Abstract
Purpose: Eosinophilic cystitis (EC) is a rare disease and remains a poorly understood. We explored the potential etiology, clinical and radiological presentation, diagnosis and therapeutic experience with EC. Materials and Methods: A pooled ten patients diagnosed with EC were retrospectively studied in our hospital to assess clinical presentation symptoms, radiological and pathological diagnosis, treatment and outcomes between 1998 and 2012. Results: Nine patients presented with irritative urinary symptoms, one was symptomless. Allergic history were found in 2 patients, bladder mass was detected in all by radiologic tests or cystoscopy. Radiology revealed diffuse thickening of bladder wall in 7 cases among which one with bilateral hydroureteronephrosis, solitary tumor-like lesion in other 3. Elevated serum leukocytes were evident in 4 cases while elevated peripheral eosinophilias were observed in 3. One was asymptomatic and without specific therapy (group 1, 10%), transurethral resection of the lesions in one tumor-like case (10%). The other 8 cases were treated with corticosteroid and/or antihistaminics and 5 patients had excellent outcome with symptom resolution (62.5%). Conclusions: EC usually follows a benign course, most treated with corticosteroids and resulted in relief of symptoms while some may relapse. Surgery is an available choice for drug refractory or localized EC.
Keywords: Eosinophilic cystitis, diagnosis, therapy, radiology
Introduction
Eosinophilic cystitis (EC) is a rare urological disease simulating bladder tumor. It was first reported by Palubinskas and Brown independently in 1960 [1]. The etiology of eosinophilic cystitis remains unclear. It is considered to be immunological and self limiting disease. It is characterized by inflammation, mainly by eosinophils, throughout all layers of the bladder wall and fibrosis of the mucosa and muscularis necrosis. To the best of our knowledge, there are no more than 200 cases documented in english literature. The typical manifestations of EC are irritative bladder symptoms and most cases with large bladder mass simulating bladder carcinoma. There are no standard management, it mainly necessitate conservative therapy. Here, we performed an analysis of 10 cases treated with medicine therapy and/or surgical intervention to explore the best way for management, most cases obtained excellent results in our study.
Materials and methods
After institutional review board approval was obtained, we retrospectively reviewed the records in our institutions from 1998 to 2012. All 10 cases were diagnosed eosinophilic cystitis (EC). To confirm the diagnosis, all hematoxylin and eosin stained glass slides were reexamined by pathologists. Patient gender, age, presence of symptoms, physic examination, laboratory test, radiology, cystoscopy, pathology, treatment and outcomes were recorded.
Results
There were 6 males and 4 females. The mean age at diagnosis for the 10 patients was 16.4 years (range, 4-68 years). During this observation time, allergic or similar family history were found in 2 patients (20%), and their allergens were food or pollen (Table 1). 9 of 10 patients (90%) were symptomatic. The predominant symptoms were irritative voiding symptoms, dysuria, hematuria and abdomen pain, and the most common symptom was frequency (6/9, 67%). while another one patient (10%) were asymptomatic but claimed history of transurethral resection of bladder tumor 2 years ago and Mitomycin (MMC) intravesical instillation for one year. The asymptomatic one was found in routing surveillance (Table 1). Bladder masses were palpable during digital rectal examination or abdomen palpation in 2 patients. The rest had no positive founding in physical examination. Elevated serum leukocytes were evidently higher in 4 cases while elevated peripheral eosinophilias were observed in 3 cases. Urinalysis results indicated microscopic hematuria in 5 patients (50%) (Table 2). Urine cultures and cytology were negative in all patients. Ultrasonography presented bilateral hydronephrosis with bladder wall thickening in one case, and irregular wall thickening or tumor-like lesion were showed in the other 9 cases. CT scan revealed diffuse or irregular thickening of bladder wall in 7 cases (Figures 1, 2 and 3), among which one patient had restricted filling of bladder and bilateral upper tract dilation. Solitary tumor-like lesion with wide base appeared in three cases (Figures 4, 5). Urography, consisting of excretory urography (IVP) was performed in three cases and revealed abnormal findings of a small contracted bladder and bilateral hydronephrosis that had progressed for 2 years in one patient. All of them were considered to have bladder tumor, chronic or interstitial cystitis of bladder according to radiological reports. Cystoscopy or diagnostic TUR were introduced in 9 cases and open operation biopsy in one case. Abnormalities were found in all the patients, including inflammatory lesions (edema, erythema) in six patients, among which 2 patients accompanied with ulceration, and 4 with tumor-like lesion (Table 2). All cases were diagnosed pathologically by biopsy or lesion resection.
Table 1.
Clinical and demographic characteristics at presentation
| Patient | Sex | Age (years) | Symptoms | Onset time | allergic history | physic examination |
|---|---|---|---|---|---|---|
| 1 | M | 4 | 1, 2 | 2 months | No | Mass |
| 2 | M | 11 | 1 | 1 week | Pollen | Negative |
| 3 | M | 8 | 2, 3 | 6 days | No | Mass |
| 4 | M | 68 | No | No | No | Negative |
| 5 | M | 15 | 1, 2 | 2 weeks | Fish | Negative |
| 6 | M | 5 | 1 | 2 months | No | Negative |
| 7 | F | 6 | 1 | 5 days | No | Negative |
| 8 | F | 28 | 1 | 1 month | No | Negative |
| 9 | F | 12 | 1 | 1 week | No | Negative |
| 10 | F | 7 | 1 | 6 days | No | Negative |
Symptoms: 1: Irritative voiding symptoms, 2: Abdomen pain, 3: Intermittent hematuria.
Table 2.
Radiology, cystoscopy and laboratory finding of patients
| No | Leucocyte count (*109) | Eosinophilic leucocyte (%) | Urinalysis | Radiology | Cystoscopy or TUR |
|---|---|---|---|---|---|
| 1 | 8.6 | 0 | Negative | Diffuse | Diffuse |
| 2 | 43.8 | 16 | Negative | Diffuse | Diffuse |
| 3 | 11.9 | 1 | RBC ++ | Irregular | 4cm |
| 4 | 6.6 | 2 | RBC ++++ | Diffuse | Diffuse |
| 5 | 14.3 | 7 | RBC +++ | Diffuse, bilateral hydronephrosis | Diffuse, BC < 100 ml |
| 6 | 7.1 | 3 | RBC + | Tumor-like 4 cm | 3.5 cm |
| 7 | 12.5 | 9 | RBC ++ | Tumor-like , 6 cm | 6 cm |
| 8 | 5.8 | 0 | Negative | Tumor-like, 2.5 cm | 2.5 cm, papillary |
| 9 | 7.2 | 1 | Negative | Tumor-like Irregular | 3.5 cm (OP) |
| 10 | 6.7 | 4 | Negative | Diffuse | Diffuse |
Cystoscopy: BC = bladder capacity, OP = open operation biopsy.
Figure 1.
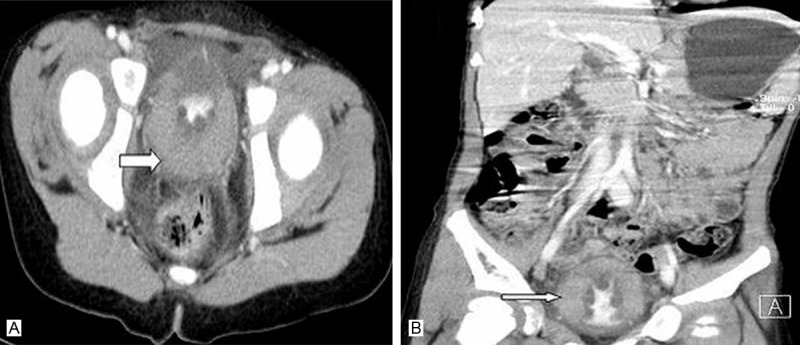
A. Axial CT of pelvis with intravenous contrast material reveals diffuse thickening of the bladder wall without solid mass (White arrow). B. In coronal view, the lesion is well defined and involving the entire bladder wall without enhancement and with low bladder capacity (White arrow).
Figure 2.
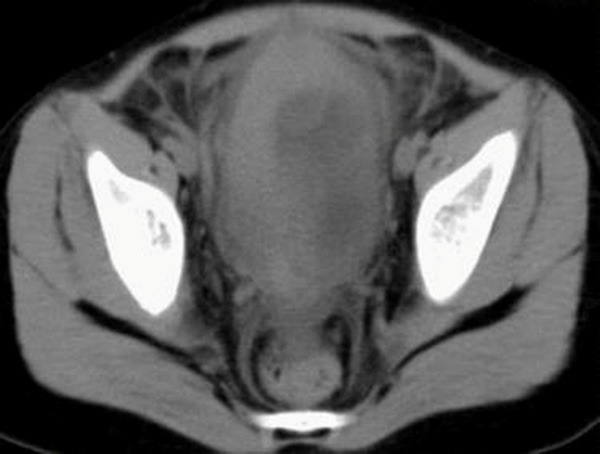
Abdominal CT without intravenous contrast material revealed diffuse thickening of the bladder wall without mass.
Figure 3.
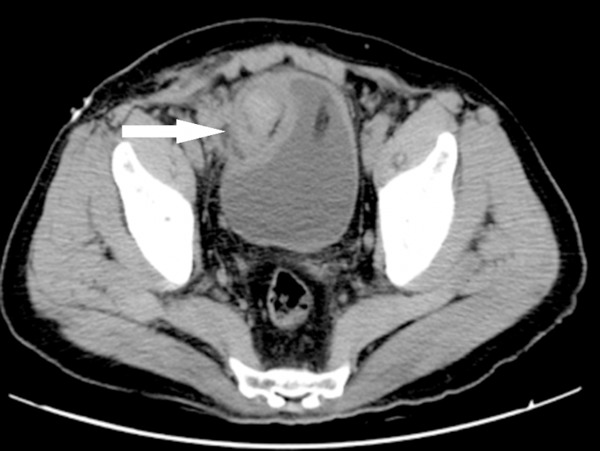
Pelvic CT revealed a well defined inhomogeneous mass with hemorrhage (White arrow), the mass protrudes into bladder on right side of dome measuring about 3 cm in diameter.
Figure 4.
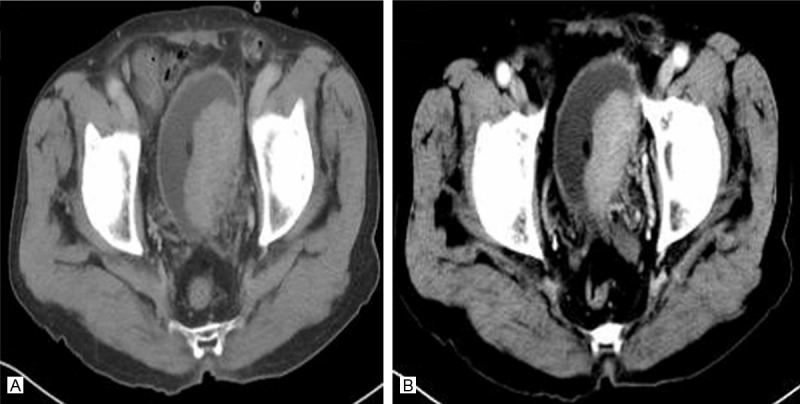
A. Axial CT image showed a large, smooth, regular, solid mass arising from the left bladder wall and bulging into the bladder lumen (arrow). B. Axial contrast-enhanced CT image showed obvious enhancement of the mass without extravesicular extension (arrow).
Figure 5.
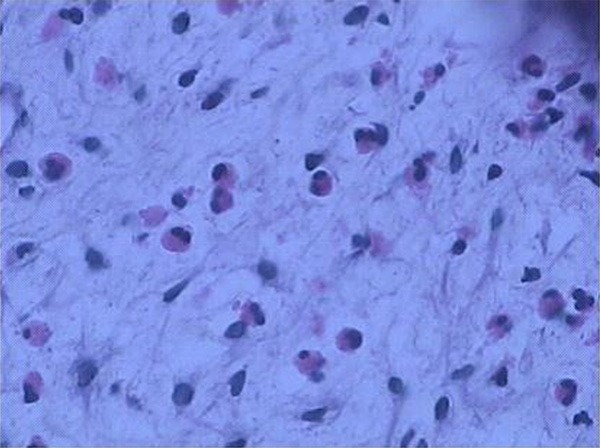
Biopsy of the bladder mucosa demonstrated dense inflammatory infiltration of predominant eosinophils with characteristic red granules (H & E, ×400).
Histologically, diffuse infiltration of eosinophilic granulocytes in the stroma and musculature was found, and most of them with muscle necrosis. Fibrosis of the bladder wall with numerous scattered eosinophils can be found in contracted bladder. The diagnosis of eosinophilic cystitis was confirmed in all cases. After the diagnosis of eosinophilic cystitis were confirmed, one asymptomatic patient was just under watchful observation (10%), while another one was given transurethral resection (TUR) of the lesion (10%). Oral regimens were given to the rest 8 patients, prednisone daily for 2-8 weeks that was tapered to 5 mg with or without antihistamines (loratadine, 5 to 10 mg, daily or terfenadine 30 to 60 mg, twice daily) for the first line (Table 3). Mean follow-up time for the 10 subjects was 17.4 months (range, 2-30 months). Among 8 oral regimens used cases, 5 patients had satisfactory outcome with symptom resolution (62.5%), including one case underwent TUR who had symptom relapse and subsequently take another period of corticosteroid treatment without relapse for 2 years follow-up. Partial cystectomy was performed on 2 refractory cases, and another unremitting deterioration case with contracted bladder secondary to eosinophilic cystitis that eventually received augmentation ileocystoplasty to increase bladder volume and compliance (Table 3).
Table 3.
Treatment methods for patients
| No. | First line treatment | Course (weeks) | Follow-up (months) | Results |
|---|---|---|---|---|
| 1 | C + A | 6 | 15 | Good |
| 2 | C | 6 | 24 | Good |
| 3 | C + A | 4 | 4 | Partial cystectomy |
| 4 | No | No | 36 | Good |
| 5 | C + A | 2 | 0.5 | Augmentation ileocystoplasty |
| 6 | C + A | 4 | 3 | Partial cystectomy |
| 7 | C | 8 | 30 | TUR Two course, Good |
| 8 | TUR | No | 20 | Good |
| 9 | C + A | 4 | 24 | Good |
| 10 | C | 6 | 17 | Good |
First line treatment: C = corticosteroid, A = antihistamine.
Discussion
Eosinophilic cystitis (EC) is a rare form of bladder inflammation characterized by extensive eosinophilic infiltration of the bladder wall. The etiology of EC is uncertain and several hypotheses such as allergens, parasites, bladder injuries, and drugs especial intravesical instillation have been postulated in the genesis of eosinophilic cystitis [2-4]. Allergy associated risk factors include atopic and environmental allergens contribute to the disease. Meanwhile, eosinophilic gastrointestinal disorder is associated with eosinophilic cystitis in 4.5% of cases. Nevertheless, the exact mechanism needs to be explored. No underlying cause is recognized in about one third of patients. Although early study revealed EC is self-limited [5], while it eventually may progress to sclerosis and complete fibrosis of the bladder with clinical condition and radiological findings continued to deteriorate [6]. Eosinophilic cystitis can be overlooked clinically and radiologically, especially without elevated peripheral blood eosinophilia.
The incidence of EC is uncertain, it affects patient of all ages, especial adults [7], 20% occurring in children, it has a female predominance in some studies while other studies showing equal sex incidence or slight male pre-dominance. The most common clinical presentations including frequency, urgency, enuresis and incontinence, suprapubic pain, recurrent hematuria and urinary retention or even spontaneous bladder perforation in children [8,9]. The most severe complication of EC is hydroureteronephrosis or even uremia, possibly due to inflammation obstructing of the intramural ureter or bladder fibrosis. Peripheral eosinophilia can be seen in 43% of patients. It is associated with eosinophilic disease of the gastrointestinal tract sometimes [10]. The periopheral blood elevated eosinophil count and marked elevation of serum IgE were highly suggestive of an underlying allergic cause, while 80% of our cases was normal and allergy tests failed to identify any specific antigen. Commonly, the urine specimen is positive for protein, red cells and white cell which are similar to non-specific cystitis. Urine cytology demonstrated no atypia and no tumor cells in all 10 cases. Urine cultures in our patients were no positive finding.
The cystoscopic examination revealed papillary and tumor-like appearance or diffusely oedematous and gross necrotic bladder mucosa, submucosal hemorrhage and erythematous plaques. It is very difficult to distinguish from bladder tumor and nonspecific cystitis. Pathologically, a transurethral biopsy specimen showed edema, microhaemorragic focuses grossly. Biopsy specimen showed edema, an inflammatory infiltrate containing eosinophils, lymphocytes, rarely plasma cells, with more prominent eosinophilic infiltrate under the lamina propria and muscularis or all layers of the bladder wall with few necroses with more prominent eosinophilic infiltrate under the surface of urothelium which is denude in most areas. It is imperative to obtain adequate deep biopsies; otherwise, the diagnosis can be missed. Diagnosis can be reached only by the microscopic demonstration of eosinofilic infiltration of the whole bladder wall. EC may be classified as either acute or chronic phase. It is characterized by fibrosis while eosinophilia is not conspicuous in the chronic phase. Massive eosinophilic infiltrates are noted in the acute phase but are absent in areas of muscle wall scarring in chronic phase. In our study, two initial transurethral biopsies failed to obtain adequate tissue sampling. Therefore, TUR or open biopsy was performed and made the final diagnosis. Pathological diagnosis of EC should raise consideration of the possibility of concurrent bladder tumor and other associated inflammation conditions. However, it can be misleading because of resembling nonspecific inflammation. Giemsa stain is helpful in the diagnosis of EC. Previous studies suggest that activated eosinophils release cytotoxic proteins that can induce tissue damage. The elevated eosinophilia of peripheral blood is helpful in diagnosis. Imaging studies of EC can mimic bladder tumor, some cases presented with infiltrating solid mass involving the entire bladder wall and multifocal segmental thickening. Occasionally, mucosal mass formation mimicking papillary tumors. Bladder wall thickening is characteristic in computed tomography (CT). Contrast-enhanced CT showed intense, progressive enhancement on delayed scan sand and preservation of the mucosal line, which is a characteristic finding [10].
Because of the rareness of this disease standardized therapy regimens do not exist. Some EC cases with an unpredictable course,medical therapies were given in different periods. In some cases, EC was asymptomatic and the diagnosis was made by cystoscopy done because of a history of bladder carcinoma [11]. Some symptoms were reversible after stopping predisposition [12]. In our study, the lesion was occasionally found after transurethral resection bladder tumor and intravesical MMC instillation, the patient did not take further treatment after cystoscopy and biopsy and completely recovered when MMC instillation was terminated. In earlier studies, therapy begins with removal of any identifiable offending antigen, a formal allergen skin test may help to identify an antigenic stimulus. The majority of patients were treated conservatively with combinations of corticosteroids, antihistaminics, anticholinergic, nonsteroidal anti-inflammatory drugs. Nonsteroidal anti-inflammatory agents and antihistamines are favored first-line agents in some cases, in refractory cases corticosteroids is recommended with considerations stabilizing lysosomal membranes. There was excellent symptomatic improvement in most patients. Corticosteroids refractory case treated with Cyclosporin A, with a complete clinical cure [13]. Intravesical dimethyl sulfoxide instillations can be useful in the treatment of eosinophilic cystitis [14]. Leukotriene receptor antagonist (Montelukast sodium) may be helpful while require long-term treatment [15]. Watchful waiting policy is reasonable for mild symptoms tend to disappear spontaneously [16]. In some rare cases, glandular cystitis or bladder tumor are complicating an EC, transurethral resection of all lesions and a combination of corticosteroids and/or antihistamines got a good result [17].
Based on changes in differential reaction of corticosteroid and/or antihistaminics, patients were divided into 3 groups. Group 1 was asymptomatic and no need treatment, Group 2 had good response and cured by oral corticosteroid and/or antihistaminics, and Group 3 had deterioration symptom although intense medicine therapy and need surgery. Recurrence is possible, a second course of steroid and/or anthistaminics therapy was inevitable [18]. A case of recurrent eosinophilic cystitis which responded successfully to initial and secondary steroid treatment [19]. In some refractory cases, surgery is an alternative. Invasive therapies including transurethral resection, partial or total cystectomy augmentation ileocystoplasty may be taken into consideration when conservative therapy fails [20,21]. Augmentation ileocystoplasty and total cystoprostatectomy with ileal bladder replacement can increase bladder volume and correct urgency incontinence and prevent renal failure [22]. Total cystoprostatectomy was inevitable while severe fibrosis of the bladder wall which could endanger renal function. In a 135 cases report, 17% of patients finally need surgery (4%) [23]. In our study, surgical rate was 40%, higher than former report.
In all, many treatments have been proposed, individualized treatment and close follow-ups are highly recommended [24]. Early study divided EC into three groups. Group I is constituted of good response to steroids. Group II included recurrent case especially poor response to medicine treatment. Group III comprises elderly patients without clinical symptoms and requiring no treatment [25]. In our experience, the lesion with large extent seemed to be more suitable for steroids treatment. Medicinal therapy refractory cases surgery were advised as soon as possible to relieve symptom and prevent renal failure. Based on the literature and our experience, the clinical and imaging studies are not specific, clinical suspicion of EC is often crucial to the correct diagnosis and proper management of EC while antibiotic medicine was useless. Although prognosis of EC is favorable with conservative treatment, while this course can occasionally progress relentlessly, resulting in complete fibrosis of the bladder with secondary obstructive uropathy and variable degrees of renal insufficiency.
Conclusions
EC is a rare condition without specific clinical and radiological characters. It usually follows a benign course, most treated with corticosteroids and resulted in relief of symptoms while some may relapse. Surgery is an available choice for drug refractory or localized EC.
Acknowledgements
This study was supported by the National Natural Science Foundation for Young Scholars of China (Grant 81302211) and Tianjin Research Program of Application Foundation and Advanced Technology (NO: 14CYBJC29800).
Disclosure of conflict of interest
None.
References
- 1.Brown EW. Eosinophilic granuloma of the bladder. J Urol. 1960;83:665–668. doi: 10.1016/S0022-5347(17)65773-2. [DOI] [PubMed] [Google Scholar]
- 2.Choe JM, Kirkemo AK, Sirls LT. Intravesical thiotepa-induced eosinophilic cystitis. Urology. 1995;46:729–731. doi: 10.1016/S0090-4295(99)80312-4. [DOI] [PubMed] [Google Scholar]
- 3.Hidoussi A, Slama A, Jaidane M, Zakhama W, Youssef A, Ben Sorba N, Mosbah AF. Eosinophilic cystitis induced by bacillus Calmette-Guerin (BCG) intravesical instillation. Urology. 2007;70:591.e9–10. doi: 10.1016/j.urology.2007.07.032. [DOI] [PubMed] [Google Scholar]
- 4.Oh SJ, Chi JG, Lee SE. Eosinophilic cystitis caused by vesical sparganosis: a case report. J Urol. 1993;149:581–583. doi: 10.1016/s0022-5347(17)36154-2. [DOI] [PubMed] [Google Scholar]
- 5.Sutphin M, Middleton AW Jr. Eosinophilic cystitis in children: a self-limited process. J Urol. 1984;132:117–119. doi: 10.1016/s0022-5347(17)49493-6. [DOI] [PubMed] [Google Scholar]
- 6.Mitas JA 2nd, Thompson T. Ureteral involvement complicating eosinophilic cystitis. Urology. 1985;26:67–70. doi: 10.1016/0090-4295(85)90261-4. [DOI] [PubMed] [Google Scholar]
- 7.Ebel Sepulveda LF, Foneron A, Troncoso L, Canoles R, Carrasco C, Hornig A, Gil G, Corti D. [Eosinophilic cystitis: review and two case reports] . Actas Urol Esp. 2009;33:443–446. doi: 10.1016/s0210-4806(09)74174-9. [DOI] [PubMed] [Google Scholar]
- 8.Ficarra V, Beltrami P, Giusti G, Tontodonati M, Zanon G, Malossini G. [Spontaneous bladder perforation due to eosinophilic cystitis: a case report] . Prog Urol. 1997;7:1012–1014. [PubMed] [Google Scholar]
- 9.Hwang EC, Kwon DD, Kim CJ, Kang TW, Park K, Ryu SB, Ma JS. Eosinophilic cystitis causing spontaneous rupture of the urinary bladder in a child. Int J Urol. 2006;13:449–450. doi: 10.1111/j.1442-2042.2006.01320.x. [DOI] [PubMed] [Google Scholar]
- 10.Kim MS, Park H, Park CS, Lee EJ, Rho MH, Park NH, Joh J. Eosinophilic cystitis associated with eosinophilic enterocolitis: case reports and review of the literature. Br J Radiol. 2010;83:e122–125. doi: 10.1259/bjr/36109223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Itano NM, Malek RS. Eosinophilic cystitis in adults. J Urol. 2001;165:805–807. [PubMed] [Google Scholar]
- 12.Tsakiri A, Balslev I, Klarskov P. Eosinophilic cystitis induced by penicillin. Int Urol Nephrol. 2004;36:159–161. doi: 10.1023/b:urol.0000034663.53795.25. [DOI] [PubMed] [Google Scholar]
- 13.Pomeranz A, Eliakim A, Uziel Y, Gottesman G, Rathaus V, Zehavi T, Wolach B. Eosinophilic cystitis in a 4-year-old boy: successful long-term treatment with cyclosporin A. Pediatrics. 2001;108:e113. doi: 10.1542/peds.108.6.e113. [DOI] [PubMed] [Google Scholar]
- 14.Sibert L, Khalaf A, Bugel H, Sfaxi M, Grise P. Intravesical dimethyl sulfoxide instillations can be useful in the symptomatic treatment of profuse hematuria due to eosinophilic cystitis. J Urol. 2000;164:446. [PubMed] [Google Scholar]
- 15.Sterrett S, Morton J, Perry D, Donovan J. Eosinophilic cystitis: successful long-term treatment with montelukast sodium. Urology. 2006;67:423.e419–423.e421. doi: 10.1016/j.urology.2005.08.059. [DOI] [PubMed] [Google Scholar]
- 16.Verhagen PC, Nikkels PG, de Jong TP. Eosinophilic cystitis. Arch Dis Child. 2001;84:344–346. doi: 10.1136/adc.84.4.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baldi A, Di Marino MP, Persichetti P, Ferrara N, Baldi F. Cystitis glanduralis complicating an eosinophilic cystitis: a case report. In Vivo. 2003;17:651–653. [PubMed] [Google Scholar]
- 18.Kilic S, Erguvan R, Ipek D, Gokce H, Gunes A, Aydin NE, Baydinc C. Eosinophilic cystitis. A rare inflammatory pathology mimicking bladder neoplasms. Urol Int. 2003;71:285–289. doi: 10.1159/000072680. [DOI] [PubMed] [Google Scholar]
- 19.Barese CN, Podesta M, Litvak E, Villa M, Rivas EM. Recurrent eosinophilic cystitis in a child with chronic granulomatous disease. J Pediatr Hematol Oncol. 2004;26:209–212. doi: 10.1097/00043426-200403000-00014. [DOI] [PubMed] [Google Scholar]
- 20.Cardini S, Smulevich E, Salvadori A, Lombardi M. Augmentation ileocystoplasty in a case of eosinophilic cystitis. Minerva Urol Nefrol. 1997;49:219–223. [PubMed] [Google Scholar]
- 21.Martino G, Torcasio A, Iavarone C, Cardarelli A, Monti M. Eosinophilic cystitis associated with urethral stricture disease from pelvic trauma. Case report and literature review. G Chir. 2005;26:425–429. [PubMed] [Google Scholar]
- 22.Kayigil O, Ozbagi T, Cakar S, Metin A. Contracted bladder secondary to eosinophilic cystitis. Int Urol Nephrol. 2001;33:341–342. doi: 10.1023/a:1015201410322. [DOI] [PubMed] [Google Scholar]
- 23.van den Ouden D. Diagnosis and management of eosinophilic cystitis: a pooled analysis of 135 cases. Eur Urol. 2000;37:386–394. doi: 10.1159/000020183. [DOI] [PubMed] [Google Scholar]
- 24.Nofal R. [Eosinophilic cystitis as symptom of an idiopathic hypereosinophilic syndrome] . Aktuelle Urol. 2007;38:148–151. doi: 10.1055/s-2006-932169. [DOI] [PubMed] [Google Scholar]
- 25.Server Pastor G, Lopez Cubillana P, Garcia Hernandez JA, Hita Rosino E, Asensio Egea L, Rigabert Montiel M. [Eosinophilic cystitis: a single anatomopathologic entity and three different presentation forms. Proposed classification] . Actas Urol Esp. 1996;20:155–161. [PubMed] [Google Scholar]


