Abstract
Background: To illustrate whether the steroid-antivirals treatment could acquire a better recovery in patients with Bell’s palsy than the steroids alone treatment. Materials and methods: We conducted an exhaustive search over Pub med/Medline, Ovid, Elsevier search engines and the Cochrane library thereby collecting the randomized controlled trials in the treatment of patients with Bell’s palsy with steroid-antivirals and steroids. The qualities of relevant articles were assessed by GRADE, which was used to present the overall quality of evidence as recommended by the Cochrane Handbook for Systematic Reviews of Interventions. Results: Two investigators evaluated these papers independently, and resolved the disagreements by discussion. At last 8 eligible papers (1816 patients included: 896 treated with steroid-antivirals and 920 treated with steroids alone) match the criteria. Owing to the result (chi2 = 12.57, P = 0.08, I2 = 44%) presented by the formal test for heterogeneity, the fixed effect meta-analysis model was chosen. The facial muscle recovery between the steroids-antivirals group and the steroids alone group show significant differences (OR = 1.52, 95% CI: 1.20-1.94), while the statistical outcome of adverse effect shows no statistical significance (OR = 1.28, 95% CI: 0.71-2.31). Conclusions: The present meta-analysis indicates that the steroid-antivirals treatment could improve the recovery rate in patients with Bell’s palsy when comparing with the steroid alone treatment. Clinical significance: This meta-analysis showed that the steroid-antivirals treatment achieved the better outcomes in patients with Bell’s palsy. Clinicians should consider that steroid-antivirals therapy is an alternative choice for the patients with Bell’s palsy.
Keywords: Steroid-antivirals, steroids, Bell’s palsy, meta-analysis
Introduction
Bell’s palsy is an acute peripheral facial paresis caused by unknown factors. [1] Usually, the diagnosis is established without difficulty in patients presenting with unexplained unilateral isolated facial weakness [2]. It affects 11 to 40 people per 100,000 in the population per annum, most commonly in the age group 30 to 45 [3]. The etiology of Bell’s palsy remains indistinct, but the reactivation of herpes simplex virus (HSV) which can be detected by the polymerase chain reaction (PCR) to analyze the saliva of patients with Bell’s palsy for the presence of shed HSV-1 in about 31-79% of patients of Bell’s palsy might be one reason [4-6]. Moreover, the injection of HSV into the auricle or tongue could induce Bell’s palsy in an animal model [7]. Thus, patients are prescribed with antivirals, such as aciclovir, famciclovir and valaciclovir [8]. Inflammation and edema of the facial nerve are implicated in causing Bell’s palsy and steroids have a potent anti-inflammatory action which should minimize nerve damage and thereby improve the outcome [9,10]. Therefore, steroids was used to treat Bell’s palsy and ended up with significantly improving outcomes [11].
Some studies showed that the addition of antiviral agents could effectively enhance the curative effect of steroids [12-14]. However, Kawaguchi K et al and Engstrom, M et al found that the combination of prednisolone and valacyclovir did not achieve significantly better performance than prednisolone alone [15,16]. With the dispute existing, the present meta-analysis was made to illustrate whether the steroid-antivirals treatment could acquire a better recovery in patients with Bell’s palsy than the steroids alone treatment.
Materials and methods
Search strategy and study selection
We searched the electronic databases Pubmed, Medline, Web of science and the Cochrane library to collect the eligible original papers from 1984 (the year that aciclovir was licensed for clinical use) to July 2014 using the following Medical Subject Heading (MeSH) terms: “bell’s palsy”, “bell palsy”, “bell’s palsies”, “idiopathic facial paralyses”, “idiopathic facial paralysis”, “herpetic facial paralysis”, “acyclovir”, “valacyclovir”, “famcyclovir”, “famciclovir”, “Antiviral agents”, “steroids”, “methylprednisolone”, “prednisolone”. Search strategies for both databases are described in the Table 1.
Table 1.
Search strategies for collecting papers
| Search MeSH terms | |
|---|---|
| 1# | “bell’s palsy,” or “bell palsy,” or “bell’s palsies,” or “idiopathic facial paralyses,” or “idiopathic facial paralysis,” or “herpetic facial paralysis |
| 2# | “acyclovir,” or “valacyclovir,” or “famcyclovir,” or “famciclovir.” or “antiviral agents” |
| 3# | “Steroids”, or “methylprednisolone”, or “Prednisolone” |
| 4# | 1# and 2# |
| 5# | 1# and 3# |
| 6# | 4# or 5# |
The eligible papers were limited to the following criteria:
(a) Randomized controlled trials (RCT) or controlled clinical trials (CCTs);
(b) The original papers were written in English;
(c) Studied subjects aged 15 years or older with sufficient data;
(d) At least 25 patients in total were included;
(e) An assessment of facial muscle recovery determined by a recognized scoring system, such as the House-Brackmann grade, Yanagihara score or the facial paralysis recovery index.
No controlled clinical trials, editorial letters, pilot studies, historical reviews, cohort, observational and descriptive studies, case reports, In vitro studies, review, editorial letters were excluded. In addition, animal studies were also excluded.
Furthermore, we reviewed citations in the retrieved articles to search for additional relevant studies. The criteria of collecting papers were reviewed by two authors (Y.B.D and Y.Z) and checked by another author (C.M).
Two investigators evaluated these papers independently, and then resolved the disagreements by discussion. Literature selection was present in the PRISMA flow chart, a four-phase flow diagram, (Figure 1) according to the PRISMA guidelines [17,18].
Figure 1.
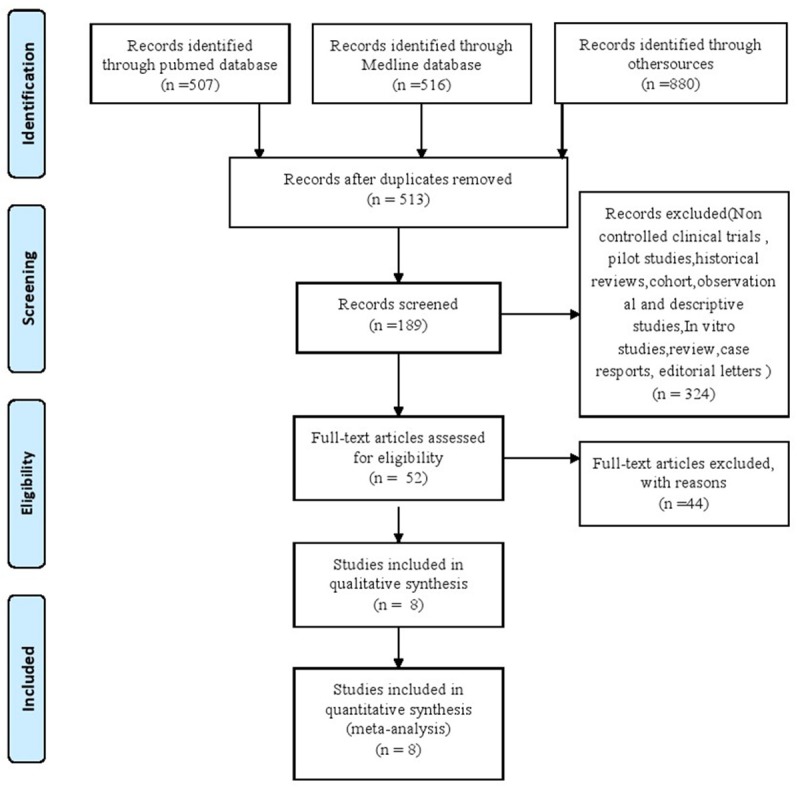
Flow Chart of Study Selection Process.
Data extraction & quality assessment (QA)
Data extraction was independently carried out by two investigators (Y.B.D and Y.Z) using a standardized data extraction form which included (a) study design, (b) year of publication, (c) sample size, (d) patient characteristics (i.e. gender, age, type of treatment, study style, Mean disease score and so on), (e) type of intervention and comparator, (f) outcomes, and (g) follow-up time. Quality of studies was assessed by two independent investigators (Y.B.D and Y.Z) based on a modified Jadad score, which takes into account of randomization technique, allocation concealment, blinding, intention to treat, and patient attrition [19]. All controversies between the two reviewers were resolved by discussion.
Statistical analyses
The outcome of facial muscle recovery from Bell’s palsy and the adverse effects were summarized. Odds ratio (OR) and 95% CI (confidence intervals) were employed to evaluate each outcome for the proportion of patients with at least partial facial muscle recovery who were treated with steroid-antivirals compared with those who received steroids alone. The OR would be considered statistically significant at the P < 0.05 level if the 95% CI does not include the value 1. Heterogeneity of the trails was assessed by I2, which gives a better measure of the consistency opposed to random error or chance between trials in a meta-analysis. According to I2 values (low: 0-25%; moderate: 25-50%; high: 50-75%; extreme: 75-100%) , we calculated the OR using fixed effect model or random effect model (Mantel-Haenszel method) [20]. The test seeks to determine whether there are genuine differences underlying the results of the studies (heterogeneity), or whether the variation in findings is compatible with chance alone (homogeneity) [21].
The meta-analysis was carried out with the RevMan 5.2 software (http://ims.cochrane.org/revman/download). Effect sizes were calculated as Hedges’ adjusted with 95% CI.
Results
Search results
We searched 298 potentially relevant papers in relation to Bell’s palsy. 189 articles were available after duplicates removed. We excluded 137 articles because of the inconformity to the criteria (a), (b) and (c). Finally, after assessing the full-text articles for eligibility, we adopted 8 studies in total in the present meta-analysis [11-14,16,22-24]. The cross-sectional or RCT studies were included based on our eligibility criteria. The detailed steps of paper collection are shown in the Flow Chart (Figure 1).
Description of included studies
The 8 eligible papers include 896 patients treated with steroid-antivirals and 920 patients treated with steroids alone. The RCTs showed a low risk of bias according to the Cochrane Collaboration’s tool for assessing the risk of bias [25]. The quality score of studies ranged from 6 to 8 according to the Newcastle-Ottawa Scale [26]. General characteristics of the patients in the articles are present in Table 2, and the patients were followed up at the longest follow-up point. The retrieved studies and the levels of evidence were described referring to Oxford Centre for Evidence-based Medicine [27]. The study quality was also estimated according to the GRADE system, which was used to present the overall quality of evidence as recommended by the Cochrane Handbook for Systematic Reviews of Interventions [28]. Figure 2 shows the outcomes of ‘summary of findings’, which was moderate for the new outcome.
Table 2.
Characteristics of the included studies
| Author | N | % Male Participants | Age | Type of treatment | Study style | Mean disease severity score | Full time (month) | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Intervention (dosage) | Control (dosage) | |||||||
| Adour, Kedar K (1996) | 99 | 55*,a | 44.6*,a | Valaciclovir 2000 mg/d × 10 d | Prednisolone with the same dosage | RCT (double blind) | House-Brackmann grade (II-V) | 4 |
| 43#,a | 41.9#,a | Prednisolonee 60 mg/d × 5 d | ||||||
| 50 mg/d × 6 d, | ||||||||
| 40 mg/d × 7 d, | ||||||||
| 30 mg/d × 8 d | ||||||||
| 20 mg/d × 9 d | ||||||||
| 10 mg/d × 10 d | ||||||||
| Axelsson, S (2012) | 415 | 50.7*,a | 18-75 | Valaciclovir 3000 mg/d × 7 d | Prednisolone with the same dosage | RCT (double blind) | Sunnybrook facial grading system severe-moderate | 12 |
| 52#,a | Prednisolonee 60 mg/d × 5 d | |||||||
| 50 mg/d × 6 d, | ||||||||
| 40 mg/d × 7 d, | ||||||||
| 30 mg/d × 8 d | ||||||||
| 20 mg/d × 9 d | ||||||||
| 10 mg/d × 10 d | ||||||||
| Engstrom, M (2008) | 416 | 61*,a | 18-75 | Valaciclovir 3000 mg/d × 7 d | Prednisolone with the same dosage | RCT (double blind) | House-Brackmann grade (II-V) | 12 |
| 1#,a | Prednisolone 60 mg/d × 5 d | |||||||
| 50 mg/d × 6 d, | ||||||||
| 40 mg/d × 7 d, | ||||||||
| 30 mg/d × 8 d | ||||||||
| 20 mg/d × 9 d | ||||||||
| 10 mg/d × 10 d | ||||||||
| Lee, H. Y (2013) | 206 | 47.7*,a | 48.6*,a | Famciclovir 750 mg/d × 7 d | Methylprednisolon with the same dosage | RCT (double blind) | House-Brackmann grade ≥ V | 6 |
| 50.5#,a | 48.7#,a | Methylprednisolone 64 mg/d × 4 d | ||||||
| 48 mg/d × 5-6 d, | ||||||||
| 32 mg/d × 7-8 d, | ||||||||
| 16 mg/d × 9-10 d | ||||||||
| Minnerop, M. (2008) | 117 | 50.7*,a | 40.6*,a | Valaciclovir 750 mg/d × 7 d | Prednisolone with the same dosage | RCT (not blind) | House-Brackmann grade (II-V) | 3 |
| 52#,a | 42.6#,a | Prednisolone (1 mg/kg body weight) for 4 days and tapered subsequently over the following 8 days | ||||||
| Yeo, S. G (2008) | 91 | 42.6*,a | 40.2*,a | Acyclovir 2400 mg/d × 5 d | Prednisolone with the same dosage | RCT (double blind) | House-Brackmann grade (II-V) | 6 |
| 47.7#,a | 42.7#,a | Prednisone, at a dose of 1 mg/kg per day (maximum, 80 mg/d) × 4 days | ||||||
| 60 mg/d × 5-6 d, | ||||||||
| 40 mg/d × 7-8 d, | ||||||||
| 20 mg/d × 9-10 d | ||||||||
| Hato, N. (2007) | 221 | 47*,a | 52.3#,a | Valacyclovir 1,000 mg/d × 5 d | Prednisolone with the same dosage | RCT (singleblind) | House-Brackmann grade (II-V) | 6 |
| 48#,a | 48.4*,a | Prednisolonee 60 mg/d × 5 d | ||||||
| 50 mg/d × 6 d, | ||||||||
| 40 mg/d × 7 d, | ||||||||
| 30 mg/d × 8 d | ||||||||
| 20 mg/d × 9 d | ||||||||
| 10 mg/d × 10 d | ||||||||
the steroids alone group;
the steroids-antivirals group;
mean age.
Figure 2.
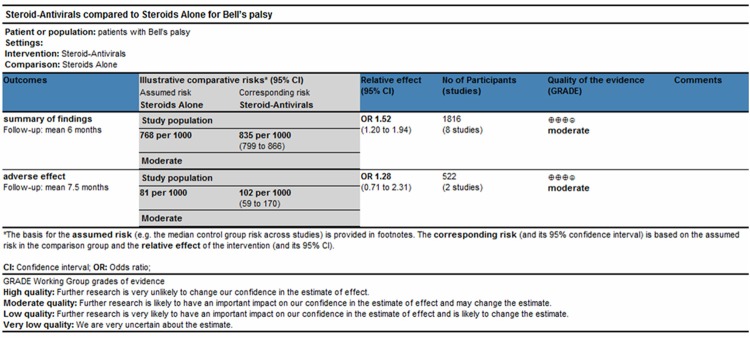
Summary of findings table and adverse effect using GRADE methodology.
Results of the meta-analysis
Owing to the result (chi2 = 12.57, P = 0.08, I2 = 44%) presented by the formal test for heterogeneity, the fixed effect meta-analysis model was chosen. The pooled proportion of patients recovery was 83.6% (749/896) among those who received steroids-antivirals, while the steroids alone group was 76.8% (707/920). There were significant differences in facial muscle recovery between the steroids-antivirals group and steroids alone group (OR = 1.52, 95% CI: 1.20-1.94) (Figure 3).
Figure 3.
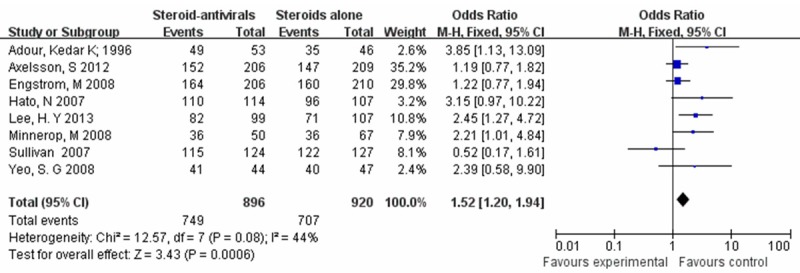
The steroids-antivirals group versus the steroids alone group (fixed effects model).
To assess the effect of study quality on our results, we further performed a sensitivity analysis by changing the effect model into random effect model. Consistently with the fixed effect model, the differences between the steroids-antivirals group and the steroids alone group stayed statistically significant (OR = 1.65, 95% CI: 1.14-2.38) (Figure 4).
Figure 4.
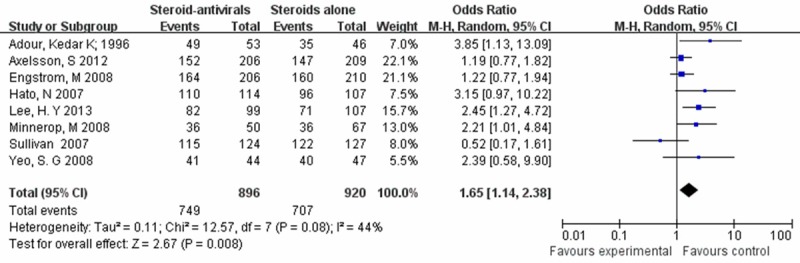
The steroids-antivirals group versus the steroids alone group (random effects model).
Two studies (Engström 2008 and Minnerop 2008) [13,16] reported the adverse effects of the two therapies, such as headache and fatigue and so on. There was a low heterogeneity among the trials (I2 = 0%). So we calculate the statistically outcome using the fixed effect model, and no statistically significant difference was observed (OR = 1.28, 95% CI: 0.71-2.31) (Figure 5).
Figure 5.
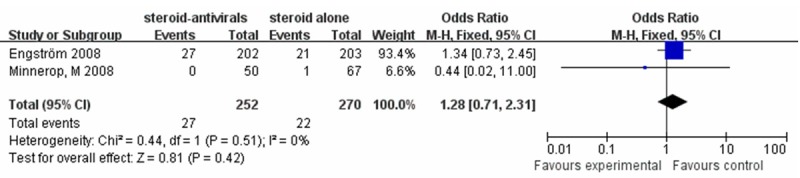
The adverse effect of steroids-antivirals group versus the steroids alone group (fixed effects model).
Discussion
In general, Bell’s palsy affects each individual differently. The treatments for Bell’s palsy are different. Recent studies have shown that steroids such as the steroid prednisone which was used to reduce inflammation and swelling are effective in treating Bell’s palsy [9]. Some studies showed that the addition of antiviral agents such as acyclovir could effectively enhance the curative effect of steroids [12-14].
Taking account of the different treatments for Bell’s palsy, we performed a Meta-analysis to get a better treatment for the patients with Bell’s palsy. Eight randomized controlled trials include 1816 patients in our meta-analysis. A high facial recovery rate at least partial recovery were achieved, no matter the patients were given steroid-antivirals or steroids alone. Our pooled odds ratio for facial muscle recovery (OR = 1.52, 95% CI: 1.20-1.94) (Figure 3) indicate that the steroid-antivirals treatment improves the recovery rate in patients with Bell’s palsy. The quality of this study was moderate which was estimated by the GRADE system, supporting the new outcome.
Furthermore, oral antivirals are well tolerated if administered at standard doses. The rate of adverse effects of antiviral agents is low in all cases. We observe the adverse effects of included studies. Two trials (Engström 2008 and Minnerop 2008) reported the adverse effect of two therapies [13,16]. The similar complications were presented in both groups. The statistically outcome shows no statistically significant difference between the steroid-antivirals group and the steroid alone group (OR = 1.28, 95% CI: 0.71-2.31) (Figure 5).
Several meta-analysis studies reported the different outcomes about whether combination treatment is effective on patients with Bell’s palsy. Goudakos JK’s study included 5 studies with 709 patients reporting no benefit of the steroid-antivirals treatment (OR = 1.03; 95% CI: 0.74-1.42) when compared with the steroid alone group [29]. But the small numbers in this meta-analysis may lead to lack of power. Numthavaj P’ net meta-analysis suggests that the current practice of treating Bell’s palsy with antiviral treatment plus corticosteroid may lead to slightly higher recovery rates compared with treating with prednisone alone. But their pooled estimates were quite heterogeneous. Moreover this study does not quite reach statistical significance [30]. The heterogeneity of included studies is moderate according to I2 values (I2 = 44%) in our Meta-analysis. Then the fixed effect meta-analysis model was chosen. At last we got the significant differences between the steroids-antivirals group and steroids alone group.
The two latest studies in our Meta-analysis which were double blind, had an effect on the result [14,23]. The House-Brackmann grade of the facial muscle paralysis in Lee, H. Y’s study is severe to complete. And Yeo, S. G’s study shows that physical therapy in the department of rehabilitation was adopted to treat the patients [23]. All of these may have a great effect on the last outcome. So sensitivity analysis was performed showing similar findings (OR = 1.76, 95% CI: 1.25-2.46) (Figure 4), that the steroid-antivirals treatment improves the recovery rate in patients with Bell’s palsy.
The steroid-antivirals treatment could improve the recovery rate in patients with Bell’s palsy in our Meta-analysis. Given the truly existing heterogeneity detected between the low number of studies, before making final conclusion, caution is still needed to determine whether the steroid-antivirals treatment can achieve a better curative effect than the steroids alone on patients with Bell’s palsy.
Conclusions
There were significant differences in facial muscle recovery between the steroids-antivirals group and the steroids alone group. This meta-analysis shows that the steroid-antivirals treatment achieves a recovery rate in patients with Bell’s palsy than the steroid alone treatment, supporting the routine addition of antivirals to steroids in Bell’s palsy. Clinicians should consider that steroid-antivirals therapy is a better choice for the patients with Bell’s palsy.
Acknowledgements
This study was supported by the Science and Technology Development Plan of Shandong Province (2012G0021833) and Lu Cai Jiao Zhi ([2013]171). The authors would like to thank the contributors of trails who agreed to share their data. We also thank Gaoyi Wu and Jinlong Chen for their invaluable help in the editing of this meta-analysis.
Disclosure of conflict of interest
None.
References
- 1.Hauser WA, Karnes WE, AnnisL J, Kurland T. Incidence and prognosis of Bell’s palsy in the population of Rochester, Minnesota. Mayo Clin Proc. 1971;46:258–64. [PubMed] [Google Scholar]
- 2.Katusic SK, Beard CM, Wiederholt WC, BergstralhL ET, Kurland T. Incidence, clinical features, and prognosis in Bell’s palsy, Rochester, Minnesota, 1968-1982. Ann Neurol. 1986;20:622–7. doi: 10.1002/ana.410200511. [DOI] [PubMed] [Google Scholar]
- 3.Lockhart P, Daly F, Pitkethly M, Comerford N. Sullivan F. Antiviral treatment for Bell’1(1):s palsy (idiopathic facial paralysis) Cochrane Database Syst Rev. 2009:CD001869. doi: 10.1002/14651858.CD001869.pub4. [DOI] [PubMed] [Google Scholar]
- 4.Murakami S, Mizobuchi M, Nakashiro Y, Doi T, Hato N, Yanagihara N. Bell palsy and herpes simplex virus: identification of viral DNA in endoneurial fluid and muscle. Ann Intern Med. 1996;124:27–30. doi: 10.7326/0003-4819-124-1_part_1-199601010-00005. [DOI] [PubMed] [Google Scholar]
- 5.Furuta Y, Fukuda S, Chida E, Takasu T, Ohtani F, Inuyama Y, Nagashima K. Reactivation of herpes simplex virus type 1 in patients with Bell’s palsy. J Med Virol. 1998;54:162–6. doi: 10.1002/(sici)1096-9071(199803)54:3<162::aid-jmv3>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 6.Abiko Y, Ikeda M, Hondo R. Secretion and dynamics of herpes simplex virus in tears and saliva of patients with Bell’s palsy. Otol Neurotol. 2002;23:779–83. doi: 10.1097/00129492-200209000-00028. [DOI] [PubMed] [Google Scholar]
- 7.Sugita T, Murakami S, Yanagihara N, Fujiwara Y, Hirata Y, Kurata T. Facial nerve paralysis induced by herpes simplex virus in mice: an animal model of acute and transient facial paralysis. Ann Otol Rhinol Laryngol. 1995;104:574–81. doi: 10.1177/000348949510400713. [DOI] [PubMed] [Google Scholar]
- 8.Allen D, Dunn L. Aciclovir or valaciclovir for Bell’s palsy (idiopathic facial paralysis) Cochrane Database Syst Rev. 2004:CD001869. doi: 10.1002/14651858.CD001869.pub2. [DOI] [PubMed] [Google Scholar]
- 9.Kim IS, Shin SH, Kim J, Lee WS, Lee HK. Correlation between MRI and operative findings in Bell’s palsy and Ramsay Hunt syndrome. Yonsei Med J. 2007;48:963–8. doi: 10.3349/ymj.2007.48.6.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jackson CG, von Doersten PG. The facial nerve. Current trends in diagnosis, treatment, and rehabilitation. Med Clin North Am. 1999;83:179–95. x. doi: 10.1016/s0025-7125(05)70096-1. [DOI] [PubMed] [Google Scholar]
- 11.Sullivan FM, Swan IR, Donnan PT, Morrison JM, Smith BH, McKinstry B, Davenport RT, Vale LD, Clarkson JE, Hammersley V, Hayavi S, McAteer A, Stewart K, Daly F. Early treatment with prednisolone or acyclovir in Bell’s palsy. N Engl J Med. 2007;357:1598–607. doi: 10.1056/NEJMoa072006. [DOI] [PubMed] [Google Scholar]
- 12.Adour KK, Ruboyianes JM, Von Doersten PG, Byl FM, Trent CS, Quesenberry CP Jr, Hitchcock T. Bell’s palsy treatment with acyclovir and prednisone compared with prednisone alone: a double-blind, randomized, controlled trial. Ann Otol Rhinol Laryngol. 1996;105:371–8. doi: 10.1177/000348949610500508. [DOI] [PubMed] [Google Scholar]
- 13.Minnerop M, Herbst M, Fimmers R, Kaabar P, Matz B, Klockgether T, Wullner U. Bell’s palsy: combined treatment of famciclovir and prednisone is superior to prednisone alone. J Neurol. 2008;255:1726–30. doi: 10.1007/s00415-008-0008-6. [DOI] [PubMed] [Google Scholar]
- 14.Lee HY, Byun JY, Park MS, Yeo SG. Steroid-antiviral treatment improves the recovery rate in patients with severe Bell’s palsy. Am J Med. 2013;126:336–41. doi: 10.1016/j.amjmed.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 15.Kawaguchi K, Inamura H, Abe Y, Koshu H, Takashita E, Muraki Y, Matsuzaki Y, Nishimura H, Ishikawa H, Fukao A, Hongo S, Aoyagi M. Reactivation of herpes simplex virus type 1 and varicella-zoster virus and therapeutic effects of combination therapy with prednisolone and valacyclovir in patients with Bell’s palsy. Laryngoscope. 2007;117:147–56. doi: 10.1097/01.mlg.0000248737.65607.9e. [DOI] [PubMed] [Google Scholar]
- 16.Engstrom M, Berg T, Stjernquist-Desatnik A, Axelsson S, Pitkaranta A, Hultcrantz M, Kanerva M, Hanner P, Jonsson L. Prednisolone and valaciclovir in Bell’s palsy: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet Neurol. 2008;7:993–1000. doi: 10.1016/S1474-4422(08)70221-7. [DOI] [PubMed] [Google Scholar]
- 17.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 20.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–48. [PubMed] [Google Scholar]
- 21.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hato N, Yamada H, Kohno H, Matsumoto S, Honda N, Gyo K, Fukuda S, Furuta Y, Ohtani F, Aizawa H, Aoyagi M, Inamura H, Nakashima T, Nakata S, Murakami S, Kiguchi J, Yamano K, Takeda T, Hamada M, Yamakawa K. Valacyclovir and prednisolone treatment for Bell’s palsy: a multicenter, randomized, placebo-controlled study. Otol Neurotol. 2007;28:408–13. doi: 10.1097/01.mao.0000265190.29969.12. [DOI] [PubMed] [Google Scholar]
- 23.Yeo SG, Lee YC, Park DC, Cha CI. Acyclovir plus steroid vs steroid alone in the treatment of Bell’s palsy. Am J Otolaryngol. 2008;29:163–6. doi: 10.1016/j.amjoto.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 24.Axelsson S, Berg T, Jonsson L, Engström M, Kanerva M, Stjernquist-Desatnik A. Bell’s palsy - the effect of prednisolone and/or valaciclovir versus placebo in relation to baseline severity in a randomised controlled trial. Clin Otolaryngol. 2012;37:283–90. doi: 10.1111/j.1749-4486.2012.02526.x. [DOI] [PubMed] [Google Scholar]
- 25.Higgins JPT, Green S. The Cochrane Collaboration, 2011. 2011. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011] Available from: www.cochrane-handbook.org. [Google Scholar]
- 26.Wells GA, Shea B, O’Connell D, Peterson D, Welch V, Losos M, Tugwell M, editors. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Hospital Research Institute (OHRI) 2012. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 27.Philips B, Ball C, Sackett D, Badenoch D, Straus S, Haynes B, Dawes M, editors. Oxford Centre for Evidence-based Medicine Levels of Evidence. 2009. Available: http://www.cebm.net/index.aspx?o=1025.
- 28.Higgins J, C R, Cumpston M, Chandler J. Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0. 2013. Available: http://www.cochrane.org/training/cochrane-handbook. [Google Scholar]
- 29.Goudakos JK, Markou KD. Corticosteroids vs corticosteroids plus antiviral agents in the treatment of Bell palsy: a systematic review and meta-analysis. Arch Otolaryngol Head Neck Surg. 2009;135:558–64. doi: 10.1001/archoto.2009.44. [DOI] [PubMed] [Google Scholar]
- 30.Numthavaj P, Thakkinstian A, Dejthevaporn C, Attia J. Corticosteroid and antiviral therapy for Bell’s palsy: a network meta-analysis. BMC Neurol. 2011;11:1. doi: 10.1186/1471-2377-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


