Abstract
Objective: Whole human genome oligo microarrays were employed to systematically investigate the mRNA expression profile of 5-HT synthetase, transporter, receptor, and factors in 5-HT signaling pathway in peripheral blood karyocytes from pulmonary embolism (PE) patients. Methods: A total of 20 PE patients and 20 healthy subjects matched in gender and age were recruited. The human genome microarrays were performed to detect the mRNA expression profile of 5-HT synthetase, transporter, receptor, and factors in 5-HT signal pathway of two groups. The random variance model corrected t-test was used for analysis. Results: Our results showed (1) tryptophan hydroxylase (TPH1)-related gene expression was markedly down-regulated in PE patients (P < 0.01); (2) monoamine oxidases (MAO)-related gene (MAOB) expression was significantly up-regulated in PE patients (P < 0.01); (3) the expression of 17 genes of 7 5-HT receptors showed a down-regulated tendency in PE patients, and significant difference was observed in the expression of HTR1E, HTR3B, HTR4 and HTR5A between them (P < 0.05); (4) the expression of DalDAG-GEF I, Tubby, PKA and EPAC in 5-HT signal pathways was dramatically up-regulated in PE patients (P < 0.05); the expression of SPA1, RIAM, RAPL, Talin, PKC, PLC and Pyk2 was remarkably up-regulated in PE patients (P < 0.05); (5) the expression of integrin genes ITGA2B, ITGB1 and ITGB3 was significantly up-regulated in PE patients (P < 0.05). Conclusion: In PE patients, the expression of TPH1 and HTR4 was down-regulated as a negative feedback; the MAOB expression was up-regulated. Consistent with the expression of 5-HTR1E and 5-HTR4 and the abnormally activated Tubby, the expression of integrins in platelets was activated.
Keywords: 5-HT receptor, Tubby, integrin, pulmonary embolism, mRNA
Introduction
Both pulmonary embolism (PE) and deep venous thrombosis (DVT) are known as venous thromboembolism (VTE). It has become one of the major health problems worldwide because of its high incidence, high mortality and high misdiagnose rate [7]. Strudsholm et al found that psychiatric patients suffered higher risk for VTE than healthy people in clinical practice [11,18]. Dehydration, mental stress, and physical restriction can increase this risk [12]. Antipsychotic drugs, especially antidepressants, can increase the risk for VTE significantly [1,13,17]. Study has showed that antipsychotic drugs in unclassified psychiatric patients lead to an increase in the incidence of VTE to 50% [2]. Selective serotonin reuptake inhibitors (SSRIs) and 5-HT2A receptor inhibitors, which serve as the most common clinical antipsychotic drugs, involve in the 5-HT function. This suggests that endogenous 5-HT and its receptors and transporters may take part in the occurrence of VTE. This study aimed to investigate the differentially expressed genes related to 5-HT between symptomatic PE patients and healthy controls by using human genome oligo microarrays.
Material and methods
Patients’ characteristics
Twenty patients with PE who were admitted in 2007 were recruited, and included 11 males and 9 females, with an average age of 70 ± 14 years (range: 44-89 years). PE was diagnosed on the basis of at least 2 of following criteria: 1) selective pulmonary arteriography shows a filling defect or blockage; 2) pulmonary ventilation perfusion scanning exhibits single or multiple blood flow perfusion defects with normal or abnormal ventilation and mismatched ratio of ventilation/perfusion; 3) there are other clinical characteristics of PE, including a typical manifestation of PE, arterial blood gas analysis, and D-dimer test, and ultrasound cardiogram (UCG) and chest computerized tomography (CT) support the diagnosis and exclude other cardiac and pulmonary disorders. Another 20 healthy subjects admitted in the same period were also recruited as controls. These subjects had no PE, DVT and other congenital bleeding and thrombosis diseases. There were 11 males and 9 females with a mean age of 72 ± 14 years (range: 44-91 years). The demographics were matched between 2 groups. This study was approved by the Ethics Committee of our hospital and an informed consent was obtained from each patient in accordance with the declaration of Helsinki.
Total RNA isolation
A total of 5 ml of venous EDTA anti-coagulated blood was obtained from patients of both groups and mononuclear cells were isolated by density gradient centrifugation. Red blood cell lysis buffer (Qiagen, Hilden, Germany) was used to isolate mononuclear cells and total RNA was extracted from mononuclear cells with TRIzol (Invitrogen, Carlsbad, USA) fol-lowed by purification with RNeasy column (QIAGEN). Treatment with DNase was performed to avoid the influence by genomic DNA. Quantification of extracted RNA was performed with Nanodrop ND-1000 spectrophotometer (Nanodrop Technology, Cambridge, UK).
Gene expression clip
Agilent G4112A Whole Human Genome Oligo Microarrays were purchased from Agilent (USA). A microarray is composed of 44,290 spots including 41675 genes or transcripts, 314 negative control spots, 1924 positive control spots and 359 blank spots. The functions of more than 70% of genes in the microarray have been known. All patients of both groups were subjected to microarray analysis.
Detection of gene expression
About 1 μg of total RNA was reversely transcribed into double strand cDNA. After purification, in vitro amplification was performed with Agilent Low RNA Input Linear Amplification Kit (Agilent, Pal alto, USA) and modified UTP [aaUTP, 5-(3-aminoally1)-UTP] was used to replace UTP. The integrated aaUTP can interact with Cy3 ester forming fluorescent products which are then used for hybridization. The integration rate of fluorescence can be determined with a NanodropND-1000 spectro-photometer. Then, hybridization mixture was pre-pared with Agilent oligonucleotide microarray in situ hybridization plus kit. About 750 ng of fluorescent products were fragmented at 60°C and hybridization was conducted in Human Whole-Genome 60-mer oligo-chips (G4112F, Agilent Technologies) at 60°C for 17 h at 10 rpm. After hybridization, the chips were washed with Agilent Gene Expression Wash Buffer according to manufacturer’s instructions. Original signals were obtained Agilent scanner and Feature Extraction software. The standardization of original signals was carried out with RMA standardized method and standardized signal values were used for screening of differentially expressed genes.
RT-PCR
The spots in the microarray were randomly selected and their expressions were confirmed by RT-PCR. Among genes with differential expressions, 3 genes were randomly selected and these genes and housekeeping gene (GAPDH) were subjected to RT-PCR. The relative expressions were expressed as the expressions of target genes normalized by that of GAPDH (2-ΔΔCt). Melting curve and 2-ΔΔCt method were used to compare the difference in the expressions between control group and PE group. Results from RT-PCR were consistent with microarray analysis.
Statistical analysis
Agilent Feature extraction software was used to collect original data from microarray followed by analysis with robust multichip average (RMA). Results were expressed as average ± standard deviation. Gene intensity data between PE group and control group were compared with t test after calibration with a stochastic variance model. Differentially expressed genes were identified from whole genomes. Independent-Samples T Test was used to compare mRNA levels in samples from PE patients and controls. Statistical tests were performed using SPSS 14.0, and p values < 0.05 were considered significant. Before t test, test for equality of variances was performed, if variances were not equal, t test result would be corrected. Multiple linear regression analysis was performed to determine the correlation between expressions of various 5-HT mRNA and platelet membrane glycoprotein mRNA.
Results
Gene expression clip results
All patients of both groups were subjected to microarray analysis. 40 Agilent G4112A Whole Human Genome Oligo Microarrays were employed in this study. Each of them was conform to the quality control standard. The experimental system was stable. The intense fluorescence signal was uniform. The detection rate of gene expression was high with less coefficient variation (Table 1; Figure 1).
Table 1.
The detection rate of the chip
| Number | PE group (%) | Control group (%) |
|---|---|---|
| 1 | 72.95 | 65.92 |
| 2 | 74.38 | 68.84 |
| 3 | 67.07 | 69.81 |
| 4 | 68.40 | 59.48 |
| 5 | 71.58 | 51.26 |
| 6 | 69.38 | 74.05 |
| 7 | 63.84 | 72.95 |
| 8 | 68.44 | 65.91 |
| 9 | 65.04 | 57.45 |
| 10 | 71.51 | 71.96 |
| 11 | 79.63 | 67.08 |
| 12 | 66.69 | 70.22 |
| 13 | 70.68 | 67.78 |
| 14 | 68.51 | 57.38 |
| 15 | 71.03 | 69.97 |
| 16 | 66.91 | 66.88 |
| 17 | 73.34 | 64.67 |
| 18 | 74.11 | 53.05 |
| 19 | 65.53 | 64.36 |
| 20 | 65.00 | 69.72 |
Figure 1.
The intense fluorescence signal was uniform.
A total of 44 related genes were tested
When compared with controls, TPH1 expression was significantly down-regulated in peripheral blood (P < 0.01); TPH2 expression was down-regulated with no significant difference (P > 0.05); monoamine oxidase MAO related gene MAOB was markedly up-regulated (P < 0.01); the expressions of 5-HT transporter SERT and monoamine oxidase MAO related gene MAOA was up-regulated without statistically significant difference (P > 0.05) in PE patients. The mRNA expressions of genes related to TPH, SERT and MAO are showed in Figure 2.
Figure 2.
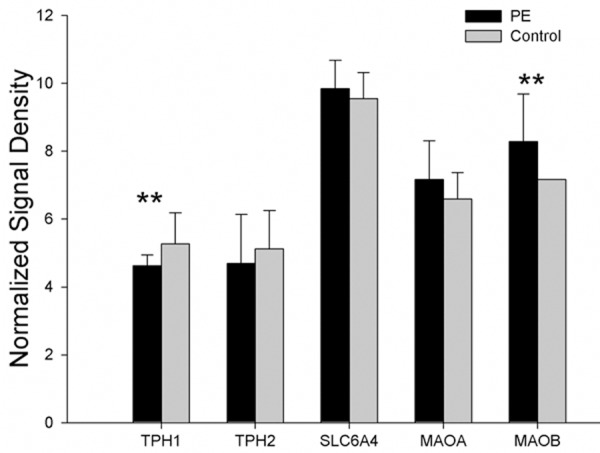
The mRNA expressions of TPH, SERT and MAO showed that the expressions of TPH1 and MAOB mRNA were regulated with significant difference (*P < 0.05, **P < 0.01).
When compared with controls, 17 genes of 7 5-HT receptors showed a down-regulated tendency, and the expressions of 4 genes (HTR1E, HTR3B, HTR4, and HTR5A) were significantly down-regulated in PE patients (P < 0.05). The mRNA expressions of genes related to HTR are showed in Figure 3.
Figure 3.
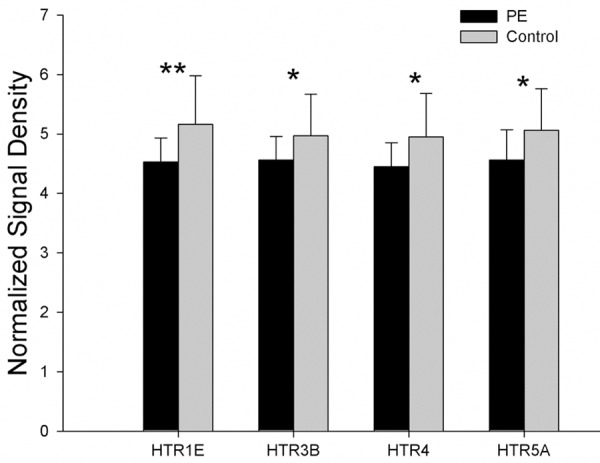
The mRNA expression of genes related to 5-HT receptors showed that the expressions of 4 genes (HTR1E, HTR3B, HTR4, and HTR5A) were significantly down-regulated in PE patients (*P < 0.05, **P < 0.01).
When compared with control group, the expressions of DalDAG-GEF I, Tubby, EPAC, and PKA (factors in 5-HT receptor signaling pathway) were down-regulated (P < 0.05), and the expressions of PLCγ, PLCβ, SPA1, RIAM, RAPL, Talin, PKC and Pyk2 were up-regulated (P < 0.05) in PE patients. The mRNA expressions of genes related to 5-HT receptor signaling pathway are showed in Figure 4.
Figure 4.
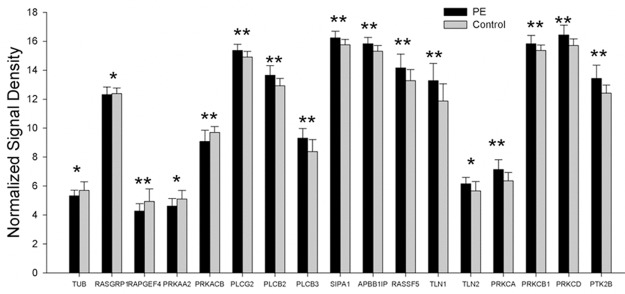
The mRNA expression of genes related to receptor signaling pathway showed that the expressions of DalDAG-GEF I, Tubby, EPAC, and PKA (factors in 5-HT receptor signaling pathway) were down-regulated, and the expressions of PLCγ, PLCβ, SPA1, RIAM, RAPL, Talin, PKC and Pyk2 were up-regulated in PE patients (*P < 0.05, **P < 0.01).
When compared with control group, the expressions of integrin α2β1 and α2bβ3 related genes (ITGB1, ITGB3 and ITGA2B) were up-regulated (P < 0.01) in PE patients, and the ITGA2 expression was comparable between two groups (P > 0.05). The mRNA expressions of genes related tointegrin α2β1 and α2bβ3 are showed in Figure 5.
Figure 5.
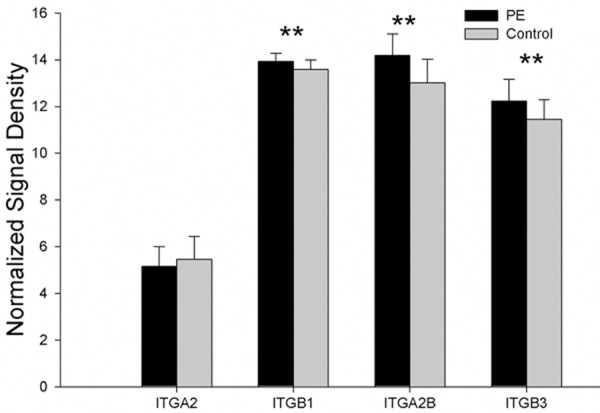
The mRNA expression of genes related to integrin α2β1 and α2bβ3 showed that the expressions of integrin α2β1 and α2bβ3 related genes (ITGB1, ITGB3 and ITGA2B) were up-regulated in PE patients, and the ITGA2 expression was comparable between two groups (*P < 0.05, **P < 0.01).
Discussion
5-hydroxytryptamine (5-HT) is a monoamine transmitter. A tryptophan was hydroxylated into 5-Hydroxytryptophan by tryptophan hydroxylase, and then dehydroxylated by 5-Hydroxytryptophan dehydroxylase, and finally the 5-hydroxytryptamine (10% located in central neurons and 90% located in intestinal pheochromocytoma) is synthesized. Blood-brain barrier is not permeable to 5-HT, and so the central nervous system and peripheral tissues should be seen as separate systems. There are two subunits (TPH1 and TPH2) in TPH. TPH1 is rate limiting enzyme in the peripheral 5-HT synthesis. 5-HT was inactivated after exhibiting its physiological function, because the accumulation of 5-HT may cause toxicity and 5-HT receptor desensitization. About 2/3 of 5-HT is degraded in the liver (together with sulfuric acid or glucose) and 1/3 is degraded in the endothelial cells (deaminized by MAO), and then excreted though urine. Some investigators have shown that, in monkeys, MAOB takes a major part in the 5-HT degradation. Our study demonstrated that, in PE patients, TPH1 expression was down-regulated and MAOB expression up-regulated. This suggests that 5-HT may be accumulated in PE patients.
SERT is 5-HT transporter. It locates on the surface of neural cells, platelets, intestinal epithelial cells, and pulmonary vascular endothelial cells [14,15]. SERT from epithelial cells and endothelial cells transports 5-HT from peripheral tissues into cells for degradation. SERT from platelets re-uptakes 5-HT. SERT is regulated through multiple factors in vivo. Brenner and his colleagues found the plasma 5-HT has dual roles in the regulation of platelet membrane SERT [4]. Plasma 5-HT may increase in prehypertension, and so does platelet membrane SERT. When during hypertension, plasma 5-HT further increases, but platelet membrane SERT reduces. This suggests 5-HT can regulate the platelet membrane SERT expression. Jess et al foundthat PKC could phosphorylate SERT after SERT combining with MARCKS protein of macrophages [9]. This phenomenon leads 5-HT re-uptake and exocytosis. Extracellular 5-HT regulates PKC-dependent 5-HT. Benmansour et alfound that SERT of intestinal epithelial cells was up-regulated through the EGF-induced activation of AP-1 [3]. Latorre et al showed SERT of intestinal epithelial cells wasup-regulated after P13K pathway activation by IL-10 at a high concentration [10]. Previous studies have shown that multiple factors are involved in the regulation of SERT in vivo. Our findings showedthe expression of SERT related gene was comparable between PE patients and controls, whichsuggests the regulation of SERT is related to multiple factors in vivo.
Plasma 5-HT can only exhibit physiological functions after binding to 5-HT receptors. There are 7 5-HT receptors in human body which are G protein-coupled receptors (except for 5-HT3R belonging to ligand-gated ion channel). 5-HT receptor contains 7 transmembrane segments, 3 cytoplasmic loops, and 3 extracellular loops. Our study demonstrated that the expression of all the 17 genes showed a down-regulated tendency, and significant difference in the expression of HTR1E, HTR3B, HTR4 and HTR5A was observed between two groups. (1) HTR1E is a Gi protein-coupled receptor, and mainly locates in cytoplasm and on platelets. HTR1Ecan cause a release of Ca2+, IL-6, and IL-8 after binding to 5-HT receptors, which may suppress adenylate cyclase (AC) and inhibit cAMP [20]. (2) HTR4 is a Gi protein-coupled receptor, and mainly locates in platelets of peripheral tissues. HTR4 can activate AC, increase the cAMP synthesis, and elevate the EPAC and PKA activities. (3) HTR2Ais a Gq protein-coupled receptor, and mainly locates in the platelets and livers. HTR2A can induce signal transduction only with the help of Tubby [16,19]. After binding to 5-HT, HTR2A can dehydrate PIP2 and release DAG, with the help of Tubby and PLC. (4) HTR5A locates only in the central nervous system [6]. HTR3B cannot function as HTR3 without HTR3A, and suppress by Ca2+ [8].
HTR1E, HTR2A and HTR4-activated Ca2+ release and activated DAG, EPAC and PKA may up-regulate DalDAG-GEFI expression, resulting in the activation of Rap-1. Rap-1 is Ras-related small molecular GTP enzyme and a key protein of the integrin “inside-out” signal pathway. DalDAG-GEFI can activate Rap-1, and its downstream RIAM, RAPL. SPA1 protein effectors can inactivate Rap-1. RIAM can regulate Talin. This protein binds to the β subunit of integrin and extends the β subunit through regulating cytoskeletal proteins (such as α-actinin). RAPL can extend α subunit of integrin. These processes can increase the affinity of integrin. In addition, DAG can activate PKC and its downstream Pyk2 (FAK family). Pyk2 can regulate PI3Kβ in the α2β1 signaling pathway. In this situation, the combination of α and β subunit of α2bβ3 integrin is disrupted, α2bβ3 is activated, and thrombosis occurs [5].
Our study showed that the expression of Tubby, DalDAG-GEFI, EPAC and PKA of 5-HT signal pathway were down-regulated in PE patients as a negative feedback, and the expression of its downstream genes (SPA1, RIAM, RAPL, Talin, PKC, Pyk2) was significantly up-regulated (P < 0.05), and that of ITGA2B, ITGB1, ITGB3 up-regulated markedly (P < 0.01). This suggests that 5-HT may be activated after binding to 5-HTR1E and HTR4 with the help of Tubby, which activates related GPCRs and then the key protein Rap-1 of the “inside-out” signaling pathway. Finally, the α2bβ3 signal pathway (related to platelet aggregation) is activated; α and β subunit then are extended, and the affinity increases.
A study conducted from 1987 to 2009 showed that family history was an important risk factor of VTE [21]. Family aggregation of VTE has a strong genetic characteristic. Genetic factors of VTE are mainly the coagulation inhibiting protein mutation and procoagulant function acquired-mutation, but they are very rare [21]. In a pilot study, investigators found that I93V mutation of HTR2A gene in PE/DVT patients of a VTE family. This mutation causes a 5-HTR2A dysfunction. In 100 healthy people, HTR2A DNA mutation was undetectable. This suggests this kind of mutation holds a family history characteristic. Our study showed that, in PE patients, plasma 5-HT at a high concentration was detected but 5-HTR2A mRNA expression remained unchanged, and HTR2A expression was not up-regulated. This suggests that HTR2Awas insensitive to high level peripheral 5-HT. This is similar in VTE family.
In the presence of high level 5-HT, Tubby expression was up-regulated as a negative feedback (P < 0.05) but 5-HTR2A remained unchanged in PE patients. 5-HTR2A dysfunction or insensitive, despite of high level 5-HT, may abnormally activate Tubby, and then activate α2bβ3 integrin signaling pathway, which is a final common pathway of platelet aggregation and increases the risk for venous thrombosis.
Acknowledgements
The study was granted by “12th Five-year” National Science & Technology Supporting Program (2011BAI11B16).
Disclosure of conflict of interest
None.
References
- 1.Allenet B, Schmidlin S, Genty C, Bosson JL. Antipsychotic drugs and risk of pulmonary embolism. Pharmacoepidemiol Drug Saf. 2012;21:42–48. doi: 10.1002/pds.2210. [DOI] [PubMed] [Google Scholar]
- 2.Barbui C, Conti V, Cipriani A. Antipsychotic Drug Exposure and Risk of Venous Thromboembolism: A Systematic Review and Meta-Analysis of Observational Studies. Drug Saf. 2014;37:79–90. doi: 10.1007/s40264-013-0127-6. [DOI] [PubMed] [Google Scholar]
- 3.Benmansour S, Owens WA, Cecchi M, Morilak DA, Frazer A. “Serotonin clearance in vivo is altered to a greater extent by antidepressant-induced downregulation of the serotonin transporter than by acute blockade of this transporter”. J Neurosci. 2002;22:6766–6772. doi: 10.1523/JNEUROSCI.22-15-06766.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brenner B, Harney JT, Ahmed BA, Jeffus BC, Unal R, Mehta JL, Kilic F. Plasma serotonin levels and the platelet serotonin transporter. J Neurochem. 2007;102:206–215. doi: 10.1111/j.1471-4159.2007.04542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Consonni A, Cipolla L, Guidetti G, Canobbio I, Ciraolo E, Hirsch E, Falasca M, Okigaki M, Balduini C, Torti M. Role and regulation of phosphatidylinositol 3-kinase beta in platelet integrin alpha 2 beta 1 signaling. Blood. 2012;119:847–856. doi: 10.1182/blood-2011-07-364992. [DOI] [PubMed] [Google Scholar]
- 6.Grailhe R, Grabtree GW, Hen R. Human 5-HT5 receptors: the 5-HT5A receptor is functional but the 5-HT5B receptor was lost during mammalian evolution. Eur J Pharmacol. 2001;418:157–167. doi: 10.1016/s0014-2999(01)00933-5. [DOI] [PubMed] [Google Scholar]
- 7.Heit JA. The epidemiology of venous thromboembolism in the community. Arterioscler Thromb Vasc Biol. 2008;28:370–372. doi: 10.1161/ATVBAHA.108.162545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002;71:533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- 9.Jess U, El Far O, Kirsch J, Betz H. Interaction of the C-terminal region of the rat serotonin transporter with MacMARCKS modulates 5-HT uptake regulation by protein kinase C. Biochem Biophys Res Commu. 2002;294:272–279. doi: 10.1016/S0006-291X(02)00460-6. [DOI] [PubMed] [Google Scholar]
- 10.Latorre E, Mendoza C, Matheus N, Castro M, Grasa L, Mesonero JE, Alcalde AI. IL-10 modulates serotonin transporter activity and molecular expression in intestinal epithelial cells. Cytokine. 2013;61:778–784. doi: 10.1016/j.cyto.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 11.Malý R, Masopust J, Hosák L, Konupcíková K. Assessment of risk of venous thromboembolism and its possible prevention in psychiatric patients. Psychiatry Clin Neurosci. 2008;62:3–8. doi: 10.1111/j.1440-1819.2007.01773.x. [DOI] [PubMed] [Google Scholar]
- 12.Malý R, Masopust J, Konupcíková K. Prevention of venous thromboembolism in psychiatry. Vnitr Lek. 2006;52(Suppl 1):73–78. [PubMed] [Google Scholar]
- 13.Parker C, Coupland C, Hippisley-Cox J. Antipsychotic drugs and risk of venous thromboembolism: nested case-control study. BMJ. 2010:341. doi: 10.1136/bmj.c4245. [DOI] [PubMed] [Google Scholar]
- 14.Pavone LM, Mithbaokar P, Mastellone V, Avallone L, Gaspar P, Maharajan V, Baldini A. Fate map of serotonin transporter-expressing cells in developing mouse heart. Genesis. 2007;45:689–695. doi: 10.1002/dvg.20343. [DOI] [PubMed] [Google Scholar]
- 15.Pavone LM, Tafuri S, Mastellone V, Morte RD, Lombardi P, Avallone L, Maharajan V, Staiano N, Scala G. Expression of the serotonin transporter (SERT) in the choroid plexuses from buffalo brain. Anat Rec (Hoboken) 2007;290:1492–1499. doi: 10.1002/ar.20610. [DOI] [PubMed] [Google Scholar]
- 16.Santagata S, Boggon TJ, Baird CL, Gomez CA, Zhao J, Shan WS, Myszka DG, Shapiro L. G-protein signaling through tubby proteins. Science. 2001;292:2041–2050. doi: 10.1126/science.1061233. [DOI] [PubMed] [Google Scholar]
- 17.Schmedt N, Garbe E. Antipsychotic Drug Use and the Risk of Venous Thromboembolism in Elderly Patients With Dementia. J Clin Psychopharmacol. 2013;33:753–758. doi: 10.1097/JCP.0b013e3182a412d5. [DOI] [PubMed] [Google Scholar]
- 18.Strudsholm U, Johannessen L, Foldager L, Munk-Jørgensen P. Increased risk for pulmonary embolism in patients with bipolar disorder. Bipolar Disord. 2005;7:77–81. doi: 10.1111/j.1399-5618.2004.00176.x. [DOI] [PubMed] [Google Scholar]
- 19.Sun X, Haley J, Bulgakov OV, Cai X, McGinnis J, Li T. Tubby is required for trafficking G protein-coupled receptors to neuronal cilia. Cilia. 2012;1:21. doi: 10.1186/2046-2530-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zgombick JM, Schechter LE, Macchi M, Hartig PR, Branchek TA, Weinshank RL. Human gene S31 encodes the pharmacologically defined serotonin 5-hydroxytryptamine1E receptor. Mol Pharmacol. 1992;42:180–185. [PubMed] [Google Scholar]
- 21.Zöller B, Ohlsson H, Sundquist J, Sundquist K. Familial risk of venous thromboembolism in first-, second- and third-degree relatives: a nationwide family study in Sweden. Thromb Haemost. 2013;109:458–463. doi: 10.1160/TH12-10-0743. [DOI] [PubMed] [Google Scholar]


