ± 2°C with a relative humidity of 50 ± 5% and a 12 h light-dark cycle. Standard laboratory chow and water were provided ad libitum. All the animal experiment protocols in the present study were approved by the Animal Care Committee of China Medical University.
Induction of TNBS-induced UC and ALA administration
Mice were randomized into four groups, the control group, the ALA group, the UC group, and the UC + ALA group, each consisting of 10 animals. In the UC group, UC was induced with trinitrobenzene sulfonic acid (TNBS) enema according to a published method [14,15]. Briefly, BALB/c mice were anaesthetized by ether after a 24 h fast. A catheter was inserted into the anus of the mice, and the tip was advanced approximately 4 cm. 3 mg of TNBS (Sigma-Aldrich, St Louis, MO, USA) dissolved in 0.12 ml of 50% ethanol was slowly instilled into colon through the catheter. Following the instillation of the TNBS solution, the mice were maintained in a head-down position for 2-3 min. In the control group and the ALA group, mice received 50% ethanol alone using the same technique. 24 h after the instillation of TNBS, the ALA group and the UC + ALA group were given ALA (80 mg/kg bw/day; Sigma-Aldrich) by gastric gavage. The control group and the UC group were given the same volume of 2% DMSO without ALA. All treatment regimens were continued for 7 days. On the 7th day, mice were sacrificed and weighed up, and colonic segments were excised. The colon mass index was calculated from the ration of colon to body mass, and the colon tissues were collected.
Histopathology
After excision, the colonic tissues were fixed in 4% paraformaldehyde, dehydrated through the graded ethanol, embedded in paraffin, and cut into 5 μm sections. Sections were hydrated, stained with hematoxylin and eosin (H & E) and examined microscopically to evaluate tissue damages. The mucosal damage was scored according to Ho H. Luk, et al [16] as follows: 0, normal histological appearance; 1, damage limited to surface epithelium; 2, cell disruption and focal ulceration limited to mucosa; 3, transmural inflammation and focal ulceration; 4, extensive inflammation and transmural ulceration bordered by areas of normal mucosa; 5, extensive inflammation and transmural ulceration involving entire section from epithelium to serosa.
Localization of apoptotic cells
Rehydrated sections were stained for apoptotic cells using the terminal deoxynucleotidyl transferase (TdT)-mediated dUTP-biotin nick end labeling (TUNEL, Roche, Basel, Switzerland) method in accordance with the manufacturer’s protocol. The sections were examined under light microscopy to evaluate tissue apoptosis.
Measurement of caspase-3, caspase-9, nitric oxide (NO), glutathione (GSH), malondialdehyde (MDA) and superoxide dismutase (SOD) in colon tissues
Activities of caspase-3 and caspase-9 were measured by caspase-3 and caspase-9 activity kits (Beyotime, Haimen, China). Concentration of NO was detected using NO concentration detection kit (Beyotime). Concentrations of GSH and MDA were measured by GSH and MDA concentration detection kit (Jiancheng Bioengineering Institute, Nanjing, China) and activity of SOD was detected using SOD activity detection kit (Jiancheng Bioengineering Institute). Briefly, frozen colon tissues were thawed and homogenized in cold PBS. The supernatant was collected by centrifuge for the measurement of caspase-3, caspase-9, NO, SOD, GSH, MDA.
Immunohistochemistry
Sections of colon were dewaxed in xylene, hydrated with decreasing concentrations of ethanol, followed by antigen retrieval in citrate buffer under microwave treatment for 10 min. Endogenous peroxidase was quenched by 3% H2O2, and unspecific sites were blocked by normal goat serum. Then, the sections were incubated overnight at 4°C with primary antibody against iNOS (Bioss, Beijing, China) at a dilution of 1:50 in PBS, followed by incubation with secondary antibody and streptavidin-HRP conjugate. Thereafter, the sections were incubated with a 3, 3’-diaminobenzidine (DAB), stained with hematoxylin and observed in an inverted digital image light microscopy.
Quantitative real-time PCR (qPCR)
Total RNA was isolated using RNA simple Total RNA Kit (Tiangen, Beijing, China) according to the manufacturer’s instructions, and reversed transcribed to synthesize complementary DNA (cDNA) using a Super M-MLV reverse transcriptase (BioTeke, Beijing, China). qPCR was performed by SYBR-GREEN (Solarbio, Beijing, China) in a PCR system (Exicycler 96, Bioneer, Daejeon, Korea). The primer sequence information was shown in Table 1 and the relative mRNA levels were quantified via 2-ΔΔCT and normalized to β-actin.
Table 1.
Oligonucleotide primer sets for quantitative real-time PCR
| Gene symbol | Sequence (5’-3’) | Product size (bp) |
|---|---|---|
| iNOS-F | GCAGGGAATCTTGGAGCGAGTTG | 139 |
| iNOS-R | GTAGGTGAGGGCTTGGCTGAGTG | |
| Bcl-2-F | CTCTGGTTGGGATTCCTACGG | 233 |
| Bcl-2-R | CGGCATGATCTTCTGTCAAGTTTA | |
| Bax-F | CCAGGATGCGTCCACCAAGAA | 199 |
| Bax-R | AGCAAAGTAGAAGAGGGCAACCAC | |
| β-actin-F | CTGTGCCCATCTACGAGGGCTAT | 155 |
| β-actin-R | TTTGATGTCACGCACGATTTCC |
Western blot
Protein samples were isolated from frozen colon tissue specimens using RIPA buffer (Beyotime) containing 1% PMSF. Protein concentrations were determined by a commercial BCA protein assay kit (Beyotime). Equal quantity of each sample was fractionated on sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene fluoride (PVDF) membranes (Millipore, Bedford, MA, USA). The membranes were blocked with 5% fat-free milk or 5% bovine serum albumin and then incubated with antibody against bcl-2, bax (1: 500, Boster, Wuhan, China), iNOS (1: 200, Bioss), extracellular signal regulated kinase (ERK), phosphorylated ERK (p-ERK) (1: 1000, Beyotime), c-Jun N-terminal kinase (JNK), phosphorylated JNK (p-JNK) (1: 1000, wanleibio, Shenyang, China), p38 kinase (p38, 1: 200) and phosphorylated p38 (p-p38, 1: 100) (Santa Cruze, CA, USA) at 4°C overnight, followed by incubating with horseradish peroxidase-conjugated secondary antibodies (1: 5000, Beyotime) at 37°C for 45 min. The protein blots on the membranes were visualized with an enhanced chemiluminescence (ECL) detection kit (Millipore) and the density values of the protein bands were calculated as a ratio to β-actin.
Statistical analysis
Data were presented as mean ± standard deviation (SD). Multiple comparisons were analyzed by one-way analysis of variance (ANOVA) followed by Bonferroni post test, and P < 0.05 was considered to be significant.
Results
ALA ameliorates TNBS-induced ulcerative colitis
The therapeutic efficacy of ALA on experimental UC was assessed by histological analysis, body weight change and colon mass index. Histological analysis showed that colon of TNBS-treated mice had thickening of the colon wall, high level of inflammatory cell infiltration in the mucosa, and loss of goblet cells. Nevertheless, treatment with ALA inhibited these pathological symptoms and histopathological score and showed progressive restoration, improvement of normal intestinal architecture (Figure 1; Table 2). Furthermore, TNBS also induced severe weight loss, which was partially improved by the ALA treatment. Otherwise, the colon mass index was significantly increased in mice after TNBS administration, while ALA treatment attenuated TNBS-induced elevation of colon mass index (colon mass indexes were 12.15 and 10.23 of UC group and UC + ALA group, respectively). No remarkable differences of body weight and colon mass index were observed between control mice and mice treated with ALA alone (Table 2). The above results indicate a protective role of ALA in TNBS-induced UC.
Figure 1.
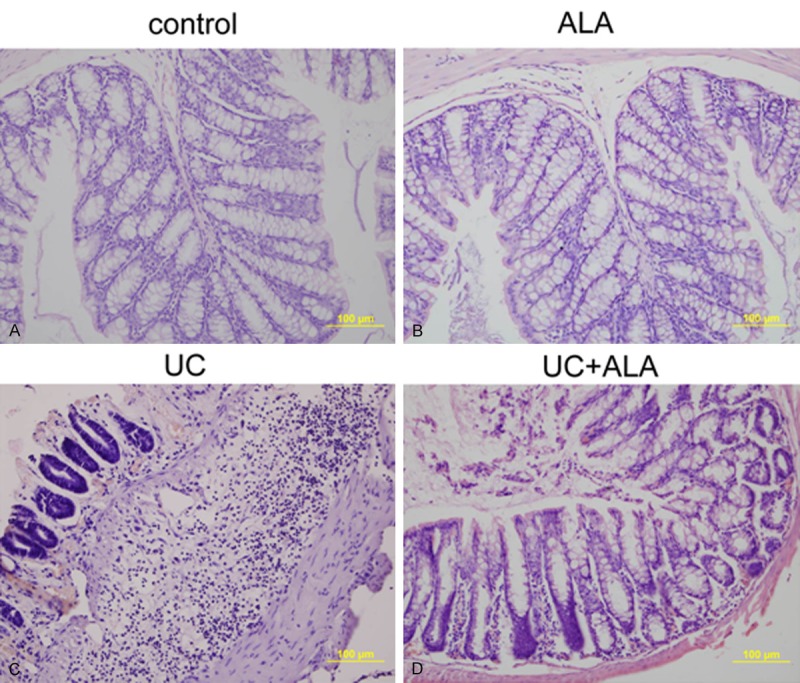
Histological sections of colonic mucosa from colitic mice 7 days after TNBS-induced UC. Mice were given ether 2% DMSO as a control (A), or 80 mg/kg bw/day of ALA (B), or 3 mg of TNBS (C), or 3 mg of TNBS and 80 mg/kg bw/day of ALA (D). Colonic mucosa were stained with H&E and observed under the microscope.
Table 2.
Effects of ALA on body weight, colon mass index and histopathological score
| Groups | Body weight (g) | Colon mass index | Histopathological Score |
|---|---|---|---|
| Control | 22.8 ± 1.48 | 9.02 ± 0.52 | 0.2 ± 0.42 |
| ALA | 23.0 ± 1.76 | 8.86 ± 0.27 | 0.1 ± 0.32 |
| UC | 19.3 ± 1.34** | 12.15 ± 1.31** | 4.1 ± 0.88** |
| UC + ALA | 21.2 ± 1.14# | 10.23 ± 0.94*,## | 2.2 ± 0.63**,## |
Data are presented as the means ± SD.
P < 0.05 vs. control group;
P < 0.01 vs. control group;
P < 0.05 vs. UC group;
P < 0.01 vs. UC group.
ALA inhibits TNBS-induced cell apoptosis in colon
To assess the effect of ALA on TNBS-induced apoptosis, TUNEL staining was performed. As shown in Figure 2A, few apoptotic cells were observed in the control group and the ALA group, whereas colon tissues demonstrated a remarkable appearance of dark brown apoptotic cells after treatment with TNBS. In contrast, administration of ALA reduced apoptotic responses. To further reveal the molecular mechanism of protective effects of ALA on TNBS-induced apoptosis, bax and bcl-2 levels, caspase-3 and caspase-9 activities in the colon homogenate were analyzed. As results shown, TNBS induced bax expression increase and bcl-2 expression decrease in the colon of mice (Figure 2B-D). ALA treatment significantly decreased bax level and increased bcl-2 level. In addition, we also found the activities of caspase-3 and caspase-9, which play critical roles in the modulation of apoptosis, obviously rose in the UC group compared with the control group, and the treatment of ALA lowered the activities dramatically. These present findings reveal that ALA attenuates TNBS-induced colonic apoptosis.
Figure 2.
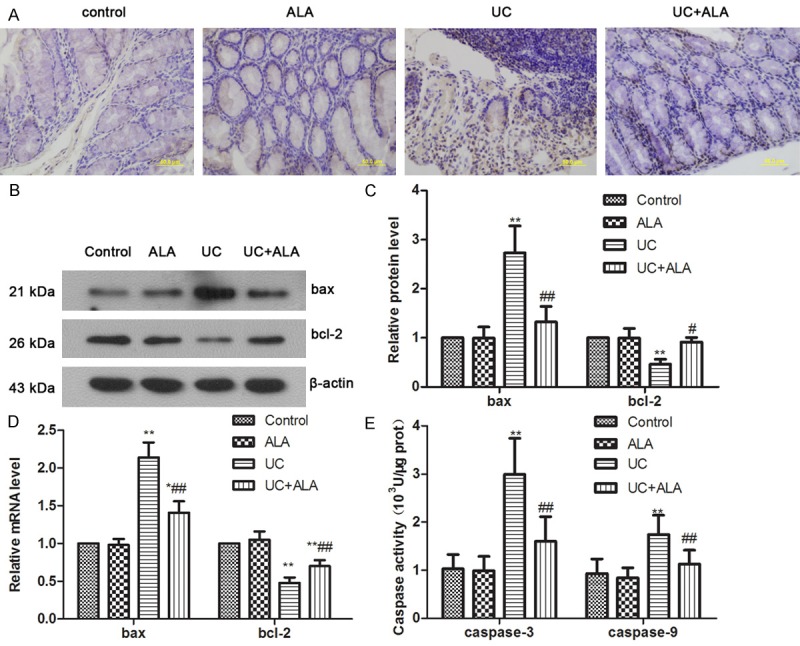
Effects of ALA on TNBS-induced apoptosis in colon. TUNEL staining of colon from mice sacrificed 7 days after TNBS-induced UC (A). Bax and Bcl-2 representative blots are shown and the protein size is expressed in kDa (B). Protein levels were calculated as a ratio to β-actin, and data were subjected to densitometric quantification (C). Quantitative real-time PCR was used for analysis of bax and bcl-2 mRNA expression (D). Caspase-3 and Caspase-9 activities in colon tissues were detected by Activity Assay Kit (E). Data are presented as the means ± SD. *P < 0.05 vs. control group, **P < 0.01 vs. control group; #P < 0.05 vs. UC group; ##P < 0.01 vs. UC group.
ALA protects against TNBS-induced oxidative stress in colon
Regarding to the important roles of oxidative stress in colon inflammatory responses [17,18], we examined the effect of ALA on SOD activity and NO, GSH, MDA, iNOS levels in the colon. As shown in Figure 3, colonic NO and MDA levels were obviously increased in mice administered with TNBS; however, ALA treatment significantly attenuated TNBS-induced increase in NO and MDA levels (Figure 3A, 3B). Otherwise, the SOD activities and GSH levels were markedly reduced after TNBS instillation, but these decreases were dramatically restored by ALA treatment (Figure 3C, 3D). Meanwhile, iNOS expression levels were measured at 7 days after TNBS-induced UC. Immunohistochemistry and western blot results showed that TNBS-induced protein expression elevation of iNOS was significantly inhibited by ALA treatment (Figure 4A-C). Real-time PCR data were accordant with results from the prior protein analysis (Figure 4D). These results suggest that ALA treatment reduces colonic oxidative stress induced by TNBS.
Figure 3.
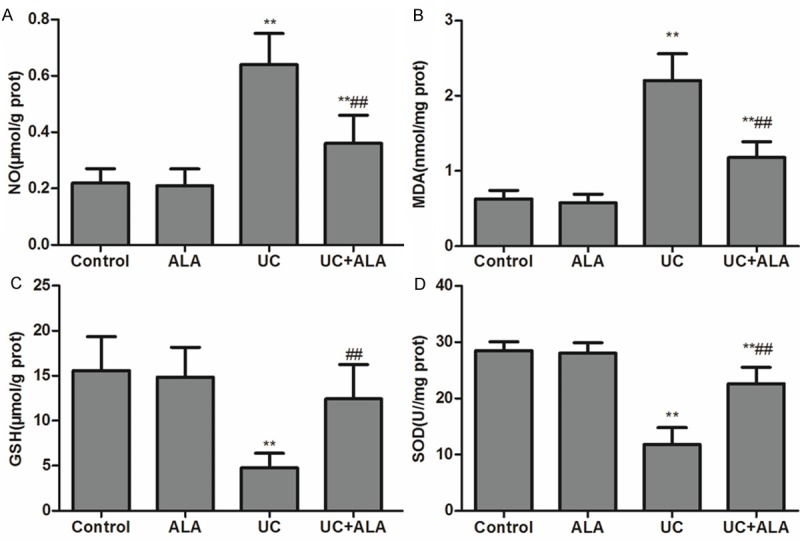
Effects of ALA on lipid peroxide and antioxidant parameters in colon tissues. Mice were treated with ALA (80 mg/kg bw/day) or vehicle for 7 days after TNBS-induced UC. NO (A), MDA (B), GSH (C) contents and SOD activity (D) in colon tissues were determined. Data are presented as the means ± SD. **P < 0.01 vs. control group; ##P < 0.01 vs. UC group.
Figure 4.
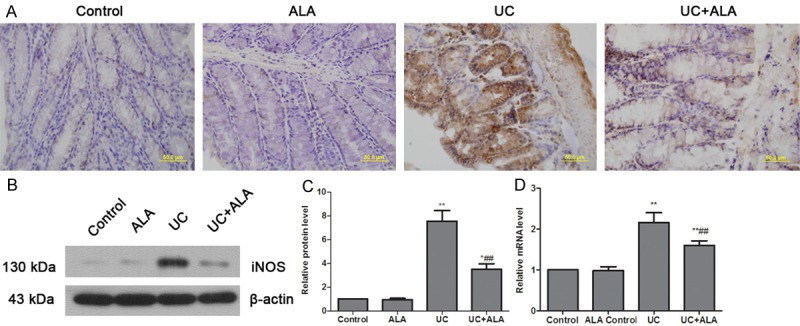
Effects of ALA on the expression of iNOS in colon tissues. Mice were treated with ALA (80 mg/kg bw/day) or vehicle for 7 days after TNBS-induced UC. Protein levels of iNOS in colon were detected by immunohistochemical analysis (A) and western blot (B). Protein levels were normalized with β-actin, and data were subjected to densitometric quantification (C). Quantitative real-time PCR was used for analysis of iNOS mRNA expression (D). Data are presented as the means ± SD. *P < 0.05 vs. control group, **P < 0.01 vs. control group; ##P < 0.01 vs. UC group.
ALA functions through MAPK signaling pathway in colon
Mitogen-activated protein kinase (MAPK) involves in numerous physiological and pathological signaling and responds to cellular proliferation and apoptosis regulation. Western blot analysis was performed to detect the phosphorylation levels of the 3 proteins of MAPK including extracellular signal regulated kinase (ERK), c-Jun N-terminal kinase (JNK) and p38 kinase (p38) in this study. As indicated in Figure 5, TNBS obviously enhanced protein phosphorylation of JNK, ERK and p38. After ALA treated, these phosphorylation levels were evidently decreased. Our data indicate that TNBS-induced activation of MAPK signaling pathway in colon is partially inhibited by ALA treatment.
Figure 5.
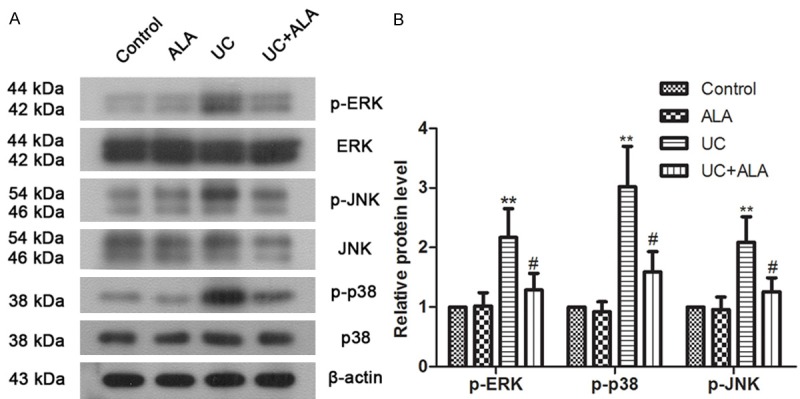
Effects of ALA on MAPK signaling in colon tissues. Mice were treated with ALA (80 mg/kg bw/day) or vehicle for 7 days after TNBS-induced UC. Protein levels of ERK, phosphorylated ERK, JNK, phosphorylated JNK, p38 and phosphorylated p38 in colon tissues were examined by western blot analysis (A). Representative blots are shown, and β-actin is used as a loading control. Quantitative analysis of protein expression was performed by densitometry (B). Data are presented as the means ± SD. **P < 0.01 vs. control group; #P < 0.05 vs. UC group.
Discussion
Induction of colitis by TNBS in mice is used widely as an experimental model of UC which can mimic human UC and can be extensively used to explore novel pharmacological approaches for preventing UC. In the current study, we investigated the role of ALA in TNBS-induced UC in vivo. Our results showed that ALA inhibited TNBS-induced colon inflammation and damages. Furthermore, ALA also remarkably inhibited apoptosis, oxidative stress, and MAPK activation in the colons from TNBS-induced mice, indicating a possible mechanism responsible for the protective role of ALA against TNBS-induced UC.
ALA is a multifunctional antioxidant and has been shown to possess potent bioactivity both in vivo and in vitro. It has been reported that ALA exhibits anti-inflammatory effects in carrageenan-induced acute inflammations and cotton pellet-induced chronic inflammations [6]. The effect of ALA on TNBS-induced colon injury was detected via histological analysis and colon mass index detection. In the present study, we found that ALA treatment attenuated TNBS-induced colon injury, as confirmed by histological evaluation. The beneficial actions of ALA observed in our study are consistent with a previous research showing that ALA improves gut mucosal lesions induced by TNBS in rats [19]. Furthermore, ALA treatment significantly restored mice body weight and reduced colon mass index. These results indicated a protective role of ALA in TNBS-induced UC, and the possible mechanisms needed to be further elucidated.
Apoptosis or programmed death, defined as an active death process of cell controlled by gene, is thought to be a key contributor to UC [20,21]. The aim of this research was to investigate the anti-apoptosis mechanism of ALA in TNBS-induced UC. Our findings suggested that TNBS significantly induced epithelial apoptosis in colon, and ALA attenuated the TNBS-induced apoptosis, as evidenced by TUNEL staining. The above result is accordance with previous studies from Li and Ji et al who showed that ALA inhibits apoptosis [22,23]. In addition, selected proteins involved in apoptotic signaling pathway such as bax, bcl-2 and caspase activity were investigated. Bcl-2 family proteins play an important role in the modulation of apoptosis. Bcl-2, the founder member of the family, specifically blocks the morphologic features of apoptosis, including the DNA cleavage, plasma membrane blebbing, and negative regulates cell death [24]. Bax is a pro-apoptotic factor which can accelerate programmed cell death by antagonizing the apoptosis repressor bcl-2. Under stress conditions, bax undergoes a conformation change that causes translocation to the mitochondrion membrane and leads to the release of cytochrome C that triggers apoptosis [25]. Caspases are proteins that are highly conserved including initiator caspases, such as caspase 2, 8, 9, 10, and effector caspases, such as caspase 3, 6, 7. The initiator caspases binds to specific oligomeric adaptin, activates effector caspases through proteolytic cleavage, and then the active effector caspases degrade a host of intracellular proteins to carry out the cell death program [26]. The current study demonstrated that TNBS increased the expression of bax and the activities of caspase-3 and caspase-9, whereas TNBS decreased the expression of bcl-2. ALA treatment significantly reduced Bax level and caspases activities and restored Bcl-2 level. Our results are in agreement with the previous studies showed that ALA could suppress apoptosis by modulating bax, bcl-2 levels and caspases activities [27-29]. These findings suggest that ALA is able to suppress TNBS-induced apoptosis via regulating bcl-2 family protein, thereby alleviating colon injury.
Excessive oxidative stress is potentially dangerous as it may alter inflammatory response, protein modifications and apoptosis. Therefore, oxidative stress has been implicated in numerous human diseases, including inflammatory bowel diseases [30,31]. Noteworthy, it is indicated that the colonic enterocytes are characterized not only by higher concentration of SOD and GPx, but also by higher ROS contents [32]. GSH and SOD are important components in protecting against the adverse reaction of ROS, whereas, MDA is a lipid peroxidative product used as an indicator of cellular oxidation status [33,34]. Moreover, it has been suggested that acute colitis may be aggravated by either too much or too little NO, which indicated that NO, especially that produced by the inducible form of iNOS, performed a critical role in the pathophysiology of colitis [35]. In this study, we found that ALA treatment significantly inhibited TNBS-induced up-regulation of MDA, NO and iNOS, whereas restored TNBS-induced depletion of GSH level and SOD activity. The previous studies showing ALA inhibits oxidative stress in mice are corresponded to our present observations [36,37]. These results illustrated that ALA suppressed TNBS-induced oxidative stress in mice.
Previous studies have documented that MAPK signaling was involved in pathogenesis of inflammatory bowel diseases [38]. ERK, JNK, and p38 are considered to be main kinases in MAPK. The results from western blot analysis showed a hyperphosphorylation of ERK, JNK, and p38 after TNBS instillation, whereas, ALA could reduce the phosphorylation levels. Several lines of evidence showed that ALA could decline the activation of MAPK [39,40], which were accordance with our above results. The findings indicate that ALA is able to suppress TNBS-induced MAPK activation, and the inhibition of MAPKs may partly contribute to the protective effects.
In conclusion, the present study showed that ALA effectively suppressed TNBS-induced UC, possible through reducing apoptosis and oxidative stress. The inhibition of MAPK activation by ALA may account for its protective effects against TNBS-induced colon injury. These findings presented here also imply that ALA have therapeutic potential in the prevention of UC and represent a promising therapeutic strategy.
Acknowledgements
This study was supported by a grant from the Science and Technology Program of Department of Science and Technology, Liaoning Province (No.: 2013020198-202).
Disclosure of conflict of interest
None.
References
- 1.Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307–317. doi: 10.1038/nature10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Irving PM, Gibson PR. Infections and IBD. Nat Clin Pract Gastroenterol Hepatol. 2008;5:18–27. doi: 10.1038/ncpgasthep1004. [DOI] [PubMed] [Google Scholar]
- 3.Irving PM, Shanahan F, Rampton DS. Drug interactions in inflammatory bowel disease. Am J Gastroenterol. 2008;103:207–219. doi: 10.1111/j.1572-0241.2007.01559.x. [DOI] [PubMed] [Google Scholar]
- 4.Karlen P, Lofberg R, Brostrom O, Leijonmarck CE, Hellers G, Persson PG. Increased risk of cancer in ulcerative colitis: a population-based cohort study. Am J Gastroenterol. 1999;94:1047–1052. doi: 10.1111/j.1572-0241.1999.01012.x. [DOI] [PubMed] [Google Scholar]
- 5.Lichtenstein GR. Current Research in Crohn’s Disease and Ulcerative Colitis: Highlights from the 2010 ACG Meeting. Gastroenterol Hepatol (N Y) 2010;6:3–14. [PMC free article] [PubMed] [Google Scholar]
- 6.Odabasoglu F, Halici Z, Aygun H, Halici M, Atalay F, Cakir A, Cadirci E, Bayir Y, Suleyman H. alpha-Lipoic acid has anti-inflammatory and anti-oxidative properties: an experimental study in rats with carrageenan-induced acute and cotton pellet-induced chronic inflammations. Br J Nutr. 2011;105:31–43. doi: 10.1017/S0007114510003107. [DOI] [PubMed] [Google Scholar]
- 7.Min AK, Kim MK, Seo HY, Kim HS, Jang BK, Hwang JS, Choi HS, Lee KU, Park KG, Lee IK. Alpha-lipoic acid inhibits hepatic PAI-1 expression and fibrosis by inhibiting the TGF-beta signaling pathway. Biochem Biophys Res Commun. 2010;393:536–541. doi: 10.1016/j.bbrc.2010.02.050. [DOI] [PubMed] [Google Scholar]
- 8.Golbidi S, Badran M, Laher I. Diabetes and alpha lipoic Acid. Front Pharmacol. 2011;2:69. doi: 10.3389/fphar.2011.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feuerecker B, Pirsig S, Seidl C, Aichler M, Feuchtinger A, Bruchelt G, Senekowitsch-Schmidtke R. Lipoic acid inhibits cell proliferation of tumor cells in vitro and in vivo. Cancer Biol Ther. 2012;13:1425–1435. doi: 10.4161/cbt.22003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaya-Dagistanli F, Tanriverdi G, Altinok A, Ozyazgan S, Ozturk M. The effects of alpha lipoic acid on liver cells damages and apoptosis induced by polyunsaturated fatty acids. Food Chem Toxicol. 2013;53:84–93. doi: 10.1016/j.fct.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 11.Lee WR, Kim A, Kim KS, Park YY, Park JH, Kim KH, Kim SJ, Park KK. Alpha-lipoic acid attenuates atherosclerotic lesions and inhibits proliferation of vascular smooth muscle cells through targeting of the Ras/MEK/ERK signaling pathway. Mol Biol Rep. 2012;39:6857–6866. doi: 10.1007/s11033-012-1511-5. [DOI] [PubMed] [Google Scholar]
- 12.Lim SY, Bae EH, Jeong MH, Kim JH, Hong YJ, Sim DS, Kim YS, Park IK, Ahn Y, Song SJ, Cho DL, Kim KS, Kang JC. Effect of alpha lipoic acid in a porcine in-stent restenosis model. J Cardiol. 2009;54:375–385. doi: 10.1016/j.jjcc.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 13.Trivedi PP, Jena GB. Role of alpha-lipoic acid in dextran sulfate sodium-induced ulcerative colitis in mice: studies on inflammation, oxidative stress, DNA damage and fibrosis. Food Chem Toxicol. 2013;59:339–355. doi: 10.1016/j.fct.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 14.He J, Liang J, Zhu S, Zhao W, Zhang Y, Sun W. Protective effect of taurohyodeoxycholic acid from Pulvis Fellis Suis on trinitrobenzene sulfonic acid induced ulcerative colitis in mice. Eur J Pharmacol. 2011;670:229–235. doi: 10.1016/j.ejphar.2011.08.036. [DOI] [PubMed] [Google Scholar]
- 15.Cromer WE, Ganta CV, Patel M, Traylor J, Kevil CG, Alexander JS, Mathis JM. VEGF-A isoform modulation in an preclinical TNBS model of ulcerative colitis: protective effects of a VEGF164b therapy. J Transl Med. 2013;11:207. doi: 10.1186/1479-5876-11-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luk HH, Ko JK, Fung HS, Cho CH. Delineation of the protective action of zinc sulfate on ulcerative colitis in rats. Eur J Pharmacol. 2002;443:197–204. doi: 10.1016/s0014-2999(02)01592-3. [DOI] [PubMed] [Google Scholar]
- 17.Piechota-Polanczyk A, Fichna J. Review article: the role of oxidative stress in pathogenesis and treatment of inflammatory bowel diseases. Naunyn Schmiedebergs Arch Pharmacol. 2014;387:605–620. doi: 10.1007/s00210-014-0985-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jena G, Trivedi PP, Sandala B. Oxidative stress in ulcerative colitis: an old concept but a new concern. Free Radic Res. 2012;46:1339–1345. doi: 10.3109/10715762.2012.717692. [DOI] [PubMed] [Google Scholar]
- 19.Kolgazi M, Jahovic N, Yuksel M, Ercan F, Alican I. Alpha-lipoic acid modulates gut inflammation induced by trinitrobenzene sulfonic acid in rats. J Gastroenterol Hepatol. 2007;22:1859–1865. doi: 10.1111/j.1440-1746.2006.04504.x. [DOI] [PubMed] [Google Scholar]
- 20.van der Woude CJ, Moshage H, Homan M, Kleibeuker JH, Jansen PL, van Dekken H. Expression of apoptosis related proteins during malignant progression in chronic ulcerative colitis. J Clin Pathol. 2005;58:811–814. doi: 10.1136/jcp.2004.017418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seidelin JB, Nielsen OH. Epithelial apoptosis: cause or consequence of ulcerative colitis? Scand J Gastroenterol. 2009;44:1429–1434. doi: 10.3109/00365520903301212. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Liu YZ, Shi JM, Jia SB. Alpha lipoic acid protects lens from H(2)O(2)-induced cataract by inhibiting apoptosis of lens epithelial cells and inducing activation of anti-oxidative enzymes. Asian Pac J Trop Med. 2013;6:548–551. doi: 10.1016/S1995-7645(13)60094-2. [DOI] [PubMed] [Google Scholar]
- 23.Ji D, Majid AS, Yin ZQ. alpha-Lipoic acid attenuates light insults to neurones. Biol Pharm Bull. 2013;36:1060–1067. doi: 10.1248/bpb.b12-00941. [DOI] [PubMed] [Google Scholar]
- 24.Ola MS, Nawaz M, Ahsan H. Role of Bcl-2 family proteins and caspases in the regulation of apoptosis. Mol Cell Biochem. 2011;351:41–58. doi: 10.1007/s11010-010-0709-x. [DOI] [PubMed] [Google Scholar]
- 25.Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parrish AB, Freel CD, Kornbluth S. Cellular mechanisms controlling caspase activation and function. Cold Spring Harb Perspect Biol. 2013:5. doi: 10.1101/cshperspect.a008672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ozturk G, Ginis Z, Kurt SN, Albayrak A, Bilen S, Fadillioglu E. Effect of alpha lipoic acid on ifosfamide-induced central neurotoxicity in rats. Int J Neurosci. 2014;124:110–116. doi: 10.3109/00207454.2013.823962. [DOI] [PubMed] [Google Scholar]
- 28.Yang Y, Wang W, Liu Y, Guo T, Chen P, Ma K, Zhou C. alpha-lipoic acid inhibits high glucose-induced apoptosis in HIT-T15 cells. Dev Growth Differ. 2012;54:557–565. doi: 10.1111/j.1440-169X.2012.01356.x. [DOI] [PubMed] [Google Scholar]
- 29.Lee SJ, Kim SH, Kang JG, Kim CS, Ihm SH, Choi MG, Yoo HJ. Alpha-lipoic acid inhibits endoplasmic reticulum stress-induced cell death through PI3K/Akt signaling pathway in FRTL5 thyroid cells. Horm Metab Res. 2011;43:445–451. doi: 10.1055/s-0031-1277182. [DOI] [PubMed] [Google Scholar]
- 30.Valko M, Morris H, Mazur M, Rapta P, Bilton RF. Oxygen free radical generating mechanisms in the colon: do the semiquinones of vitamin K play a role in the aetiology of colon cancer? Biochim Biophys Acta. 2001;1527:161–166. doi: 10.1016/s0304-4165(01)00163-5. [DOI] [PubMed] [Google Scholar]
- 31.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 32.Sanders LM, Henderson CE, Hong MY, Barhoumi R, Burghardt RC, Carroll RJ, Turner ND, Chapkin RS, Lupton JR. Pro-oxidant environment of the colon compared to the small intestine may contribute to greater cancer susceptibility. Cancer Lett. 2004;208:155–161. doi: 10.1016/j.canlet.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 33.Blokhina O, Virolainen E, Fagerstedt KV. Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot. 2003;91 Spec No:179–194. doi: 10.1093/aob/mcf118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Negre-Salvayre A, Coatrieux C, Ingueneau C, Salvayre R. Advanced lipid peroxidation end products in oxidative damage to proteins. Potential role in diseases and therapeutic prospects for the inhibitors. Br J Pharmacol. 2008;153:6–20. doi: 10.1038/sj.bjp.0707395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshida Y, Iwai A, Itoh K, Tanaka M, Kato S, Hokari R, Miyahara T, Koyama H, Miura S, Kobayashi M. Role of inducible nitric oxide synthase in dextran sulphate sodium-induced colitis. Aliment Pharmacol Ther. 2000;14(Suppl 1):26–32. doi: 10.1046/j.1365-2036.2000.014s1026.x. [DOI] [PubMed] [Google Scholar]
- 36.Abdel-Zaher AO, Mostafa MG, Farghaly HS, Hamdy MM, Abdel-Hady RH. Role of oxidative stress and inducible nitric oxide synthase in morphine-induced tolerance and dependence in mice. Effect of alpha-lipoic acid. Behav Brain Res. 2013;247:17–26. doi: 10.1016/j.bbr.2013.02.034. [DOI] [PubMed] [Google Scholar]
- 37.El-Beshbishy HA, Bahashwan SA, Aly HA, Fakher HA. Abrogation of cisplatin-induced nephrotoxicity in mice by alpha lipoic acid through ameliorating oxidative stress and enhancing gene expression of antioxidant enzymes. Eur J Pharmacol. 2011;668:278–284. doi: 10.1016/j.ejphar.2011.06.051. [DOI] [PubMed] [Google Scholar]
- 38.Sun P, Zhou K, Wang S, Li P, Chen S, Lin G, Zhao Y, Wang T. Involvement of MAPK/NF-kappaB signaling in the activation of the cholinergic anti-inflammatory pathway in experimental colitis by chronic vagus nerve stimulation. PLoS One. 2013;8:e69424. doi: 10.1371/journal.pone.0069424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Min AK, Kim MK, Kim HS, Seo HY, Lee KU, Kim JG, Park KG, Lee IK. Alpha-lipoic acid attenuates methionine choline deficient diet-induced steatohepatitis in C57BL/6 mice. Life Sci. 2012;90:200–205. doi: 10.1016/j.lfs.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 40.Deslauriers J, Desmarais C, Sarret P, Grignon S. Implication of the ERK/MAPK pathway in antipsychotics-induced dopamine D2 receptor upregulation and in the preventive effects of (+/-)-alpha-lipoic acid in SH-SY5Y neuroblastoma cells. J Mol Neurosci. 2014;52:378–383. doi: 10.1007/s12031-013-0158-6. [DOI] [PubMed] [Google Scholar]


