Abstract
Exposure to an adverse intrauterine environment increases the risk of cardiovascular disease later in adult life. It has been shown that hypoxia plays a critical role in vascular remodeling and directly affects vascular smooth muscle cells functions. In the present study, we aimed to investigate the effect of hypoxia on neonatal rat aorta smooth muscle cells (NRSMCs). Our study demonstrated that hypoxia stimulation at 2% oxygen could significantly enhance NRSMCs proliferation in a time dependent manner. Moreover, hypoxia treatment resulted in an increased percentage in the S + G2/M phase and decreased apoptosis rate in NRSMCs. On the molecular level, the protein levels of pro-apoptotic proteins BNIP3 and bax were obviously reduced, while the anti-apoptotic factor bcl-2 was enhanced under hypoxia condition. Furthermore, we found that hypoxia activated hypoxia-inducible factor-1α (HIF-1α) expression and subsequently promoted NRSMCs proliferation. Specific down-regulation of HIF-1α partly abolished the proliferative effect of hypoxia on NRSMCs growth. Taken together, the present study demonstrated that hypoxia treatment promoted NRSMCs growth through activation of HIF-1α, which may contribute to the understanding of the pathogenesis of atherosclerosis.
Keywords: Hypoxia, vascular smooth muscle cells, proliferation, HIF-1α
Introduction
Epidemiological studies suggest that adverse intrauterine environment during a critical period of fetal development will lead to the fetal adaptive changes that may result in an increased risk of cardiovascular disease in adult life [1,2]. Fetal adaptations to the adverse environment in utero include altered cell differentiation and tissue growth in order to guarantee short-term survival but, on the other hand, may lead to impaired cardiovascular function in postnatal life [3-5].
Clinical and experimental evidences indicate exposure to chronic hypoxia during gestation is one of the most common intrauterine stresses and may predispose individuals to cardiac remodeling, hypertension and vascular dysfunction [6-9]. Accumulating evidences support the possible link between prenatal hypoxia and increased risk of cardiovascular dysfunction in offspring [10,11]. Experimental investigation has demonstrated that maternal hypoxia leads to increased cardiac vulnerability to ischemia and reperfusion injury in adult offspring [12]. Recent studies in rats have shown that maternal hypoxia alters postnatal vascular function and increases blood pressure response in adult offspring [13,14].
To our knowledge, the impact of maternal hypoxia on the development of atherosclerosis still remains elusive. Pathogenic development of atherosclerosis involves a complex series of events, of which abnormal proliferation of vascular smooth muscle cells (VSMCs) contribute significantly to the progression of atherosclerosis [15,16]. Since dysfunction of VSMCs play critical roles in the development of atherosclerosis, our current study aims to investigate the impact of hypoxia on neonatal rat aorta smooth muscle cells (NRSMCs), and further explore the underlying molecular mechanism.
Materials and methods
Cell culture
Female Sprague-Dawley rats (Shanghai Experimental Animal Center, Shanghai, China) were mated at 3 months of age. A vaginal smear obtained the following morning was examined for the presence of sperm, which signaled day 0 of pregnancy (term = 22 days). On day 20, the rat thoracic aorta was quickly removed under aseptic conditions following intraperitoneal injection of 10% chloral hydrate and immediately rinsed with aseptic phosphate-buffered saline (PBS). VSMCs were prepared from thoracic aorta of adult Wistar rats as previously described [13]. More than 98% of the cells were positive for smooth muscle-specific α-actin, and exhibited the typical hill-and-valley morphology of VSMCs. Cells at passages 3 to 5 were used for experiments. Cells grown to 85%-90% confluence were divided into control group and hypoxia group. The study was conducted according to the Declaration of Helsinki. The study was reviewed and approved by the medical ethics committee of The Second Affiliated Hospital of Fujian Medical University.
MTT assay
Cell viability was measured by MTT methods. To be brief, VSMCs were seeded in 24-well plates at a density of 4 × 104 cells/ml per well. After 24 h, cells were cultured for indicated time points in a hypoxia incubator (Jouan, Saint-Nazaire, France). Then, 0.5 mg/ml MTT was added and incubated for 4 h at 37°C. The optical density of each well was measured at 490 nm using an automatic plate reader.
Cell cycle analysis
Cells were digested by trypsin, washed twice with phosphate buffered solution (PBS), and fixed in 70% ethanol at -20°C overnight. After washing with PBS, cells were suspended and incubated with propidium iodide for 1 h in the dark. Cell cycle analysis was performed with FACSCalibur flow cytometer (BD, USA).
Apoptosis assay
Cells were exposed to hypoxic condition for indicated time points. The cultured cells were washed with PBS twice and detached with trypsin. Apoptosis cells were detected with annexin V-FITC/PI according to the protocol of Annexin V-FITC cell Apoptosis Detection Kit (BD, USA).
Real time PCR
Total RNAs were isolated from cells by TRIzol reagent, and reverse transcriptions were performed by Takara RNA PCR kit (Takara, Japan) following the manufacturer’s instructions. In order to quantify the transcripts of the interest genes, real-time PCR was performed using a SYBR Green Premix Ex Taq (Takara, Tokyo, Japan) on ABI 7500 system (Applied Biosystems, Foster, CA, USA) according to the manufacturer’s construction.
Western blot analysis
After hypoxia stimulation, total cell lysates were collected and subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). For Western blotting analysis, primary antibodies including anti-BINP3, bax, bcl-2, HIF-1α, and β-actin (Cell Signaling, Beverly, MA) were used. Anti-rabbit or anti-mouse secondary antibody conjugated with horseradish peroxidase was also applied (Pierce Chromatography Cartridges, USA). Immunoreactive bands were detected by enhanced chemiluminescence (ECL) kit for Western blotting detection by using a ChemiGenius bioimaging system (SYNGENE, USA).
Statistical analysis
Each experiment was performed in triplicate, and repeated at least three times. All the data were presented as means ± SD and treated for statistics analysis by SPSS program. Comparison between groups was made using ANOVA and statistically significant difference was defined as P < 0.05.
Results
Effect of hypoxia on NRSMCs proliferation
The effect of hypoxia on NRSMCs proliferation was measured by MTT assay. Cells were exposed to different degrees of hypoxia (10, 5 and 2% oxygen) at different time points (6, 12, 24, 48 h) in DMEM medium. As shown in Figure 1A, exposure to hypoxia at 2% oxygen for 24 h obviously promoted NRSMCs proliferation, whereas hypoxia at 5 and 10% oxygen had no obvious effect on cells growth. We then used 2% oxygen treatment as hypoxia stimulation in the following experiments. In addition, NRSMCs were incubated in a hypoxic condition with 2% O2 for 6, 12, 24, 48 h, the OD values were remarkably increased in hypoxia-treated cells when compared to controls (Figure 1B). These data demonstrated that hypoxia at 2% oxygen could stimulate NRSMCs proliferation in a time dependent manner.
Figure 1.
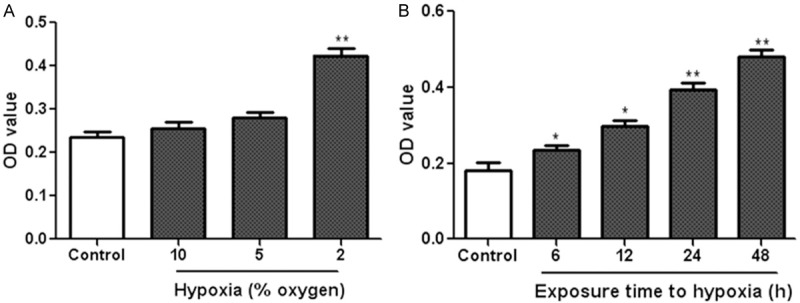
Effect of hypoxia on NRSMCs proliferation. A. NRSMCs were exposed to hypoxia at different degrees (10, 5 and 2% oxygen) for 24 h. B. NRSMCs were cultured under the hypoxic condition with 2% O2 for 6, 12, 24 and 48 h. MTT assay was used to measure cell proliferation. *P < 0.05, **P < 0.01 vs control group.
Effect of hypoxia on cell cycle changes in NRSMCs
Flow cytometry was performed to analyze cell cycle distribution in NRSMCs under hypoxia and normoxia condition. We found that the percentage of NRSMCs in the G0/G1 phase after hypoxia treatment (12, 24, 48 h) was significantly reduced compared to control cells under normoxia (Figure 2A). Moreover, the percentage of NRSMCs in the S + G2/M phase following hypoxia stimulation (12, 24, 48 h) was markedly increased compared to the control group (Figure 2B). Taken together, these data suggested that active cellular reactions had occurred and a significant proliferation was induced by hypoxia treatment.
Figure 2.
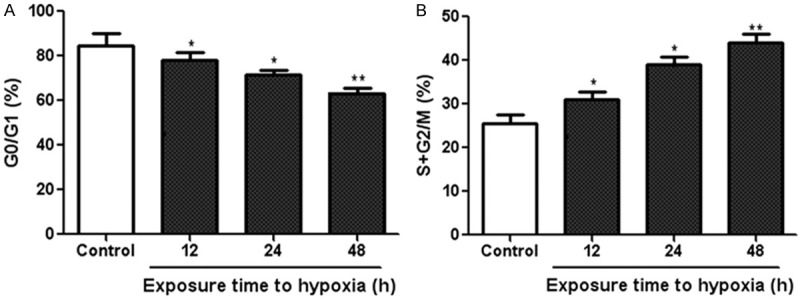
Effect of hypoxia on cell cycle changes in NRSMCs. NRSMCs were cultured under the hypoxic condition with 2% oxygen for different time points. Then, the percentage of NRSMCs in the G0/G1 (A) and S + G2/M (B) phase were analyzed by flow cytometry. *P < 0.05, **P < 0.01 vs control group.
Effect of hypoxia on NRSMCs apoptosis
We then examined the impact of hypoxia on NRSMCs apoptosis using flow cytometry analysis. As shown in Figure 3A, we found that hypoxia stimulation significantly inhibited cell apoptosis. On the molecular level, western blot analysis indicated that the protein levels of pro-apoptotic proteins BNIP3 and bax were obviously reduced, while the anti-apoptotic factor bcl-2 was enhanced under hypoxia condition compared to the control cells under normoxia (Figure 3B, 3C). These data suggested that hypoxia treatment reduced NRSMCs apoptosis in a time dependent manner.
Figure 3.
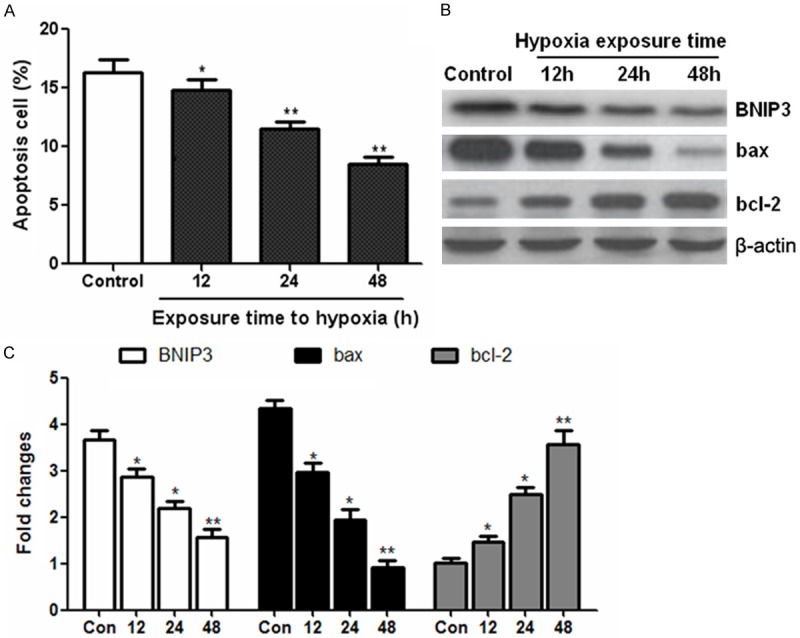
Anti-apoptotic effect of hypoxia on NRSMCs. NRSMCs were exposed to hypoxia (2% oxygen) and the apoptosis cells were determined by flow cytometry (A). Western blot was performed to detect the expression of apoptosis-related proteins such as BNIP3, bax and bcl-2 (B and C). *P < 0.05, **P < 0.01 vs control group.
Hypoxia stimulation induces HIF-1α activation in NRSMCs
HIF-1α is the key master regulator to hypoxic response [3], so we observed the possible role of HIF-1α in our experimental model. Real time PCR indicated that the HIF-1α mRNA expression was significantly increased in NRSMCs exposed to hypoxia (Figure 4A). Consistently, the protein level of HIF-1α was highly induced in NRSMCs exposed to hypoxic condition (Figure 4B, 4C). These results showed that hypoxia treatment promoted the activation of HIF-1α in NRSMCs.
Figure 4.
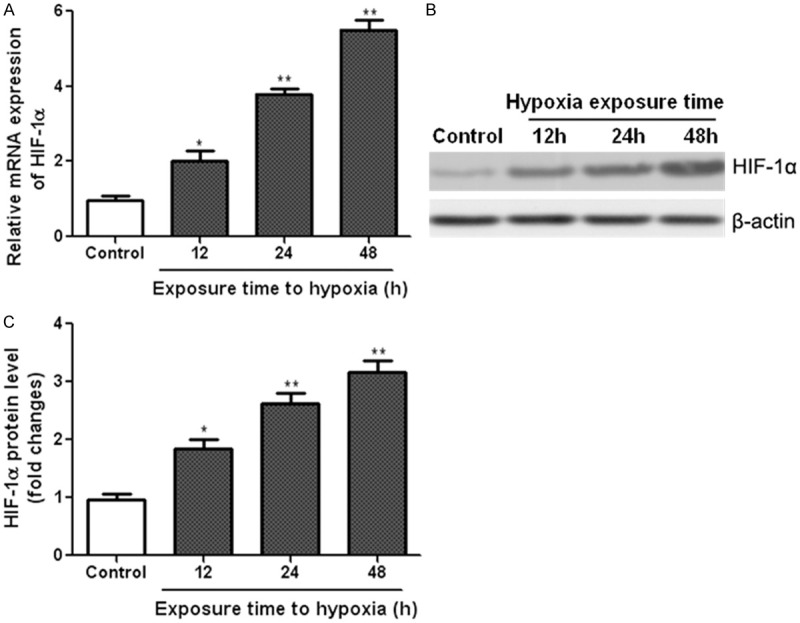
Hypoxia stimulation induces HIF-1α activation in NRSMCs. NRSMCs were exposed to hypoxia (2% oxygen) for different durations. Real time PCR and western blot were performed to measure the mRNA (A) and protein (B and C) levels of HIF-1α in NRSMCs. *P < 0.05, **P < 0.01 vs control group.
Role of HIF-1α in the hypoxia-induced proliferation of NRSMCs
In order to determine if HIF-1α plays a role in hypoxia-induced cell proliferation, we analyzed NRSMCs proliferation followed by co-treatment with specific HIF-1α-siRNA and hypoxia (2% O2) for 24 h. Western blot analysis confirmed that the specific HIF-1α-siRNA significantly suppressed HIF-1α expression in NRSMCs under both normoxia and hypoxia conditions (Figure 5A, 5B). Similar with the result in Figure 1 in which hypoxia treatment enhanced NRSMCs proliferation, exposure to hypoxia stimulated cells proliferation compared to the normoxia condition. However, down-regulation of HIF-1α in NRSMCs partly prevented the increase in cell proliferation under hypoxia condition (Figure 5C).
Figure 5.
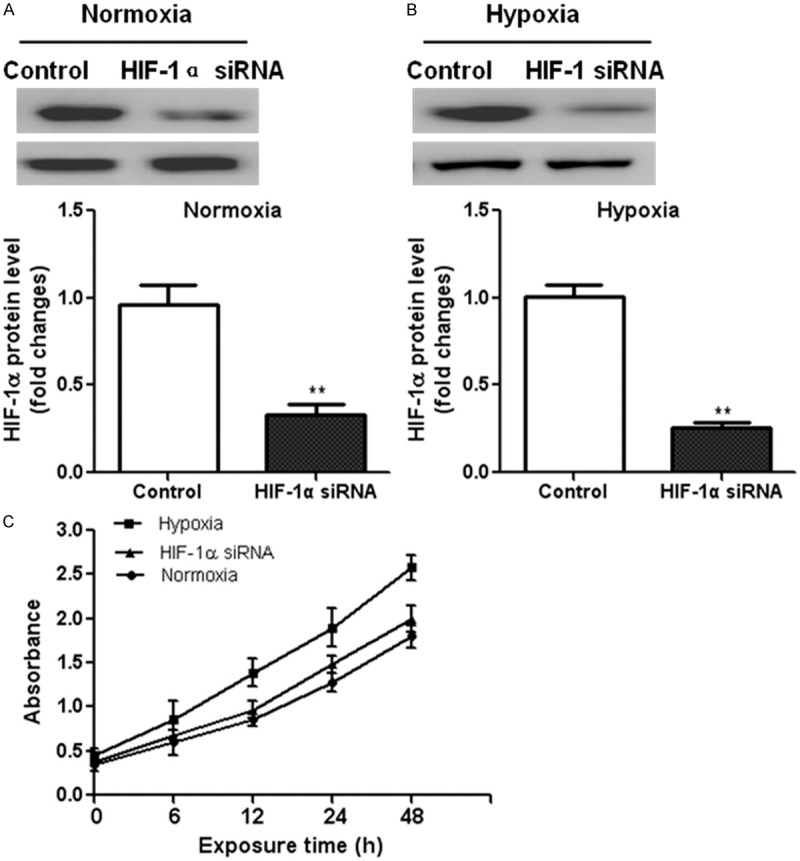
Role of HIF-1α in the hypoxia-induced proliferation of NRSMCs. NRSMCs were transfected with HIF-1α-siRNA or control siRNA for 24 h. Western blot analysis confirmed the down-regulation of HIF-1α expression both under normoxia (A) and hypoxia (B) conditions. (C) MTT assay was performed to measure the proliferation of NRSMCs or HIF-1α-siRNA transfected NRSMCs under hypoxia condition. **P < 0.01 vs. control group.
Discussion
In the cardiovascular system, hypoxia plays a crucial role in the development of atherosclerosis [17]. Hypoxia modulates key events for VSMCs functionality, including proliferation, migration and lipid accumulation [18,19]. The present study aimed to investigate the effect of hypoxia on neonatal rats-derived VSMCs, and we found that hypoxia treatment stimulated NRSMCs proliferation via HIF-1α dependent pathway.
It is well known that abnormal proliferation of vascular smooth muscle cells is closely associated with the pathogenesis of atherosclerosis. Such change in the proliferative phenotype is considered as an early event in atherogenesis and a distinctive feature in atherosclerosis-prone sites [15]. In our study, exposure to hypoxia at 2%, not 5 or 10% oxygen, stimulated NRSMCs proliferation. In addition, NRSMCs were incubated for different time points under hypoxic condition and showed remarkable enhanced proliferation, indicating that hypoxia at 2% O2 could promote NRSMCs growth in a time dependent manner.
Cell proliferation is involved in cell cycle progression and apoptosis [20], thus we assumed that abnormal proliferation of NRSMCs is due to the changed cell cycle distribution and apoptosis under hypoxia condition. Not surprisingly, data revealed that the percentage of NRSMCs in the S + G2/M phase was significantly increased following hypoxia treatment, suggesting that hypoxic stimulation resulted in more active cellular reactions in NRSMCs. Apoptosis, or programmed cell death, of VSMCs play a key role in the development and progression of the atherosclerosis and restenosis after percutaneous coronary intervention [15]. BNIP3 is a pro-death protein and a member of the BH3-only Bcl-2 family. BNIP3 overexpression induces various types of cell death via mitochondrial or non-mitochondrial death cascades [21]. In our study, we demonstrated that hypoxia inhibited NRSMCs apoptosis and, molecularly, suppressed the expression of BNIP3. Moreover, hypoxic stimulation also decreased the expression of pro-apoptotic protein bax and increased the protein level anti-apoptotic factor bcl-2. Collectively, these data demonstrated that hypoxia treatment changed cell cycle distribution and inhibited apoptosis in low oxygen-treated NRSMCs.
The cellular effects of hypoxia are primarily mediated by the hypoxia-inducible factor-1 complex which composed of α and β subunits [22]. HIF-1α is undetectable under normoxia due to the rapid proteasomal degradation. But it is stabilized under low oxygen tensions [23]. Increasing evidences indicate that the HIF-1 pathway is associated with the progression and angiogenesis of human atherosclerosis [24-26]. In experimental models of hypertension and hypercholesterolemia, HIF-1α is associated with dysfunction of VSMCs [27,28]. It is also reported that HIF-1α accumulation in macrophages promotes foam cell formation and atherosclerosis [29]. The current study demonstrated that hypoxia stimulation led to the activation of HIF-1α in NRSMCs and promoted cells proliferation. However, down-regulation of HIF-1α could partly abolish the proliferative effect of hypoxia on NRSMCs growth. Altogether, our data demonstrated that HIF-1α was an important mediator of vascular cell proliferation.
In summary, our study showed that hypoxia, and specifically HIF-1α, promoted the proliferation of NRSMCs. Specific down-regulation of HIF-1α could suppress the hypoxia-induced proliferation of vascular cells. Given the importance of hypoxia to atherosclerosis, our findings may contribute to the understanding of the development of atherosclerosis.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (No. 81271587) and the Key Program of Scientific Research of Fujian Medical University, Quanzhou, China (No. 09ZD015).
Disclosure of conflict of interest
None.
References
- 1.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359:61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zandi-Nejad K, Luyckx VA, Brenner BM. Adult hypertension and kidney disease: The role of fetal programming. Hypertension. 2006;47:502–508. doi: 10.1161/01.HYP.0000198544.09909.1a. [DOI] [PubMed] [Google Scholar]
- 3.Vuguin PM, Hartil K, Kruse M, Kaur H, Lin CL, Fiallo A, Glenn AS, Patel A, Williams L, Seki Y, Katz EB, Charron MJ. Shared effects of genetic and intrauterine and perinatal environment on the development of metabolic syndrome. PLoS One. 2013;8:e63021. doi: 10.1371/journal.pone.0063021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gallo LA, Tran M, Master JS, Moritz KM, Wlodek ME. Maternal adaptations and inheritance in the transgenerational programming of adult disease. Cell Tissue Res. 2012;349:863–880. doi: 10.1007/s00441-012-1411-y. [DOI] [PubMed] [Google Scholar]
- 5.Reynolds RM, Labad J, Buss C, Ghaemmaghami P, Raikkonen K. Transmitting biological effects of stress in utero: Implications for mother and offspring. Psychoneuroendocrino. 2013;38:1843–1849. doi: 10.1016/j.psyneuen.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 6.Menendez-Castro C, Toka O, Fahlbusch F, Cordasic N, Wachtveitl R, Hilgers KF, Rascher W, Hartner A. Impaired myocardial performance in a normotensive rat model of intrauterine growth restriction. Pediatr Res. 2014;75:697–706. doi: 10.1038/pr.2014.27. [DOI] [PubMed] [Google Scholar]
- 7.Kulandavelu S, Whiteley KJ, Bainbridge SA, Qu D, Adamson SL. Endothelial NO synthase augments fetoplacental blood flow, placental vascularization, and fetal growth in mice. Hypertension. 2013;61:259–266. doi: 10.1161/HYPERTENSIONAHA.112.201996. [DOI] [PubMed] [Google Scholar]
- 8.Zhang J, Adams MA, Croy BA. Alterations in maternal and fetal heart functions accompany failed spiral arterial remodeling in pregnant mice. Am J Obstet Gynecol. 2011;205:481–485. doi: 10.1016/j.ajog.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Villamor E, Kessels CG, Ruijtenbeek K, van Suylen RJ, Belik J, de Mey JG, Blanco CE. Chronic in ovo hypoxia decreases pulmonary arterial contractile reactivity and induces biventricular cardiac enlargement in the chicken embryo. Am J Physiol Regul Integr Comp Physiol. 2004;287:R642–R651. doi: 10.1152/ajpregu.00611.2003. [DOI] [PubMed] [Google Scholar]
- 10.Hauton D. Hypoxia in early pregnancy induces cardiac dysfunction in adult offspring of Rattus norvegicus, a non-hypoxia-adapted species. Comp Biochem Physiol A Mol Integr Physiol. 2012;163:278–285. doi: 10.1016/j.cbpa.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 11.Giussani DA, Niu Y, Herrera EA, Richter HG, Camm EJ, Thakor AS, Kane AD, Hansell JA, Brain KL, Skeffington KL, Itani N, Wooding FB, Cross CM, Allison BJ. Heart disease link to fetal hypoxia and oxidative stress. Adv Exp Med Biol. 2014;814:77–87. doi: 10.1007/978-1-4939-1031-1_7. [DOI] [PubMed] [Google Scholar]
- 12.Bourque SL, Gragasin FS, Quon AL, Mansour Y, Morton JS, Davidge ST. Prenatal hypoxia causes long-term alterations in vascular endothelin-1 function in aged male, but not female, offspring. Hypertension. 2013;62:753–758. doi: 10.1161/HYPERTENSIONAHA.113.01516. [DOI] [PubMed] [Google Scholar]
- 13.Peyronnet J, Dalmaz Y, Ehrstrom M, Mamet J, Roux JC, Pequignot JM, Thoren HP, Lagercrantz H. Long-lasting adverse effects of prenatal hypoxia on developing autonomic nervous system and cardiovascular parameters in rats. Pflugers Arch. 2002;443:858–865. doi: 10.1007/s00424-001-0766-9. [DOI] [PubMed] [Google Scholar]
- 14.Williams SJ, Hemmings DG, Mitchell JM, McMillen IC, Davidge ST. Effects of maternal hypoxia or nutrient restriction during pregnancy on endothelial function in adult male rat offspring. J Physiol. 2005;565:125–135. doi: 10.1113/jphysiol.2005.084889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rudijanto A. The role of vascular smooth muscle cells on the pathogenesis of atherosclerosis. Acta Med Indones. 2007;39:86–93. [PubMed] [Google Scholar]
- 16.Srivastava AK. High glucose-induced activation of protein kinase signaling pathways in vascular smooth muscle cells: A potential role in the pathogenesis of vascular dysfunction in diabetes (review) Int J Mol Med. 2002;9:85–89. [PubMed] [Google Scholar]
- 17.Nakano D, Hayashi T, Tazawa N, Yamashita C, Inamoto S, Okuda N, Mori T, Sohmiya K, Kitaura Y, Okada Y, Matsumura Y. Chronic hypoxia accelerates the progression of atherosclerosis in apolipoprotein E-knockout mice. Hypertens Res. 2005;28:837–845. doi: 10.1291/hypres.28.837. [DOI] [PubMed] [Google Scholar]
- 18.Hisada T, Ayaori M, Ohrui N, Nakashima H, Nakaya K, Uto-Kondo H, Yakushiji E, Takiguchi S, Terao Y, Miyamoto Y, Adachi T, Nakamura H, Ohsuzu F, Ikewaki K, Sakurai Y. Statin inhibits hypoxia-induced endothelin-1 via accelerated degradation of HIF-1alpha in vascular smooth muscle cells. Cardiovasc Res. 2012;95:251–259. doi: 10.1093/cvr/cvs110. [DOI] [PubMed] [Google Scholar]
- 19.Rose F, Grimminger F, Appel J, Heller M, Pies V, Weissmann N, Fink L, Schmidt S, Krick S, Camenisch G, Gassmann M, Seeger W, Hanze J. Hypoxic pulmonary artery fibroblasts trigger proliferation of vascular smooth muscle cells: Role of hypoxia-inducible transcription factors. FASEB J. 2002;16:1660–1661. doi: 10.1096/fj.02-0420fje. [DOI] [PubMed] [Google Scholar]
- 20.Dai XY, Zeng XX, Peng F, Han YY, Lin HJ, Xu YZ, Zhou T, Xie G, Deng Y, Mao YQ, Yu LT, Yang L, Zhao YL. A novel anticancer agent, SKLB70359, inhibits human hepatic carcinoma cells proliferation via G0/G1 cell cycle arrest and apoptosis induction. Cell Physiol Biochem. 2012;29:281–290. doi: 10.1159/000337609. [DOI] [PubMed] [Google Scholar]
- 21.Kim JY, Kim YJ, Lee S, Park JH. BNip3 is a mediator of TNF-induced necrotic cell death. Apoptosis. 2011;16:114–126. doi: 10.1007/s10495-010-0550-4. [DOI] [PubMed] [Google Scholar]
- 22.Yang M, Ge W, Chowdhury R, Claridge TD, Kramer HB, Schmierer B, McDonough MA, Gong L, Kessler BM, Ratcliffe PJ, Coleman ML, Schofield CJ. Asparagine and aspartate hydroxylation of the cytoskeletal ankyrin family is catalyzed by factor-inhibiting hypoxia-inducible factor. J Biol Chem. 2011;286:7648–7660. doi: 10.1074/jbc.M110.193540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishi K, Oda T, Takabuchi S, Oda S, Fukuda K, Adachi T, Semenza GL, Shingu K, Hirota K. LPS induces hypoxia-inducible factor 1 activation in macrophage-differentiated cells in a reactive oxygen species-dependent manner. Antioxid Redox Signal. 2008;10:983–995. doi: 10.1089/ars.2007.1825. [DOI] [PubMed] [Google Scholar]
- 24.Sluimer JC, Gasc JM, van Wanroij JL, Kisters N, Groeneweg M, Sollewijn GM, Cleutjens JP, van den Akker LH, Corvol P, Wouters BG, Daemen MJ, Bijnens AP. Hypoxia, hypoxia-inducible transcription factor, and macrophages in human atherosclerotic plaques are correlated with intraplaque angiogenesis. J Am Coll Cardiol. 2008;51:1258–1265. doi: 10.1016/j.jacc.2007.12.025. [DOI] [PubMed] [Google Scholar]
- 25.Osada-Oka M, Ikeda T, Akiba S, Sato T. Hypoxia stimulates the autocrine regulation of migration of vascular smooth muscle cells via HIF-1alpha-dependent expression of thrombospondin-1. J Cell Biochem. 2008;104:1918–1926. doi: 10.1002/jcb.21759. [DOI] [PubMed] [Google Scholar]
- 26.Luque A, Turu M, Juan-Babot O, Cardona P, Font A, Carvajal A, Slevin M, Iborra E, Rubio F, Badimon L, Krupinski J. Overexpression of hypoxia/inflammatory markers in atherosclerotic carotid plaques. Front Biosci. 2008;13:6483–6490. doi: 10.2741/3168. [DOI] [PubMed] [Google Scholar]
- 27.Herrmann J, Lerman LO, Rodriguez-Porcel M, Holmes DJ, Richardson DM, Ritman EL, Lerman A. Coronary vasa vasorum neovascularization precedes epicardial endothelial dysfunction in experimental hypercholesterolemia. Cardiovasc Res. 2001;51:762–766. doi: 10.1016/s0008-6363(01)00347-9. [DOI] [PubMed] [Google Scholar]
- 28.Kuwahara F, Kai H, Tokuda K, Shibata R, Kusaba K, Tahara N, Niiyama H, Nagata T, Imaizumi T. Hypoxia-inducible factor-1alpha/vascular endothelial growth factor pathway for adventitial vasa vasorum formation in hypertensive rat aorta. Hypertension. 2002;39:46–50. doi: 10.1161/hy1201.097200. [DOI] [PubMed] [Google Scholar]
- 29.Jiang G, Li T, Qiu Y, Rui Y, Chen W, Lou Y. RNA interference for HIF-1alpha inhibits foam cells formation in vitro. Eur J Pharmacol. 2007;562:183–190. doi: 10.1016/j.ejphar.2007.01.066. [DOI] [PubMed] [Google Scholar]


