Abstract
MicroRNA-128 is down-regulated in glioma tissues, which regulates cell proliferation, self-renewal, apoptosis, angiogenesis and differentiation. This study aims at investigating the diagnostic value of serum miR-128 in human glioma. Real-time quantitative reverse transcriptase polymerase chain reaction was used to detect the expression levels of miR-128 in serum samples from 151 glioma patients, 59 postoperative patients, 52 meningioma patients and 53 normal donors. To analyze the association of miR-128 expression with clinicopathological parameters in serum samples and matched tissues, matched 151 glioma tissues were collected in the study. Receiver operating characteristic analysis (ROC) was utilized to evaluate the value of serum miR-128 as a biomarker for the early diagnosis of glioma. Results revealed that miR-128 expression was significantly decreased in glioma preoperative serum compared with normal controls and meningioma serum samples (both P < 0.001). ROC analyses showed that serum miR-128 levels were reliable in distinguishing patients with glioma from normal controls and meningioma, with the area under the curve (AUC) values of 0.9095 and 0.8283, respectively. In addition, the AUC value for discriminating glioma II-IV from I was 0.7362. Importantly, serum miR-128 expression was significantly elevated after surgery (P < 0.001), although it didn’t reach to normal levels (P < 0.001). Furthermore, low miR-128 levels in serum and tissue were markedly correlated with high pathological grade and low Karnofsky Performance Status score (KPS). These findings proved that serum miR-128 could be a sensitive and specific biomarker of glioma.
Keywords: MicroRNA-128, glioma, serum, biomarker, diagnosis
Introduction
Human gliomas are the most common malignant central nervous system neoplasms [1]. In the order of increasing malignancy, the World Health Organization (WHO) divides gliomas into four grades: pilocytic astrocytoma (PA, WHO grade I), diffuse astrocytoma (DA, WHO grade II), anaplastic astrocytoma (AA, WHO grade III), and glioblastoma (GBM, WHO grade IV) [2]. Although there are a few certain treatments for glioma, the prognosis remains poor [3]. For GBM patiens, the 5-year relative survival is less than 5% [4]. Nowadays, computed tomography (CT) and magnetic resonance imaging (MRI) are main methods to diagnose glioma before clinical treatment. Cheap, convenient and sensitive serum markers have not been discovered in the early diagnosis of glioma.
MiRNAs play crucial roles in tumorigenesis and metastasis for various types of cancer [5]. Due to their stability and different expression levels between tumors and controls in the serum, miRNAs could be used as potential biomarkers in tumor identification, early diagnosis, classification and prognosis prediction [6].
MiR-128 is enriched in normal brain tissue [7]. Recent studies have revealed the expression levels of miR-128 were significantly lower in glioma tissues compared with normal brain tissues [8-11]. As an tumor suppressor gene in glioma, miR-128 inhibits glioma cells angiogenesis, self-renewal and proliferation by targeting p70s6k1 [8], Bmi-1 [9], E2F3a [10] and RTKs [11]. Therefore, the aim of this study was to explore the expression profile of mir-128 in glioma serum and its diagnostic value in human glioma.
Materials and methods
Clinical samples
This study was completed with the approval of Jiangsu Province Medical Center Institutional Review Board, People’s Republic of China. Written informed consents were obtained from all participants. Preoperative serum samples were obtained from patients with pathologically confirmed glioma (n = 151) and meningioma (n = 52) at the Department of Neurosurgery of Wuxi First People’s Hospital, Wuxi Second People’s Hospital and The First Affiliated Hospital of Nanjing Medical University from June 2012 to February 2014. Additionally, matched 151 glioma tissues and 59 postoperative serum samples (7 days after tumor resection) were enrolled in this study. Fifty-three serum samples from healthy donors who went to the hospital for medical examinations were collected as normal controls. The blood samples were centrifuged for 20 min at 3000 rpm within 1 h after obtaining, and the supernatant was transferred into RNase-free tubes, then stored at -80°C. All resected tissue specimens were divided into two parts: one part was fixed in formalin for pathological diagnosis according to the 2007 WHO classification, and the other one was stored in liquid nitrogen until RNA isolation. Patients received preoperative treatment, such as radiotherapy or chemotherapy, were excluded from the study. Twenty-one normal brain tissues were obtained from internal decompression of patients who underwent surgery for cerebral hemorrhage or cerebral injury. The detailed characteristics of all the specimens are summarized in Table 1.
Table 1.
Detailed characteristics of all the specimens
| Characteristic | Preoperative glioma serum and matched tissues | Postoperative glioma serum N = 59 | Meningioma serum N = 52 | Normal serum N = 53 | Normal tissues N = 21 | |||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Grade I (n = 24) | Grade II (n = 23) | Grade III (n = 43) | Grade IV (n = 61) | |||||
| Age, median (range) | 54 (15-71) | 48 (23-70) | 51 (26-75) | 53 (24-79) | 51 (36-71) | 46 (19-63) | 47 (21-83) | 48 (36-69) |
| Sex | ||||||||
| Male/Female | 16/8 | 12/11 | 23/20 | 33/28 | 36/23 | 29/23 | 27/26 | 9/12 |
RNA extraction and qRT-PCR
MiRNA extraction from serum was performed using mirVana™ PARIS™ Kit (Invitrogen, Carlsbad, CA, USA), and miRNA extraction from tisssue samples was performed with Trizol reagent (Invitrogen, Carlsbad, CA, USA). NCode™ SYBR® Green miRNA qRT-PCR Kit (Invitrogen, Carlsbad, CA, USA) was used to perform qRT-PCR. Before isolating miRNA from serum, cel-miR-39 was added until its concentration reached 100 pmol/L. MiR-128 levels in serum were normalized to cel-miR-39, whereas they were normalized to U6B in tissues. Relative expression of miR-128 was quantified using 2-ΔCT method.
Statistical analysis
We used (log2 scale) to represent relative expression of miR-128 normalized to control [12]. All data were analyzed by the software of SPSS version 21.0 (SPSS Inc., Chicago, IL, USA), and presented as mean ± standard deviation (SD). Mann-Whitney U-test was used to assess statistical differences in serum or tissue miRNA expression between unpaired groups. Multiple comparisons between more than two groups were performed utilizing Kruskal-Wallis H-test. Differences between paired serum samples obtained before and 7 days after surgery were estimated according to Wilcoxon test. Association between miR-128 expression in serum and matched glioma tissues was detected by Spearman correlation test. The feasibility of serum miR-128 as a diagnostic tool for detecting glioma was calculated by the receiver operating characteristic (ROC) curve and the area under the ROC curve (AUC). The Youden’s index was utilized to determine the optimal cutoff threshold values [13]. P value (two-side) < 0.05 was considered statistically significant.
Results
Serum miR-128 levels were down-regulated in human glioma
All the data of serum miR-128 were shown in Figure 1, including 53 normal cohorts, 52 meningioma cohorts, 24 glioma I cohorts, 23 glioma II cohorts, 43 glioma III cohorts and 61 glioma IV cohorts. Serum miR-128 expression levels were significantly lower in glioma compared with normal cohorts (mean ± SD: 5.87 ± 1.25 versus 7.60 ± 0.47, P < 0.001), associated with advanced pathological grade. Levels of serum miR-128 were significantly lower in grade III and grade IV cohorts compared to grade I cohorts (mean ± SD: 5.86 ± 1.28 and 5.51 ± 1.02 versus 6.74 ± 1.15, P = 0.037 and P < 0.001, respectively). In addition, glioma serum demonstrated a significant decrease in miR-128 transcript levels compared with the mean expression levels observed in meningioma cohorts (5.87 ± 1.25 versus 7.35 ± 0.95, P < 0.001), which often need to be distinguished from glioma. However, no significant difference was detected between meningioma cohorts and normal cohorts (P = 0.375).
Figure 1.
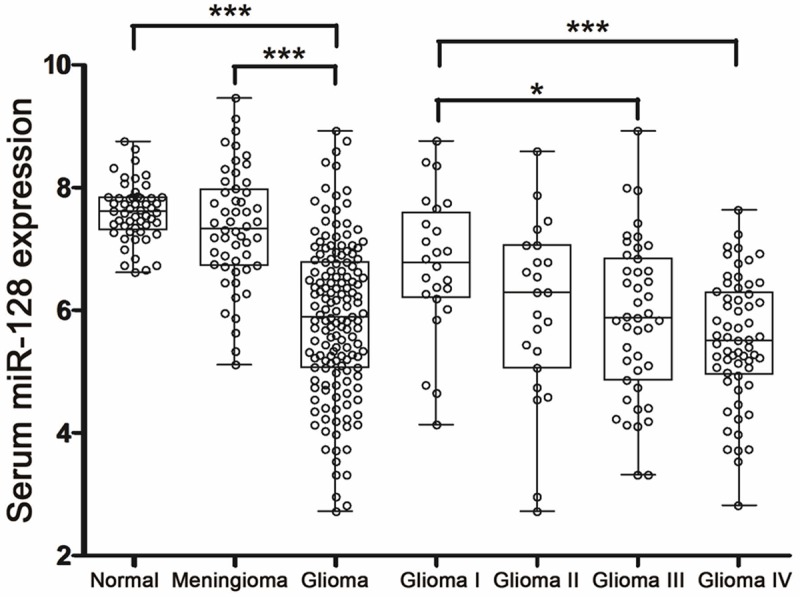
qRT-PCR results of 256 serum samples including 53 normal controls, 52 meningioma, 24 glioma I, 23 glioma II, 43 glioma III and 61 glioma IV samples. All data were normalized to cel-miR-39. Kruskal-Wallis H-test was used to assess statistical differences in multiple groups. *P < 0.05; ***P < 0.001.
Serum miR-128 levels could reliably discriminate glioma patients from control subjects
The ROC curves analysis showed that serum miR-128 levels could reliably distinguish patients with glioma from normal cohorts, with an AUC value of 0.9095 [95% confidence interval (CI): 0.8695-0.9496]. At the optimal cut-off value of 7.139, the sensitivity and specificity were 86.75% and 88.68%, respectively (Figure 2A). Importantly, serum miR-128 levels could differentiate glioma II-IV cohorts from grade I cohorts, which were always seen as benign tumors on the basis of histology and pathology. Using the optimal cut-off value of 5.840, serum miR-128 had sensitivity of 55.12% and specificity of 87.50%, and the AUC value was 0.7362 (95% CI: 0.6258-0.8466, Figure 2B). In addition, at the optimal cut-off value of 6.628, serum miR-128 yielded an AUC value of 0.8283 (95% CI = 0.7679 to 0.8888), with 72.19% sensitivity and 82.69% specificity in discriminating glioma cohorts from meningioma cohorts (Figure 2C).
Figure 2.
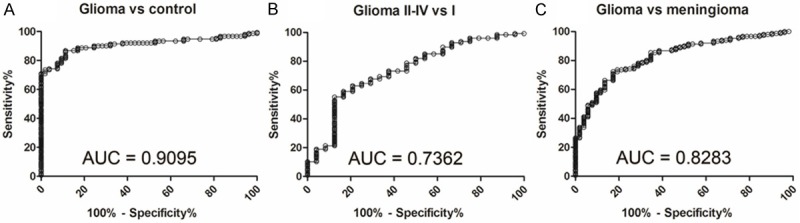
ROC curves indicate the ability of serum miR-128 levels to differentiate glioma cohorts (n = 151) from normal cohorts (n = 53) (A), glioma II-IV cohorts (n = 127) from grade I cohorts (n = 24) (B) and glioma cohorts (n = 151) from meningioma cohorts (n = 52) (C).
Univariable and multivariable logistic regression analyses showed that serum miR-128 could be a potential reliable diagnostic biomarker for glioma. All the results of logistic regression analyses were summarized in Table 2.
Table 2.
Results of univariable and multivariable logistic regression analyses
| Parameter | Univariate analysis, OR (95% CI) | P value | Multivariate analysis, OR (95% CI) | P value |
|---|---|---|---|---|
| Glioma vs normal | ||||
| Sex, male vs female | 1.207 (0.645-2.260) | 0.556 | 0.667 (0.267-1.668) | 0.387 |
| Age, ≥ 50 vs < 50 | 1.197 (0.639-2.240) | 0.574 | 1.347 (0.550-3.299) | 0.514 |
| Serum miR-128, < 7.139 vs ≥ 7.139Δ | 51.308 (19.425-135.521) | < 0.001 | 55.645 (20.427-151.578) | < 0.001 |
| Glioma II-IV vs I | ||||
| Sex, male vs female | 0.570 (0.230-1.442) | 0.239 | 0.476 (0.178-1.276) | 0.140 |
| Age, ≥ 50 vs < 50 | 0.887 (0.370-2.128) | 0.788 | 1.045 (0.405-2.701) | 0.927 |
| Serum miR-128, < 5.840 vs ≥ 5.840Δ | 8.596 (2.440-30.283) | 0.001 | 9.265 (2.597-33.053) | 0.001 |
| Glioma vs meningioma | ||||
| Sex, male vs female | 0.994 (0.527-1.875) | 0.986 | 0.734 (0.347-1.551) | 0.417 |
| Age, ≥ 50 vs < 50 | 0.989 (0.527-1.859) | 0.973 | 0.934 (0.446-1.956) | 0.857 |
| Serum miR-128, < 6.628 vs ≥ 6.628Δ | 12.399 (5.561-27.645) | < 0.001 | 12.991 (5.762-29.290) | < 0.001 |
The optimal cut-off value determined by Youden’s index.
Serum miR-128 levels of glioma patients would elevate after surgery
We chose 59 glioma patients and collected their serum in preoperation and postoperation (7 days after tumor resection). The results of qRT-PCR showed that serum miR-128 expression significantly elevated in postoperation (mean ± SD: 6.17 ± 1.04) compared with that in preoperation (mean ± SD: 5.73 ± 1.08). The P value calculated by Wilcoxon test was less than 0.001 (Figure 3). However, comparing with normal cohorts, serum miR-128 levels after surgery still did not revive to normal levels (P < 0.001).
Figure 3.
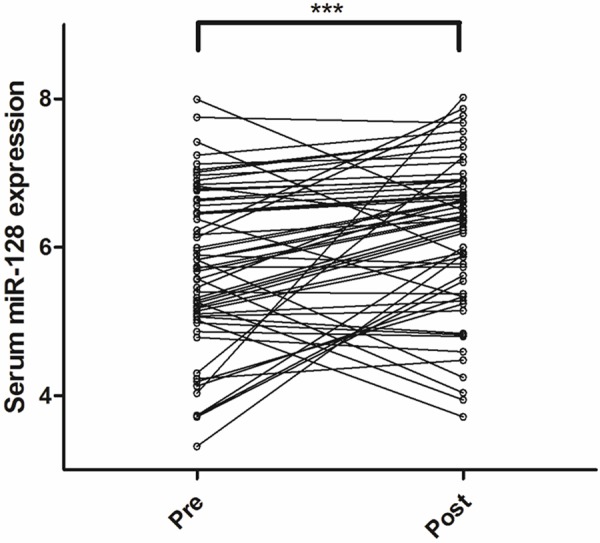
Serum miR-128 levels in 59 glioma patients before and after surgery. Differences between paired serum samples were estimated according to Wilcoxon test. ***P < 0.001.
The association between serum and tissue miR-128 expression levels in glioma patients
The miR-128 expression levels in 21 normal brain tissues and 151 matched glioma tissues were detected by qRT-PCR, the relevant values were shown in Figure 4. Similar to expression in serum, tissue miR-128 levels were significantly lower in glioma compared with normal cohorts (mean ± SD: 4.89 ± 0.87 versus 7.15 ± 1.24, P < 0.001), corresponding to glioma WHO grades. Levels of tissue miR-128 were significantly lower in grade III and grade IV cohorts than those in grade I cohorts (mean ± SD: 4.88 ± 0.85 and 4.48 ± 0.68 versus 5.49 ± 0.82, P = 0.049 and P < 0.001, respectively). However, different with expression in serum, tissue miR-128 levels were significantly decreased in grade IV cohorts compared to glioma II cohorts (mean ± SD: 4.48 ± 0.68 versus 5.35 ± 0.84, P < 0.001).
Figure 4.
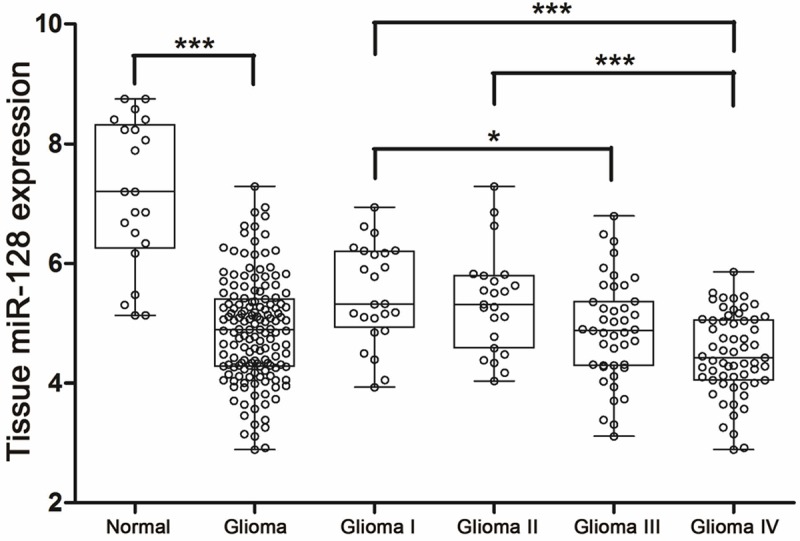
qRT-PCR results of 172 tissue samples including 21 normal controls, 24 glioma I, 23 glioma II, 43 glioma III and 61 glioma IV samples. All data were normalized to U6B. Mann-Whitney U-test was used to assess statistical differences in tissue miRNA expression between glioma and normal groups. Multiple comparisons between different WHO grade groups were performed utilizing Kruskal-Wallis H-test. *P < 0.05; ***P < 0.001.
Next, we analyzed the association of miR-128 expression with various clinicopathological features of glioma serum and matched tissue samples, and relevant data were summarized in Table 3. We found miR-128 levels in both serum and matched tissues were distinctly lower in glioma patients with advanced pathological grade (both P < 0.001) and lower KPS (P = 0.027 and P = 0.010, respectively). Similar results were not identified in other clinicopathological parameters, including sex, age, and tumor size.
Table 3.
The association of miR-128 expression with various clinicopathological features of glioma serum and matched tissue samples
| Parameter | Patients, n | Serum miR-128 expression (Mean ± SD) | P value | Tissue miR-128 expression (Mean ± SD) | P value |
|---|---|---|---|---|---|
| WHO grade | |||||
| I | 24 | 6.74 ± 1.15 | < 0.001 | 5.49 ± 0.82 | < 0.001 |
| II | 23 | 5.98 ± 1.44 | 5.35 ± 0.84 | ||
| III | 43 | 5.86 ± 1.28 | 4.88 ± 0.85 | ||
| IV | 61 | 5.51 ± 1.02 | 4.48 ± 0.68 | ||
| Sex | |||||
| Male | 84 | 5.72 ± 1.28 | 0.090 | 4.94 ± 0.91 | 0.331 |
| Female | 67 | 6.07 ± 1.20 | 4.82 ± 0.81 | ||
| Age | |||||
| < 50 | 73 | 5.75 ± 1.32 | 0.494 | 4.84 ± 0.82 | 0.545 |
| ≥ 50 | 78 | 5.99 ± 1.17 | 4.93 ± 0.91 | ||
| Tumor size | |||||
| < 5 cm | 82 | 5.78 ± 1.33 | 0.399 | 4.87 ± 0.74 | 0.842 |
| ≥ 5 cm | 69 | 5.98 ± 1.14 | 4.90 ± 1.01 | ||
| KPS | |||||
| < 90 | 103 | 5.71 ± 1.24 | 0.027 | 4.76 ± 0.78 | 0.010 |
| ≥ 90 | 48 | 6.22 ± 1.22 | 5.16 ± 0.98 |
Spearman’s correlation analysis revealed that serum miR-128 levels were significantly correlated with tissue miR-128 levels (Figure 5), with ρ value of 0.305 (P < 0.01). This result meant that circulating miR-128 levels could reliably reflect concentrations detected in glioma tissues.
Figure 5.
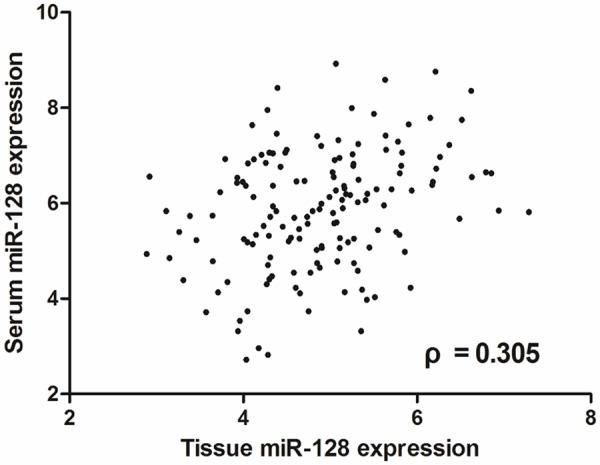
Relative expression levels of miR-128 in serum and matched tissue samples obtained from 151 glioma patients. Association between miR-128 expression in serum and matched glioma tissues was detected by Spearman correlation test.
Discussion
The early diagnosis and clinical treatment of glioma patients is very important. Previous research for circulating biomarkers of glioma had mostly focused on proteins [14], but detection of proteins is complicated and expensive. On the contrary, miRNAs have the advantage of low cost of being easily determined by qRT-PCR or microarrays [15].
The aim of this study is to investigate the diagnostic value of serum miR-128 in human glioma. In this study, we have three findings. Firstly, Serum and matched tissue miR-128 expression levels were significantly lower in glioma compared with normal cohorts, associated with advanced pathological grade and low KPS. Secondly, ROC curves analysis showed that serum miR-128 levels could reliably distinguish patients with glioma from normal or meningioma cohorts. Importantly, glioma II-IV cohorts could also be differentiated from grade I cohorts by serum miR-128 levels. Thirdly, the tumor resection could significantly elevate serum miR-128 levels in glioma patients. All the results suggested that serum miR-128 could be used as potential biomarkers in glioma identification, early diagnosis, classification and prognosis prediction.
MiR-128 function as a vital suppressor in the tumorigenesis in glioma cells. Shi et al. found that miR-128 could down-regulate p70S6K1 and its downstream signaling molecules including VEGF and HIF-1 expression, and inhibit tumor growth, proliferation and angiogenesis [8]. Another study indicated that miR-128 could bind to Bmi-1 mRNA 3’-untranslated region and cause this oncogene distinctly decrease in vitro [9]. In T98G cells, E2F3a, a transcription factor that regulates cell cycle progression, could be suppressed by overexpression of miR-128 [10]. Peruzzi et al. proved that miR-128 was an important suppressor of Polycomb Repressor Complexes (PRC), which was involved in glioma stem cell maintenance and radioresistance [16]. Therefore, further studies are deserved to explore the mechanism of miR-128 down-regulation in human glioma and the relationship with prognosis.
The origin of circulating miRNAs remains unclear. Some studies have shown that circulating miRNAs can be transported by exosomes, which are secreted from multivesicular bodies [17]. In addition, using differential centrifugation, circulating exosomes can be collected and isolated easily [18]. Due to the fact that miRNAs enrich in exosomes [19], differences between the tumors and normal controls may be more obvious. Thus detecting miRNAs derived from exosomes can be more useful in identifing preclinical patients. Although miR-128 derived from exosomes accounts for a part of in peripheral blood, it is still interesting to detect the function as potential biomarkers in glioma.
Acknowledgements
This study was supported by Science and Technology Department of Jiangsu Province (BL2013006) and Medical Management Center of Wuxi (YGZZ1101).
Disclosure of conflict of interest
None.
References
- 1.Ostrom QT, Bauchet L, Davis FG, Deltour I, Fisher JL, Langer CE, Pekmezci M, Schwartzbaum JA, Turner MC, Walsh KM, Wrensch MR, Barnholtz-Sloan JS. The epidemiology of glioma in adults: a “state of the science” review. Neuro Oncol. 2014;16:896–913. doi: 10.1093/neuonc/nou087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huse JT, Holland EC. Targeting brain cancer: advances in the molecular pathology of malignant glioma and medulloblastoma. Nat Rev Cancer. 2010;10:319–331. doi: 10.1038/nrc2818. [DOI] [PubMed] [Google Scholar]
- 4.Cloughesy TF, Cavenee WK, Mischel PS. Glioblastoma: from molecular pathology to targeted treatment. Annu Rev Pathol. 2014;9:1–25. doi: 10.1146/annurev-pathol-011110-130324. [DOI] [PubMed] [Google Scholar]
- 5.Bouyssou JM, Manier S, Huynh D, Issa S, Roccaro AM, Ghobrial IM. Regulation of microRNAs in cancer metastasis. Biochim Biophys Acta. 2014;1845:255–265. doi: 10.1016/j.bbcan.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baulande S, Criqui A, Duthieuw M. [Circulating miRNAs as a new class of biomedical markers] . Med Sci (Paris) 2014;30:289–296. doi: 10.1051/medsci/20143003017. [DOI] [PubMed] [Google Scholar]
- 7.Ciafre SA, Galardi S, Mangiola A, Ferracin M, Liu CG, Sabatino G, Negrini M, Maira G, Croce CM, Farace MG. Extensive modulation of a set of microRNAs in primary glioblastoma. Biochem Biophys Res Commun. 2005;334:1351–1358. doi: 10.1016/j.bbrc.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 8.Shi ZM, Wang J, Yan Z, You YP, Li CY, Qian X, Yin Y, Zhao P, Wang YY, Wang XF, Li MN, Liu LZ, Liu N, Jiang BH. MiR-128 inhibits tumor growth and angiogenesis by targeting p70S6K1. PLoS One. 2012;7:e32709. doi: 10.1371/journal.pone.0032709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Godlewski J, Nowicki MO, Bronisz A, Williams S, Otsuki A, Nuovo G, Raychaudhury A, Newton HB, Chiocca EA, Lawler S. Targeting of the Bmi-1 oncogene/stem cell renewal factor by microRNA-128 inhibits glioma proliferation and self-renewal. Cancer Res. 2008;68:9125–9130. doi: 10.1158/0008-5472.CAN-08-2629. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Chao T, Li R, Liu W, Chen Y, Yan X, Gong Y, Yin B, Liu W, Qiang B, Zhao J, Yuan J, Peng X. MicroRNA-128 inhibits glioma cells proliferation by targeting transcription factor E2F3a. J Mol Med (Berl) 2009;87:43–51. doi: 10.1007/s00109-008-0403-6. [DOI] [PubMed] [Google Scholar]
- 11.Papagiannakopoulos T, Friedmann-Morvinski D, Neveu P, Dugas JC, Gill RM, Huillard E, Liu C, Zong H, Rowitch DH, Barres BA, Verma IM, Kosik KS. Pro-neural miR-128 is a glioma tumor suppressor that targets mitogenic kinases. Oncogene. 2012;31:1884–1895. doi: 10.1038/onc.2011.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kroh EM, Parkin RK, Mitchell PS, Tewari M. Analysis of circulating microRNA biomarkers in plasma and serum using quantitative reverse transcription-PCR (qRT-PCR) Methods. 2010;50:298–301. doi: 10.1016/j.ymeth.2010.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bantis LE, Nakas CT, Reiser B. Construction of confidence regions in the ROC space after the estimation of the optimal Youden index-based cut-off point. Biometrics. 2014;70:212–223. doi: 10.1111/biom.12107. [DOI] [PubMed] [Google Scholar]
- 14.Gartner W, Ilhan A, Neziri D, Base W, Weissel M, Wohrer A, Heinzl H, Waldhor T, Wagner L, Preusser M. Elevated blood markers 1 year before manifestation of malignant glioma. Neuro Oncol. 2010;12:1004–1008. doi: 10.1093/neuonc/noq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hermansen SK, Kristensen BW. MicroRNA biomarkers in glioblastoma. J Neurooncol. 2013;114:13–23. doi: 10.1007/s11060-013-1155-x. [DOI] [PubMed] [Google Scholar]
- 16.Peruzzi P, Bronisz A, Nowicki MO, Wang Y, Ogawa D, Price R, Nakano I, Kwon CH, Hayes J, Lawler SE, Ostrowski MC, Chiocca EA, Godlewski J. MicroRNA-128 coordinately targets Polycomb Repressor Complexes in glioma stem cells. Neuro Oncol. 2013;15:1212–1224. doi: 10.1093/neuonc/not055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT Jr, Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muller L, Hong CS, Stolz DB, Watkins SC, Whiteside TL. Isolation of biologically-active exosomes from human plasma. J Immunol Methods. 2014;411:55–65. doi: 10.1016/j.jim.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henriksen M, Johnsen KB, Olesen P, Pilgaard L, Duroux M. MicroRNA Expression Signatures and Their Correlation with Clinicopathological Features in Glioblastoma Multiforme. Neuromolecular Med. 2014;16:565–77. doi: 10.1007/s12017-014-8309-7. [DOI] [PubMed] [Google Scholar]


