Abstract
MicroRNAs (miRNAs) are aberrantly expressed in cervical cancer. miR-7 has been demonstrated to function as both an oncogene and a tumor suppressor in some types of human cancers. In the present study, miR-7 was significantly downregulated in cervical cancer, especially metastatic tumors. Ectopic expression of miR-7 significantly inhibited metastasis and invasion in Hela and C33A cells. Upregulated miR-7 significantly suppressed focal adhesion kinase (FAK) at transcriptional and translational levels. Furthermore, the level of FAK was negatively correlated with miR-7 in cervical cancer tissues. In conclusion, miR-7 inhibited the metastasis and invasion of cervical cancer at least partially through targeting FAK. The findings of this study provide novel insight with potential therapeutic applications for the treatment of metastatic cervical cancer.
Keywords: microRNA, miR-7, focal adhesion kinase, cervical cancer, metastasis, invasion
Introduction
MicroRNAs (miRNAs) are endogenous, small (18~25 nucleotides) non-coding RNA that typically suppress gene expression by binding to 3’ untranslated regions (UTRs) of their target mRNAs for degradation [1,2]. More than 60% of human protein-coding genes has been under selective pressure to maintain pairing to miRNAs, suggesting that most mammalian mRNAs are conserved targets of miRNAs [3]. Since one miRNA can target many mRNAs, miRNAs play crucial roles in various cellular processes, including development, angiogenesis, cell cycle, and cellular migration [4]. Over the past decade, emerging evidences have demonstrated a central role for miRNAs in the occurrence and progression of human tumors [4,5]. miRNAs are abnormally expressed in human cancers and can act as either oncogenes or tumor suppressors [4-6]. Elucidating the function and mechanism of miRNAs will be particularly useful in improving cancer treatment.
Cervical cancer is the fourth leading cause of cancer mortality in women worldwide [7]. Previous studies have shown that miRNAs are aberrantly expressed in cervical cancer [8,9], which affect response to therapy and ultimately outcome [10-12]. It has been recently shown that miR-7 inhibits the expression of epidermal growth factor receptor (EGFR) in some types of cancer, such as ovarian and gastric cancers [13,14]. EGFR overexpression and activation are observed in solid tumors including cervical cancer [15], and EGFR tyrosine kinase has become an important therapeutic target for cancer therapy. Furthermore, miR-7 has been reported to act as an oncomiR in different cellular contexts in renal cell carcinoma [16]. A recent study reported that miR-7 suppressed cervical cancer cells proliferation and promoted cell apoptosis through inhibition of expression of X-linked inhibitor of apoptosis protein [17]. In this study, we showed that miR-7 inhibited cervical cancer cells metastasis and invasion by inhibiting the expression of focal adhesion kinase (FAK). These results further our understanding of the mechanisms underlying human cervical cancer.
Materials and methods
Tissue samples
Forth cervical cancer tissues and thirty normal cervical tissues were collected between 2012 and 2014 from Taixing Hospital, Jiangsu, China. All specimens were carefully reviewed by two independent pathologists to confirm the presence of cervical cancer. All patients signed an informed consent form, and the study was approved by the Institute Research Medical Ethics Committee of the Medical College of Yangzhou University.
Cell line culture
Human cervical cancer cell lines (Hela and C33A) and 293T cell line were purchased from the Chinese Peking Union Medical College Cell Bank (Beijing, China). All cell lines were maintained in DMEM and RPMI 1640 supplemented with 10% fetal bovine serum (FBS) (HyClone Victoria, Australia), 100 IU/mL penicillin, and 100 mg/mL streptomycin at 37°C in a humidified atmosphere containing 5% CO2.
Quantitative reverse-transcription polymerase chain reaction (qRT-PCR)
Total RNA was isolated using Trizol reagent (Invitrogen, San Diego, CA, USA) according to the manufacturer’s instructions. Reverse transcriptions was performed using the Takara PrimeScriptTM First Strand cDNA Synthesis kit (Takara Bio, Inc., Dalian, Japan) according to the manufacturer’s instructions. qRT-PCR was performed using the All-in-OneTM miRNA qRT-PCR Detection Kit (GeneCopoeia, Rockville, MD, USA) on an Applied Biosystems 7500 RealTime PCR system (Applied Biosystems, White Plains, NY, USA). The U6 small RNA and GAPDH mRNA were used as internal controls for miR-7 and FAK, respectively. All the reactions were run in triplicate. Primers for miR-7 and U6 were 5’-AGTCGCTAGCCACAAACC AGGAAGGGGAA-3’ and 5’-ATCGGAATTCAAATGATAAAGCCTGAAGTC-3’, and 5’-CGCTTCGGCAGCACATATAC-3’ and 5’-TTCACGAATTTGCGTGTCAT-3’, respectively. Primers for FAK and GAPDH mRNAs were 5’-AGTAAAATCCAGCCAGCCCC-3’ and 5’-GACATACTGCTGGGCCAGTT-3’, and 5’-AAGGTGAAGGTCGGAGTCAAC-3’, and 5’-GGGGTCATTGATGGCAACAATA-3’, respectively.
Transfection of cervical cancer cells
The miR-7-murine stem cell virus plasmid (MSCV) was chemically synthesized at the Department of Pathology, Medical College of Yangzhou University, and sequenced by Sangon Biotech (Shanghai) Co., Ltd. (Shanghai, China). Hela and C33A cells were transfected with 4-10 μg miR-7-MSCV plasmid which at 37°C with 5% CO2. MSCV empty vector was used as the negative control (NC).
Dual luciferase reporter assay
The full length 3’UTR of FAK (wild type, wt) was amplified by PCR from genomic DNA and cloned into the EcoRI and XhaI sites of pGL3-BS vector (Promega, Madison, WI, USA). The primer sequences used were as follows: forward, 5’-CTCTAGCCTTCCACCAGCAG-3’; reverse, 5’-AACAAGAACTTTACTGGTAA-3’. The mutant construct of FAK 3’ UTR was generated using a Quick Change mutagenesis kit (Stratagene, Heidelberg, Germany). Cotransfection of reporter vectors, and miR-7-MSCV or NCs was performed using Lipofectamine 2000 (Invitrogen, San Diego, CA, USA). After 48 h incubation, dual luciferase activity was measured using the Dual-Luciferase® reporter assay system (Promega) according to the manufacturer’s protocol.
Migration and invasion assay
In vitro cell migration and invasion assays were performed by using transwell chambers. For the migration assays, 4 × 104 cells were added into the upper chamber of 8-μm transwells (BD Bioscience, Franklin Lakes, NJ, USA). For the invasion assays, 8 × 104 cells were added into the upper chamber of 8-μm transwells pre-coated with Matrigel (BD Bioscience, USA). In both assays, cells were plated in medium without serum, and medium containing 10% FBS in the lower chamber served as the chemoattractant. After 14 h incubation, the cells that did not migrate or invade through the pores were carefully removed. Filters were fixed in 90% alcohol followed by crystal violet staining. Five random fields were counted per chamber using an inverted microscope (Olympus, Tokyo, Japan). All experiments were performed in triplicate.
Western blot analysis
Cells were collected and lysed in ice-cold RIPA buffer (Beyotime, Jiangsu, China) according to the manufacturer’s instructions. Protein concentration was quantified using an Enhanced BCA protein assay kit (Beyotime, Jiangsu, China). The proteins were separated by SDS-PAGE, and transferred onto PVDF membranes (Millipore, Bedford, MA, USA). After blocked with 5% nonfat milk for 1.5 h at room temperature, membranes were incubated with anti-FAK antibody (Cell Signaling Technologies, Boston, MA, USA) at 1:1000 dilution for overnight at 4°C, followed by incubation with horseradish peroxidase conjugated mouse and rabbit secondary antibody (Beyotime) for 2 h at room temperature. The signals were detected using a FluorChem FC2 Imaging System (Alpha Innotech, San Leandro, CA, USA).
Statistical analysis
Statistical analyses were performed using SPSS 16.0 software package (SPSS Inc., Chicago, IL, USA). All graphs were made using Microsoft Office Excel 2010 software (Microsoft Corporation, Redmond, WA, USA). Data from three independent experiments were expressed as mean ± SD. Differences were assessed by the two-tailed Student’s t test. The relationship between FAK and miR-7 expressions was tested with two-tailed Pearson’s correlation. A P value < 0.05 was considered statistically significant.
Results
miR-7 is downregulated in cervical cancer tissues and cell lines
We first evaluated the endogenous expression of miR-7 in human cervical cancer and normal cervical tissues. As shown in Figure 1A, miR-7 was downregulated in cervical cancer tissues compared to corresponding adjacent normal cervical tissues (P < 0.05) (Figure 1A). Furthermore, the level of miR-7 in metastatic cervical cancer was significantly lower compared with that in cancer without metastasis (P < 0.05) (Figure 1B). Similarly, we also found that the levels of miR-7 in two cervical cancer cell lines were more lower than that in normal cervical cells (P < 0.001) (Figure 1C).
Figure 1.
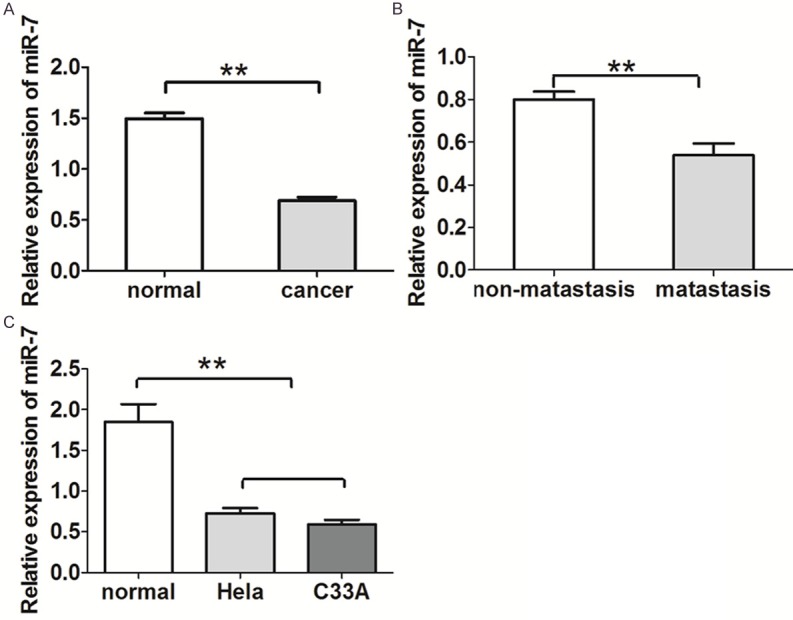
miR-7 is downregulated in cervical cancer and associated with tumor metastasis. A. The levels of miR-7 in cervical cancer tissues (n = 40) were significantly lower than those in normal cervical tissues (n = 30). B. The levels of miR-7 in non-metastatic cervical cancer tissues (n = 23) were lower than those in metastatic cervical cancer tissues (n = 17). C. The levels of miR-7 in two cervical cancer cell lines were lower than that in human cervical cells. The expression level of miR-7 was normalized to U6 small nuclear RNA. *P < 0.05, **P < 0.001.
Overexpression of miR-7 inhibits cervical cancer cell migration and invasion
Since miR-7 is downregulated in patients with metastatic cervical cancer, we next evaluated whether upregulation of miR-7 inhibits cell migration and invasion in cervical cancer. Hela and C33A cells, which possess low miR-7 levels, were transfected with miR-7-MSCV. The ectopic expression efficiency was confirmed by qRT-PCR (Figure 2). We found that overexpression of miR-7 in both Hela and C33A cells resulted in reduced migration and invasion rates (Figure 3). These results suggest that miR-7 may function as a tumor suppressor miRNA and contribute to inhibition of migration and invasion of cervical cancer cells.
Figure 2.
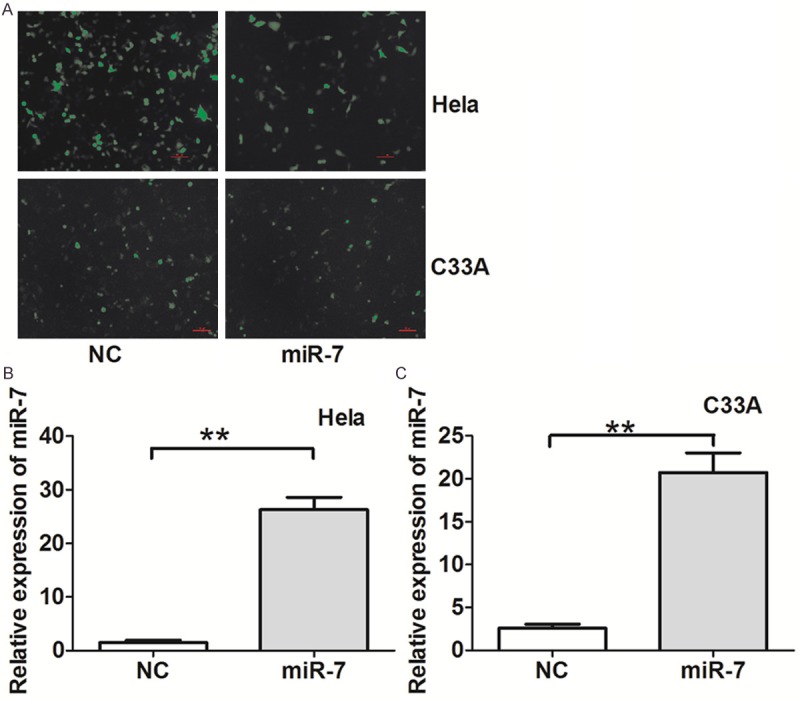
miR-7 overexpression in Hela and C33A cells transfected with miR-7-MSCV. (A) miR-7 expression in Hela and C33A cells transfected with miR-7-MSCV and control (MSCV). miR-7 was overexpressed in (B) Hela and (C) C33A cells transfected with miR-7-MSCV compared with negative controls. *P < 0.05, **P < 0.001.
Figure 3.
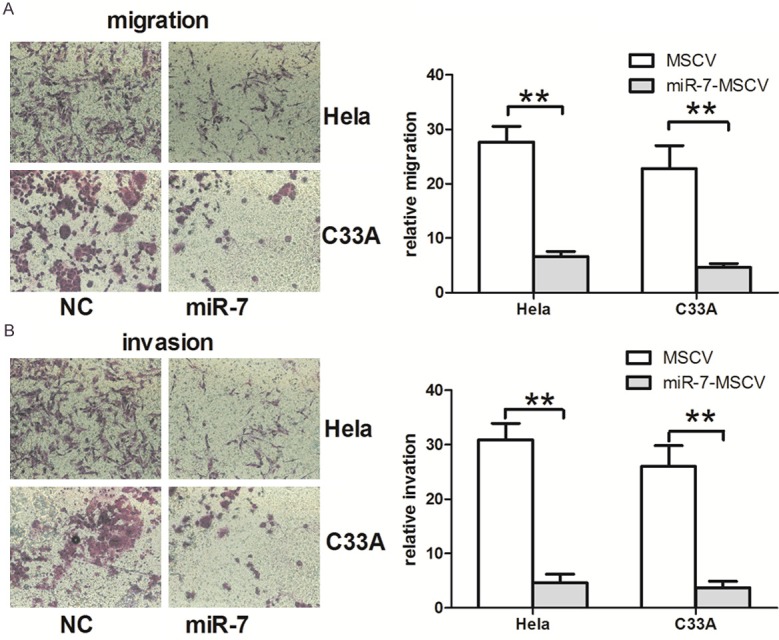
miR-7 inhibits cell migration and invasion. A. Transwell migration assays of Hela and C33A cells transfected with miR-7-MSCV and control vector. B. Transwell invasion assays of Hela and C33A cells transfected with miR-7-MSCV and control vector. *P < 0.05, **P < 0.001.
miR-7 negatively regulates FAK gene expression
Previous studies have demonstrated that FAK is the target of miR-7 [18,19]. To better elucidate the mechanisms underlying miR-7 function in cervical cancer cells, we further adopted an EGFR reporter system to confirm the direct regulation of FAK mRNA by miR-7. We constructed two vectors containing the wild-type and mutant FAK 3’UTR fragment, respectively. As shown in Figure 4, co-transfection of 293T cells with FAK-3’UTR/pGL3-BS (wt) and miR-7-MSCV caused a significant decrease in the luciferase activity compared with co-transfected FAK-3’ UTR/pGL3 (mut) with miR-7-MSCV as negative control (P < 0.05). The levels of FAK mRNA and protein in both Hela and C33A cells with miR-7 overexpression were significantly (Figure 5A and 5B). This result indicated that miR-7 exerts inhibitory effects on FAK expression via interaction with FAK 3’UTR.
Figure 4.
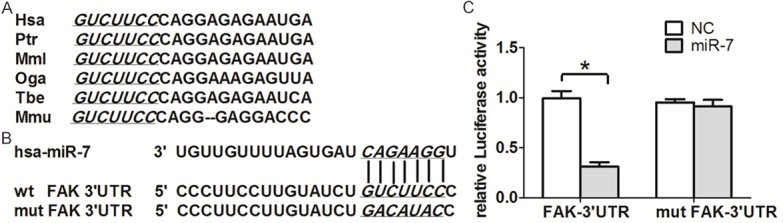
miR-7 inhibits FAK expression by directly targeting its 3’UTR. A. The potential binding sequences for miR-7 within the FAK 3’UTR of human (Hsa), chimpanzee (Ptr), rhesus (Mml), bushbaby (Oga), tree shrew (Tbe), and mouse (Mmu). Seed sequences are highlighted and underlined. B. The sequences of the predicted miR-7 binding sites (highlighted and underlined) within the human FAK 3’UTR, including wide-type full-length UTR and mutant UTR, are shown. C. Relative luciferase activity was analyzed upon transfection with reporter plasmids and miR-7-MSCV or control vector in 293T cells.
Figure 5.
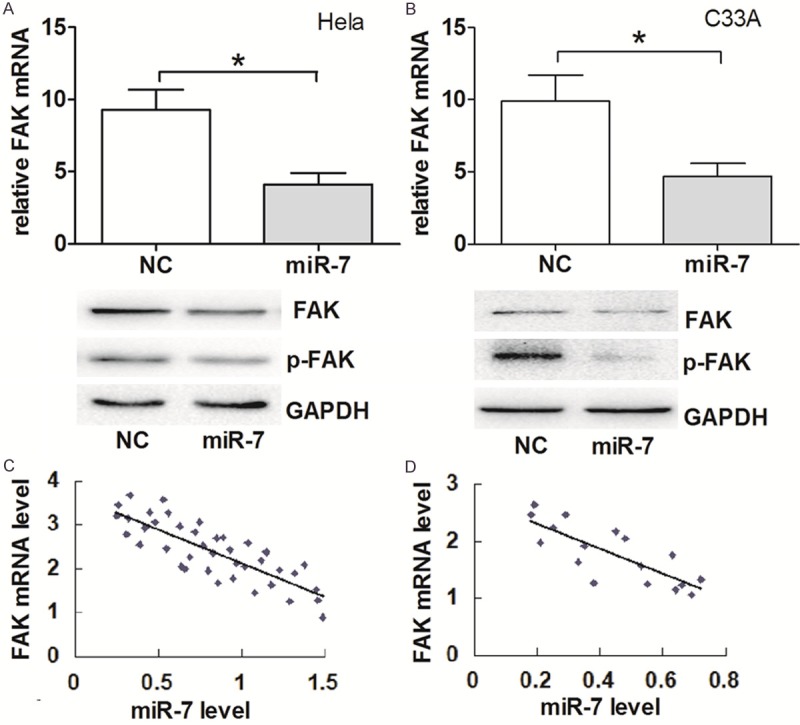
FAK is downregulated in Hela and C33A cells transfected with miR-7-MSCV.Relative expression of FAK mRNA by qPCR (top) and FAK protein expression by western blot (bottom) in Hela (A) and C33A cells (B) transfected with miR-7-MSCV and control vector. *P < 0.05, **P < 0.001. There were inverse correlations between miR-7 and FAK expression in 40 cervical cancer tissues (C) (r = 0.750; P < 0.05) and 17 metastatic cervical cancer tissues (D) (r = 0.820; P < 0.05) by detecting mRNA expression.
The mRNA level of FAK was further evaluated in cervical cancer and normal cervical tissues. As shown in Figure 5C, there was a significant inverse correlation between FAK mRNA expression and miR-7 expression (r = -0.750, P < 0.05). Furthermore, a significant inverse correlation was also observed in metastatic cases (r = -0.820; P < 0.05).
Discussion
miRNAs specifically prevent protein production by binding to the 3’UTR of target mRNAs. This recruits RISC, which facilitates nucleolytic cleavage or inhibits translation of target mRNAs. By post-transcriptional regulation of protein production, a number of miRNAs can act as oncogenes or tumor suppressor genes. In the present study, we found that miR-7 was downregulated in cervical cancer tissues and cell lines. miR-17 suppressed the metastasis and invasion of cervical cancer cell by inhibiting FAK expression. These results indicate that miR-7 play an important role in the progression of cervical cancer.
Previous studies have shown that miR-7 is involved in some human diseases. As a direct regulator of a-synuclein, miR-7 has been proposed to play a role in Parkinson’s disease [20]. Inhibition of miR-7 in pancreatic B cells induced mTOR signaling with subsequent stimulatory effects on cellular proliferation [21]. This suggests miR-7 may contribute to low B-cell renewal and could serve as a possible therapeutic target in diabetes mellitus. In addition, miR-7 directly targets and downregulates central oncogenic factors in cancer-associated signaling pathways including EGFR [13,14], FAK [18,19], Pak1 [22], and SETDB1 [23], clearly indicating a tumor-suppressive role of miR-7. In the present study, we found that miR-7 was downregulated in cervical cancer, especially metastatic tumors. In addition, there was an inverse correlation between miR-7 and FAK expression in cervical cancer. FAK is negatively regulated by miR-7, which is consistent with previous findings [18,19]. miR-7 inhibits Hela and C33A cell migration and invasion through the FAK signaling. These results suggest that dysregulation of the FAK signaling pathway by miR-7 may be an important mechanism underlying metastasis and invasion in cervical. Further studies should examine the molecular mechanism by which miR-7 function as a tumor suppressor gene in the development and progression of cervical cancer.
FAK is an important adhesion kinase that contributes to extracellular matrix (ECM) integrin signaling, cell motility, proliferation, and survival. Increased FAK expression is observed in a variety of solid tumors and associated with metastatic disease and poor prognosis [24,25]. FAK signaling regulates cancer growth and metastasis [26-28]. Increased FAK expression in precursor lesions and frankly invasive cervical cancer is an intriguing finding because an inverse relationship between p53 and FAK has been reported, which is consistent with p53 instability due to a HPV E6 viral protein as an essential etiologic factor [29]. FAK may function as an oncogene in the process of cervical cancer.
In conclusion, our study demonstrated that miR-7 suppressed cervical cancer metastasis and invasion through targeting FAK. miR-7 may be a potential therapeutic target for the treatment of metastatic cervical cancer.
Acknowledgements
This work was supported by the China National Science Foundation (Grant No. 81302041), Jiangsu Traditional Chinese Medicine Science and Technology Project Foundation for 2013 (Grant No. LZ13206), and the Jiangsu Higher Education Science Foundation (Grant No. 320007).
Disclosure of conflict of interest
None.
References
- 1.Fabian MR, Sonenberg N, Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annu Rev Biochem. 2010;79:351–379. doi: 10.1146/annurev-biochem-060308-103103. [DOI] [PubMed] [Google Scholar]
- 2.Guo H, Ingolia NT, Weissman JS, Bartel DP. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature. 2010;466:835–840. doi: 10.1038/nature09267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 4.Farazi TA, Spitzer JI, Morozov P, Tuschl T. miRNAs in human cancer. J Pathol. 2011;223:102–115. doi: 10.1002/path.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tao K, Yang J, Guo Z, Hu Y, Sheng H, Gao H, Yu H. Prognostic value of miR-221-3p, miR-342-3p and miR-491-5p expression in colon cancer. Am J Transl Res. 2014;6:391–401. [PMC free article] [PubMed] [Google Scholar]
- 6.Yu H, Li H, Qian H, Jiao X, Zhu X, Jiang X, Dai G, Huang J. Upregulation of miR-301a correlates with poor prognosis in triple-negative breast cancer. Med Oncol. 2014;31:283. doi: 10.1007/s12032-014-0283-2. [DOI] [PubMed] [Google Scholar]
- 7.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 8.Sharma G, Dua P, Agarwal SM. A Comprehensive Review of Dysregulated miRNAs Involved in Cervical Cancer. Curr Genomics. 2014;15:310–323. doi: 10.2174/1389202915666140528003249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Villegas-Ruiz V, Juarez-Mendez S, Perez-Gonzalez OA, Arreola H, Paniagua-Garcia L, Parra-Melquiadez M, Peralta-Rodriguez R, Lopez-Romero R, Monroy-Garcia A, Mantilla-Morales A, Gomez-Gutierrez G, Roman-Bassaure E, Salcedo M. Heterogeneity of microRNAs expression in cervical cancer cells: over-expression of miR-196a. Int J Clin Exp Pathol. 2014;7:1389–1401. [PMC free article] [PubMed] [Google Scholar]
- 10.Wang N, Zhou Y, Zheng L, Li H. MiR-31 is an independent prognostic factor and functions as an oncomir in cervical cancer via targeting ARID1A. Gynecol Oncol. 2014;134:129–137. doi: 10.1016/j.ygyno.2014.04.047. [DOI] [PubMed] [Google Scholar]
- 11.Yuan W, Xiaoyun H, Haifeng Q, Jing L, Weixu H, Ruofan D, Jinjin Y, Zongji S. MicroRNA-218 enhances the radiosensitivity of human cervical cancer via promoting radiation induced apoptosis. Int J Med Sci. 2014;11:691–696. doi: 10.7150/ijms.8880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang B, Chen J, Ren Z, Chen Y, Li J, Miao X, Song Y, Zhao T, Li Y, Shi Y, Ren D, Liu J. A specific miRNA signature promotes radioresistance of human cervical cancer cells. Cancer Cell Int. 2013;13:118. doi: 10.1186/1475-2867-13-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou X, Hu Y, Dai L, Wang Y, Zhou J, Wang W, Di W, Qiu L. MicroRNA-7 inhibits tumor metastasis and reverses epithelial-mesenchymal transition through AKT/ERK1/2 inactivation by targeting EGFR in epithelial ovarian cancer. PLoS One. 2014;9:e96718. doi: 10.1371/journal.pone.0096718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie J, Chen M, Zhou J, Mo MS, Zhu LH, Liu YP, Gui QJ, Zhang L, Li GQ. miR-7 inhibits the invasion and metastasis of gastric cancer cells by suppressing epidermal growth factor receptor expression. Oncol Rep. 2014;31:1715–1722. doi: 10.3892/or.2014.3052. [DOI] [PubMed] [Google Scholar]
- 15.Li Q, Tang Y, Cheng X, Ji J, Zhang J, Zhou X. EGFR protein expression and gene amplification in squamous intraepithelial lesions and squamous cell carcinomas of the cervix. Int J Clin Exp Pathol. 2014;7:733–741. [PMC free article] [PubMed] [Google Scholar]
- 16.Yu Z, Ni L, Chen D, Zhang Q, Su Z, Wang Y, Yu W, Wu X, Ye J, Yang S, Lai Y, Li X. Identification of miR-7 as an oncogene in renal cell carcinoma. J Mol Histol. 2013;44:669–677. doi: 10.1007/s10735-013-9516-5. [DOI] [PubMed] [Google Scholar]
- 17.Liu S, Zhang P, Chen Z, Liu M, Li X, Tang H. MicroRNA-7 downregulates XIAP expression to suppress cell growth and promote apoptosis in cervical cancer cells. FEBS Lett. 2013;587:2247–2253. doi: 10.1016/j.febslet.2013.05.054. [DOI] [PubMed] [Google Scholar]
- 18.Kong X, Li G, Yuan Y, He Y, Wu X, Zhang W, Wu Z, Chen T, Wu W, Lobie PE, Zhu T. MicroRNA-7 inhibits epithelial-to-mesenchymal transition and metastasis of breast cancer cells via targeting FAK expression. PLoS One. 2012;7:e41523. doi: 10.1371/journal.pone.0041523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu DG, Wang YY, Fan LG, Luo H, Han B, Sun LH, Wang XF, Zhang JX, Cao L, Wang XR, You YP, Liu N. MicroRNA-7 regulates glioblastoma cell invasion via targeting focal adhesion kinase expression. Chin Med J (Engl) 2011;124:2616–2621. [PubMed] [Google Scholar]
- 20.Junn E, Lee KW, Jeong BS, Chan TW, Im JY, Mouradian MM. Repression of alpha-synuclein expression and toxicity by microRNA-7. Proc Natl Acad Sci U S A. 2009;106:13052–13057. doi: 10.1073/pnas.0906277106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Liu J, Liu C, Naji A, Stoffers DA. MicroRNA-7 regulates the mTOR pathway and proliferation in adult pancreatic beta-cells. Diabetes. 2013;62:887–895. doi: 10.2337/db12-0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reddy SD, Ohshiro K, Rayala SK, Kumar R. MicroRNA-7, a homeobox D10 target, inhibits p21-activated kinase 1 and regulates its functions. Cancer Res. 2008;68:8195–8200. doi: 10.1158/0008-5472.CAN-08-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang H, Cai K, Wang J, Wang X, Cheng K, Shi F, Jiang L, Zhang Y, Dou J. MiR-7, Inhibited Indirectly by LincRNA HOTAIR, Directly Inhibits SETDB1 and Reverses the EMT of Breast Cancer Stem Cells by Downregulating the STAT3 Pathway. Stem Cells. 2014;32:2858–2868. doi: 10.1002/stem.1795. [DOI] [PubMed] [Google Scholar]
- 24.Chen Y, Xia X, Wang S, Wu X, Zhang J, Zhou Y, Tan Y, He S, Qiang F, Li A, Roe OD, Zhou J. High FAK combined with low JWA expression: clinical prognostic and predictive role for adjuvant fluorouracil-leucovorin-oxaliplatin treatment in resectable gastric cancer patients. J Gastroenterol. 2013;48:1034–1044. doi: 10.1007/s00535-012-0724-7. [DOI] [PubMed] [Google Scholar]
- 25.Garouniatis A, Zizi-Sermpetzoglou A, Rizos S, Kostakis A, Nikiteas N, Papavassiliou AG. FAK, CD44v6, c-Met and EGFR in colorectal cancer parameters: tumour progression, metastasis, patient survival and receptor crosstalk. Int J Colorectal Dis. 2013;28:9–18. doi: 10.1007/s00384-012-1520-9. [DOI] [PubMed] [Google Scholar]
- 26.Zhan Q, Huang RF, Liang XH, Ge MX, Jiang JW, Lin H, Zhou XL. FRAS1 knockdown reduces A549 cells migration and invasion through downregulation of FAK signaling. Int J Clin Exp Med. 2014;7:1692–1697. [PMC free article] [PubMed] [Google Scholar]
- 27.Dai Z, Zhou SL, Zhou ZJ, Bai DS, Xu XY, Fu XT, Chen Q, Zhao YM, Zhu K, Yu L, Yang GH, Wang Z, Wu WZ, Zhou J, Fan J. Capn4 contributes to tumour growth and metastasis of hepatocellular carcinoma by activation of the FAK-Src signalling pathways. J Pathol. 2014;234:316–328. doi: 10.1002/path.4395. [DOI] [PubMed] [Google Scholar]
- 28.Luedde T. MicroRNA-151 and its hosting gene FAK (focal adhesion kinase) regulate tumor cell migration and spreading of hepatocellular carcinoma. Hepatology. 2010;52:1164–1166. doi: 10.1002/hep.23854. [DOI] [PubMed] [Google Scholar]
- 29.Golubovskaya VM, Cance WG. Focal adhesion kinase and p53 signaling in cancer cells. Int Rev Cytol. 2007;263:103–153. doi: 10.1016/S0074-7696(07)63003-4. [DOI] [PubMed] [Google Scholar]


