Abstract
As signal transducer and activator of transcription 3 (STAT3)-mediated signaling cascade directly contributes to tumor metastasis, numerous agents targeting STAT3 are in clinical development. However, reported data on the prognostic impact of STAT3 expression vary considerably. We aim to quantitatively summarize available evidences for evaluating the association between STAT3 and STAT3-regulated target gene, matrix metalloproteinase 9 (MMP9), and the prognosis of Chinese patients with gastric cancer. Searches were applied to PubMed and the Chinese National Knowledge Infrastructure database without any language restriction. A total of 5,757 patients were included in the final analyses. All results favored an association between high STAT3 expression and poor 5-year overall survival (risk ratio = 1.845, 95% confidence interval [CI] = 1.027-3.315). The reduced survival was heavily influenced by advanced tumor invasion (OR = 2.885, 95% CI = 2.034-4.094), lymph node metastasis (OR = 5.349, 95% CI = 3.807-7.516), distant metastasis (OR = 5.873, 95% CI = 2.641-13.062), dedifferentiation (OR = 2.516, 95% CI = 1.814-3.491), tumor size (OR = 1.918, 95% CI = 1.246-2.954), and higher TNM stage (OR = 4.171, 95% CI = 2.840-6.126). Similar results were observed in the meta-analyses of MMP9, with the magnitude of effect OR > 2. Our findings indicate that STAT3 and MMP9, as measured by IHC, are associated with worse survival and potentially mark invasion and metastasis in gastric cancer, especially in Chinese patients. More significantly, these two biomarkers may be converted from candidates to the routine clinical evaluation to help predict the outcome of gastric carcinoma patients.
Keywords: Gastric cancer, STAT3, MMP9, prognostic factor
Introduction
Despite a decline in gastric cancer incidence in many Western countries, a report published in 2005 revealed that the disease remains the most common cancer in Eastern Asia [1-4]. Most patients with advanced disease die from complications by metastases rather than the primary tumor. Therefore, identifying novel markers involved in the key steps of metastasis would promote early prediction of recurrence and survival in such patients.
Growth and metastasis are often linked to angiogenesis in various cancers, including gastric cancer. More than 90% of solid tumors depend on a functional vascular network for their supply of oxygen and nutrients. Increasing evidence has indicated that tumor metabolism may be regulated by various growth factors/receptors and oncogenes, including vascular endothelial growth factor and receptor (VEGF/VEGFR), epidermal growth factor and receptor (EGF/EGFR), Src, Ras, etc [5]. Constitutive and aberrant activation of these factors often transmits signals through signal transducer and activator of transcription 3 (STAT3). Upon activation, STAT3 undergoes phosphorylation, homodimerization, nuclear translocation, and DNA binding, which subsequently leads to the transcription of various target genes, including Survivin, VEGF, matrix metalloproteinases (MMPs), E-cadherin, etc. to regulate cell proliferation, survival, angiogenesis, metastasis, immune evasion, inflammation, and drug resistance in a tumor microenvironment [6-8]. Among these, MMP9, one of the most important members of MMP, is well known to degrade the extracellular matrix (ECM) and basement membrane (BM), thus promoting disease progression in various cancers through increased migration, invasion, metastasis, and angiogenesis [9]. High levels of MMP9 have been shown to strongly correlate with tumor aggressiveness and poor prognosis in various human cancers [10]. Currently, routine phase 1 and phase 2 clinical trials that target STAT3 function or expression have been completed, including trials of ISIS-STAT3Rx for the treatment of advanced cancers expressing STAT3 and the effects of OPB-31121 on solid tumor (Clinical Trials: NCT01563302, NCT00955812; http://clinicaltrials.gov/). In addition, positive results from clinical practice have further reinforced the interest of drug development targeting STAT3-mediated signaling pathway.
However, despite the clinical development of anti-STAT3 therapies, whether STAT3 overexpression has any prognostic and clinical values remains controversial. Deng et al. reported that STAT3 overexpression was associated with lymph node metastasis in gastric cancer [11]. However, Xiong et al. found that increased levels of STAT3 did not relate to differentiation and tumor-node-metastasis [12]. It is unclear whether the conflicting results from these investigations are due to their limited sample size or genuine heterogeneity. Almost two-thirds of gastric cancer cases are estimated to occur in Asia, especially in China, where the date from the 7th Chinese Symposium on Medical Oncology and Chinese Cancer Registry Annual Report predicted a 1.6% annual rate of gastric cancer incidence in 2015 [13]. Therefore, we herein presented a meta-analysis on the prognostic impact of STAT3 and STAT3-regulated MMP9 abnormal expression in Chinese patients with gastric cancer. We believe that understanding the relationship between gene expression profiles and prognosis may allow more rational development of therapeutic strategies against these two markers in clinical practice.
Materials and methods
Study identification and selection
The present meta-analysis was conducted according to the statement on preferred reporting items for systematic reviews and meta-analyses [12,14,15]. PubMed and the Chinese National Knowledge Infrastructure (CNKI) databases were searched for studies evaluating the expression of STAT3 and MMP9 in gastric cancer from 1995 to 2013. Subject heading terms such as STAT3, prognosis, and gastric cancer or all other synonyms for gastric cancer were used to screen for potentially relative studies. Similar searching process was performed for MMP9.
Inclusion criteria were as follows: (1) study selection was based on the association between STAT3 or MMP9 and prognosis in humans; (2) protein expression was evaluated via immunohistochemical (IHC) methods; (3) data were collected from Chinese study cohorts; and (4) data were available for the number of cases and controls, patients’ age, sex, tumor size, venous invasion, lymph node status, distant metastasis, TNM stage, histo-differentiation, and 5-year overall survival (OS). Citation lists of the retrieved articles were manually reviewed to ensure sensitivity of the search strategy.
Data collection and compilation
Two authors (Chen J and Liu XX) independently extracted information from search results using predefined forms. Information collected included an article’s first author name, year of publication, nation, language, the cut-off values for determining STAT3 and MMP9 positivity, blinded reading, the numbers of controls and cases, association data between STAT3/MMP9 expression and 5-year OS, and the number of events in each category of STAT3/MMP9 expression on different clinicopathological factors as described above. In most cases, survival data were extracted from Kaplan-Meier curves.
Owing to the applicable clinical characteristics, each examined parameters were divided into two groups: well and moderate differentiation vs. poor and undifferentiation, T1 and T2 vs. T3 and T4, stage I and II vs. stage III and IV, tumors larger than 5 cm in size vs. those of less than 5 cm, and above vs. below 60 years of age. Disagreement was resolved by consensus in all items.
Statistical analysis
Three categories of stratified models were analyzed. The first stratified multivariate model was performed to confirm whether STAT3/MMP9 highly expressed in gastric cancer patients compared to normal gastric mucosa. The second outcome meta-analysis aimed to measure the impact of STAT3/MMP9 expression on survival by estimating the risk ratio (RR) between the positive and negative groups. The third analysis was to examine the prognostic value of STAT3/MMP9 expression in various clinical factors, such as age, sex, tumor size, location and histo-differentiation, depth of invasion, vascular invasion, lymph node status, distant metastasis, and TNM stage.
Statistical Analysis System Software (STATA SE 9.0) was used to combine collected data for meta-analyses. All studies were assessed by RR or odds ratio (OR) using different models as previously described [16]. The Egger’s linear regression test and Begg’s test were performed to examine publication bias. All statistical analyses were two-sided, and a P-value of less than 0.05 was considered significant.
Results
Study description
We identified 74 studies [12,17-89] that employed IHC assay for assessing the association between STAT3/MMP9 expression and prognosis in Chinese patients with gastric cancer. A total of 5,757 patients were included in those studies. Thirteen out of 16 studies compared the expression of STAT3 between gastric cancer and normal gastric mucosa, whereas 3 out of 16 studies evaluated the impact of STAT3 expression on OS. For all patients, measurements were obtained from the primary tumor, and all specimens were collected before chemotherapy or radiotherapy. The main features of eligible studies included in our meta-analyses and their results are summarized in Tables 1, 2.
Table 1.
Main characteristics of the 74 studies included in the final meta-analysis
| First author | Year of publication | language | Study from PubMed | Number of patients (M/F) | Median age (years) | Antibody used for the evaluation | Cutoff for MMP9 positivity (%) | Blinded reading | Reader (s) (n) | Survival analysis | Results |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Liang, et al | 2000 | Chinese | NO | 25/11 | 60.5 | FIK, Japan, 1:100 | - | - | - | - | - |
| Li, et al | 2000 | Chinese | YES | - | - | Santa Cruz, USA, 1:80 | - | - | - | - | - |
| Zhang, et al | 2000 | Chinese | YES | 82/26 | 54.8 | Maixin Fuzhou, China, 1:150 | > 0 | - | - | OS | negative |
| Wang, et al | 2001 | Chinese | NO | 28/12 | 58.6 | Dako, Denmark + I18 | - | - | - | - | - |
| Zhou, et al | 2001 | Chinese | NO | 28/19 | 54.6 | Maixin, Fuzhou, China | > 10% | - | - | - | - |
| Li, et al | 2002 | Chinese | YES | 186/70 | - | Maxim, USA | > 25% | - | - | OS | negative |
| Xue, et al | 2002 | Chinese | NO | 28/19 | 54.6 | Maixin Fuzhou, China | > 10% | - | - | - | - |
| Guan, et al | 2002 | Chinese | YES | 64/36 | 58 | Maixin Fuzhou, China | - | - | - | OS | negative |
| Wang, et al | 2003 | Chinese | NO | 48/26 | - | NeoMarkers, USA, 1:1 | > 10% | - | - | - | - |
| Jiang, et al | 2003 | Chinese | NO | 62/25 | 55.6 | Zhongshan, Beijing, China | > 10% | - | - | - | - |
| Shen, et al | 2003 | Chinese | NO | 30/10 | 57.5 | Maixin Fuzhou, China | > 0 | YES | 2 | - | - |
| Zuo, et al | 2003 | Chinese | NO | 39/28 | 56.5 | Maxim, USA | > 25% | - | - | - | - |
| Sun, et al | 2003 | Chinese | NO | 36/24 | 42 | Zhongshan, Beijing, China, 1:300 | - | - | - | - | - |
| Gao, et al | 2004 | Chinese | NO | 32/9 | 53 | Maixin Fuzhou, China | > 5% | - | - | - | - |
| Chen, et al | 2004 | Chinese | NO | 59/21 | 60 | Maixin Fuzhou, China | > 10% | - | - | - | - |
| Li, et al | 2004 | Chinese | NO | - | - | Maixin Fuzhou, China | > 10% | - | - | - | - |
| Wang, et al | 2004 | Chinese | NO | 43/20 | 55.6 | Maixin Fuzhou, China | > 30% | - | - | - | - |
| Lu, et al | 2005 | Chinese | NO | 140/120 | 53 | Maixin Fuzhou, China | > 25% | YES | 2 | - | - |
| Xie, et al | 2005 | Chinese | NO | 47/23 | 48.6 | Zhongshan, Beijing, China | > 5% | - | - | - | - |
| Wu, et al | 2005 | Chinese | NO | 67/38 | 53 | Changdao, Shanghai, China | > 0 | - | - | OS | negative |
| Gao, et al | 2005 | Chinese | NO | 56/31 | 56.5 | Maixin Fuzhou, China | > 25% | - | - | - | - |
| Zhang, et al | 2005 | Chinese | YES | 45/39 | 52.6 | RIBOBIO, Wuhan, China | > 5% | - | - | - | - |
| Chen, et al | 2006 | Chinese | NO | - | - | - | > 0 | - | - | - | - |
| Zhu, et al | 2006 | Chinese | NO | 61/19 | 56 | RIBOBIO, Wuhan, China, 1:200 | > 25% | - | - | OS | negative |
| Gao, et al | 2006 | Chinese | NO | 48/22 | 51 | Maixin Fuzhou, China | > 5% | - | - | - | - |
| Tang, et al | 2006 | Chinese | NO | 91/37 | 58 | Maxim, USA | > 25% | YES | 2 | - | - |
| Wu, et al | 2006 | Chinese | NO | 44/16 | 59.6 | Maixin Fuzhou, China | > 10% | - | - | OS | negative |
| Ye, et al | 2006 | Chinese | NO | 54/26 | 47.6 | Maixin Fuzhou, China | > 10% | - | - | - | - |
| Feng, et al | 2006 | Chinese | NO | - | 55 | Maixin Fuzhou, China | > 10% | - | - | - | - |
| Yan, et al | 2006 | Chinese | NO | 44/11 | 56.4 | Santa Cruz, USA | > 0 | - | - | - | - |
| Lv, et al | 2006 | Chinese | NO | 49/34 | 55 | Zymed, USA | > 5% | - | - | - | - |
| Yu, et al | 2006 | Chinese | NO | 32/20 | 48.6 | Maixin Fuzhou, China | > 0 | YES | 2 | - | - |
| Sun, et al | 2006 | Chinese | NO | 67/29 | 62 | NeoMarkers, USA, 1:100 | > 4% | - | - | - | - |
| Gao, et al | 2006 | Chinese | NO | 26/14 | 55.2 | Changdao, Shanghai, China | > 5% | - | - | - | - |
| Liu, et al | 2006 | Chinese | NO | 55/19 | 60.9 | ZSGB-BIO, Beijing, China | - | - | - | - | - |
| Hu, et al | 2006 | Chinese | YES | 50/26 | 48. 3 | Maixin Fuzhou, China | > 5% | - | - | - | - |
| Wang, et al | 2007 | Chinese | NO | 96/24 | 59.14 | Santa Cruz, USA, 1:1 | > 0 | - | - | - | - |
| Pan, et al | 2007 | Chinese | NO | 54/33 | 51.6 | ZSGB-BIO, Beijing, China | > 0 | - | - | - | - |
| Hu, et al | 2007 | Chinese | NO | 44/16 | 57 | Maixin Fuzhou, China | > 40% | - | - | - | - |
| Wang, et al | 2007 | Chinese | NO | 36/18 | 58 | Maixin Fuzhou, China | > 10% | - | - | - | - |
| Song, et al | 2007 | Chinese | NO | 37/17 | 60.4 | 1:100 | > 0 | - | - | - | - |
| Zhang, et al | 2007 | Chinese | NO | 87/12 | 58.2 | Santa Cruz, USA, 1:60 | > 0 | - | - | - | - |
| Yuan, et al | 2007 | Chinese | NO | 43/17 | 60.0 | ZSGB-BIO, Beijing, China | > 10% | - | - | - | - |
| Zhou, et al | 2008 | Chinese | NO | 48/19 | 56.5 | Maixin Fuzhou, China | > 30% | - | - | - | - |
| Guo, et al | 2008 | Chinese | NO | 30/15 | 60.4 | Maixin Fuzhou, China | > 10% | - | - | - | - |
| Zhang, et al | 2008 | Chinese | NO | 78/42 | 61 | Maixin Fuzhou, China | > 10% | - | - | - | - |
| Chen, et al | 2008 | Chinese | NO | 48/12 | 58.6 | ZSGB-BIO, Beijing, China | > 5% | - | - | - | - |
| Ni, et al | 2008 | Chinese | NO | 34/20 | 60.4 | Zhongshan, Beijing, China | 0 | - | - | - | - |
| Zhang, et al | 2008 | Chinese | NO | 32/18 | 54 | - | > 10% | - | - | - | - |
| Li, et al | 2008 | Chinese | NO | 32/13 | 56.3 | NeoMarkers, USA | > 5% | - | - | - | - |
| Zhen, et al | 2008 | Chinese | NO | 42/18 | 57 | - | > 25% | - | - | - | - |
| Li-Yu Lee, et al | 2008 | English | YES | 52/36 | - | Lab Vision Corporation, Fremont, CA, 1:50 | > 10% | - | - | - | - |
| Chen, et al | 2009 | Chinese | NO | 34/20 | 60.4 | - | 0 | - | - | - | - |
| Zhu, et al | 2009 | Chinese | NO | 68/36 | 49.7 | Maixin, Fuzhou, China | 0 | - | - | - | - |
| Zhao, et al | 2009 | Chinese | YES | - | - | Santa Cruz, USA | - | - | - | - | - |
| Peng, et al | 2010 | English | YES | - | - | Santa Cruz, USA, 1:300 | - | - | - | OS and DFS | negative |
| Chu, et al | 2011 | English | YES | 232/54 | - | Abcan, HK, 1:200 | > 5% | - | - | OS | negative |
| Yang, et al | 2011 | English | YES | 37/17 | - | Maixin Fuzhou, China, 1:200 | - | - | - | DFS | negative |
| Zheng, et al | 2007 | Chinese | NO | 51/39 | 56 | Maixin Fuzhou, China | > 5% | - | - | - | - |
| Han, et al | 2007 | English | YES | - | - | Santa Cruz, USA, 1:100 | > 0% | YES | 2 | - | - |
| Deng, et al | 2008 | Chinese | NO | 37/23 | 44.7 | Santa Cruz, USA, 1:300 | > 0% | YES | 2 | - | - |
| Hu, et al | 2008 | Chinese | NO | 32/8 | 63.5 | RIBOBIO, Wuhan, China, 1:100 | > 10% | YES | 2 | - | - |
| Song, et al | 2008 | Chinese | NO | 120/30 | 62 | ZSGB-BIO, Beijing, China | > 10% | - | - | - | - |
| Sun, et al | 2008 | Chinese | NO | - | - | 1:50 | - | - | - | - | - |
| Li, et al | 2009 | Chinese | NO | 41/18 | 62.8 | Cell signaling, USA, 1:100 | > 0% | YES | 2 | - | - |
| Zhang, et al | 2009 | Chinese | NO | 56/35 | 60 | CST, USA | > 5% | - | - | - | - |
| Cai, et al | 2010 | Chinese | NO | 30/26 | 57.5 | RIBOBIO, Wuhan, China | > 10% | YES | 2 | - | - |
| Shang, et al | 2010 | Chinese | NO | 23/17 | 60 | Maixin Fuzhou, China, 1:100 | > 5% | - | - | - | - |
| Deng, et al | 2010 | English | YES | 37/16 | 55 | Santa Cruz, USA, 1:100 | - | YES | 2 | OS | negative |
| Deng, et al | 2012 | Chinese | NO | 42/38 | 47 | Santa Cruz, USA, 1:300 | > 40% | - | - | - | - |
| Yan, et al | 2012 | Chinese | NO | 35/20 | 51 | Bioss, Beijing, China, 1:100 | > 5% | - | - | - | - |
| Xiong, et al | 2012 | English | YES | 176/86 | - | Dako, Denmark, 1:20 | > 15% | YES | 2 | OS | negative |
| Du, et al | 2013 | Chinese | NO | 46/14 | 55.6 | 1:100 | > 10% | - | - | - | - |
| Jia, et al | 2013 | English | YES | 34/14 | - | 1:20 | - | - | - | OS | negative |
OS, overall survival; Positive, inverse relationship between specific protein expression and survival; Negative, no relationship. ‘Reader’ are readers of the histologic slides, ‘blinded reading’ means that readers of the slides without knowledge of the clinical outcome, and ‘-’ corresponds to missing data.
Table 2.
Meta-analysis of STAT3 and MMP9 expressions on gastric cancer
| Stratification of gastric cancer | MMP9 | STAT3 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||||
| Number of studies | Total patients | Model | OR (95% CI) | P-value | I2 for heterogeneity | P-value for bias | Number of studies | Total patients | Model | OR (95% CI) | P-value | I2 for heterogeneity | P-value for bias | |
| Gastric cancer -normal gastric mucosa | 33 | 4367 | Random | 14.713 (9.623-22.496) | 0.000 | 74.10% | 0.014 | 13 | 1256 | Fixed | 13.535 ( 10.087-18.162) | 0.000 | 29.50% | 0.039 |
| 5-year survival | 8 | 862 | Random | 1.515 (1.236-1.856) | 0.000 | 60.10% | 0.102 | 3 | 363 | Random | 1.845 (1.027-3.315) | 0.04 | 71.10% | 0.35 |
| The depth of invasion | 40 | 3252 | Fixed | 3.731 (3.148-4.424) | 0.000 | 34.10% | 0.000 | 11 | 725 | Fixed | 2.885 (2.034-4.094) | 0.000 | 38.20% | 0.011 |
| Lymph node status | 51 | 3957 | Fixed | 3.818 (3.285-4.436) | 0.000 | 28.00% | 0.057 | 12 | 1187 | Fixed | 5.349 (3.807-7.516) | 0.000 | 47.50% | 0.128 |
| Distant metastasis | 16 | 1322 | Fixed | 3.180 (2.236-4.524) | 0.000 | 0.00% | 0.437 | 4 | 333 | Fixed | 5.873 (2.641-13.062) | 0.000 | 30.80% | 0.325 |
| TNM stage | 28 | 2534 | Fixed | 3.733 (3.086-4.514) | 0.000 | 49.60% | 0.041 | 10 | 832 | Fixed | 4.171 (2.840-6.126) | 0.000 | 44.40% | 0.075 |
| Age | 10 | 943 | Fixed | 1.106 (0.837-1.461) | 0.479 | 40.20% | 0.710 | 9 | 815 | Fixed | 1.048 (0.743-1.479) | 0.789 | 0.00% | 0.567 |
| Sex | 22 | 1920 | Fixed | 1.130 (0.911-1.402) | 0.266 | 0.00% | 0.456 | 12 | 991 | Fixed | 1.344 (0.971-1.860) | 0.074 | 0.00% | 0.181 |
| Size | 14 | 1085 | Fixed | 1.493 (1.154-1.931) | 0.002 | 14.80% | 0.689 | 6 | 611 | Fixed | 1.918 (1.246-2.954) | 0.003 | 0.00% | 0.056 |
| Histological differentiation | 44 | 3485 | Random | 1.451 (1.124-1.872) | 0.004 | 56.10% | 0.000 | 13 | 1027 | Fixed | 2.516 (1.814-3.491) | 0.000 | 47.80% | 0.041 |
OR, odd ratio; RR, risk ratio; CI, confidence interval.
Correlation between STAT3 expression and prognostic and clinical values
The combined results showed that STAT3 expression in Asian patients with gastric cancer was significantly higher in 13 studies (717 patients and 539 controls, OR = 13.535, 95% CI = 10.087-18.162, P < 0.001 (Table 2, Supplementary Table 1). High levels of STAT3 correlated with poor OS in 3 studies (363 patients) (RR = 1.845, 95% CI = 1.027-3.315, P = 0.04 (Table 2, Figures 1 and 2). Subgroup analysis revealed that increased STAT3 expression was associated with invasion depth (11 studies, 725 patients, OR = 2.885, 95% CI = 2.034-4.094, P < 0.001), lymph node metastasis (12 studies, 1,187 patients, OR = 5.349, 95% CI = 3.807-7.516, P < 0.001), distant metastasis (4 studies, 333 patients, OR = 5.873, 95% CI = 2.641-13.062, P < 0.001), TNM stage (10 studies, 832 patients, OR = 4.171, 95% CI = 2.840-6.126, P < 0.001), tumor size (6 studies, 611 patients, OR = 1.918, 95% CI = 1.246-2.954, P = 0.003), and histological differentiation (13 studies, 1,027 patients, OR = 2.516, 95% CI = 1.814-3.491, P < 0.001) (Table 2, Supplementary Table 1).
Figure 1.
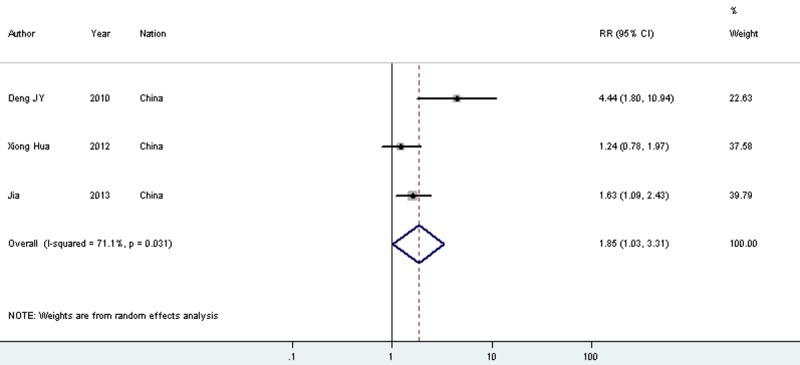
Meta-analysis on the relation between STAT3 expression and 5-year overall survival (OS).
Figure 2.
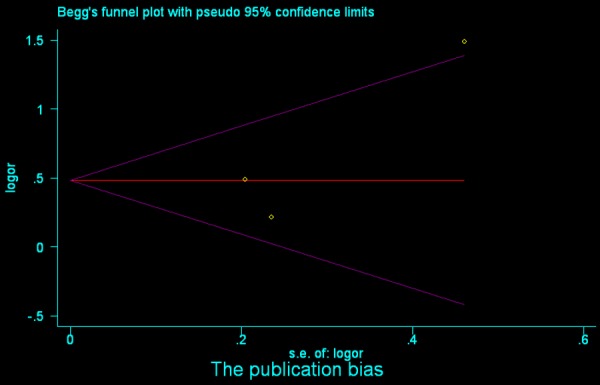
Begg’s funnel plot analysis of STAT3 to detect publication bias for overall survival (OS).
Correlation between MMP9 expression and prognostic and clinical values
When compared to normal controls, MMP9 overexpression was associated with worse outcomes for gastric cancer patients in 33 studies (2,652 patients and 1,715 controls, OR = 14.713, 95% CI = 9.623-22.496, P < 0.001 (Table 2, Supplementary Table 1). Such results from the pooled analysis were statistically significant for the detrimental 5-year OS in 8 studies (862 patients) (RR = 1.515, 95% CI = 1.236-1.856, P < 0.001 (Table 2, Figures 3 and 4). In addition, the reduced survival was heavily influenced by the depth of invasion (40 studies, 3,252 patients, OR = 3.731, 95% CI = 3.148-4.424, P < 0.001), lymph node metastasis (51 studies, 3,957 patients, OR = 3.818, 95% CI = 3.285-4.436, P < 0.001), distant metastasis (16 studies, 1,322 patients, OR = 3.180, 95% CI = 2.236-4.524, P < 0.001), TNM stage (28 studies, 2,534 patients, OR = 3.733, 95% CI = 3.086-4.514, P < 0.001), histological differentiation (44 studies, 3,485 patients, OR = 1.451, 95% CI = 1.124-1.872, P = 0.004), and tumor size (14 studies, 1,085 patients, OR = 1.493, 95% CI = 1.154-1.931, P = 0.002) (Table 2, Supplementary Table 1).
Figure 3.
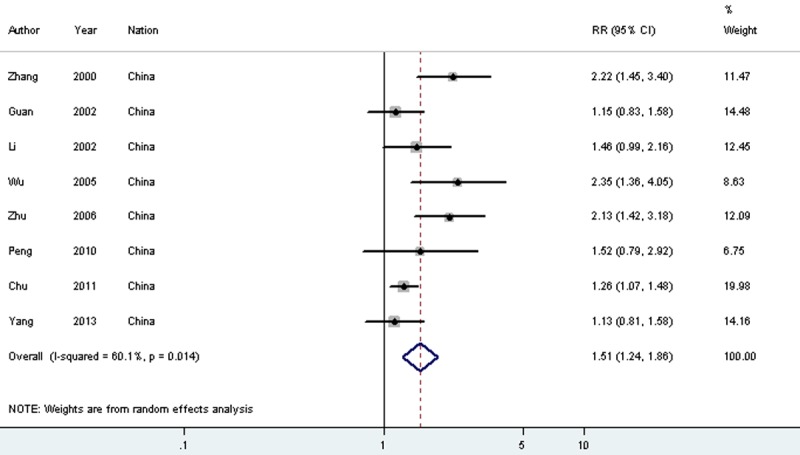
Meta-analysis on the relation between MMP9 expression and 5-year overall survival (OS).
Figure 4.
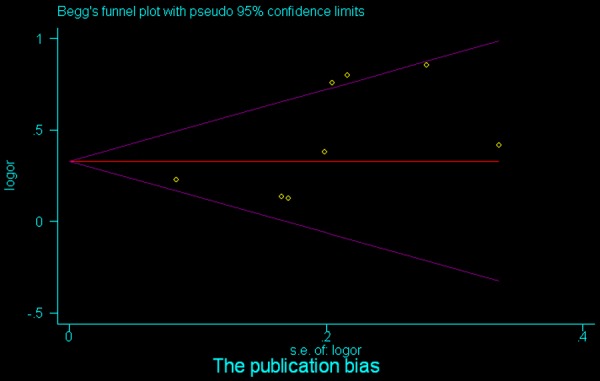
Begg’s funnel plot analysis of MMP9 to detect publication bias for overall survival (OS).
Assessment of publication bias
Our results indicated no evidence of publication bias for most subgroup analyses (Table 2). The potential bias for case-control study (P bias = 0.039), invasion depth (P bias = 0.011), and histo-differentiation (P bias = 0.041) of the STAT3 analyses could be ruled out by the Begg’s and Egger’s tests (Table 2). Similar results were observed in the MMP9 analyses regarding case-control study (P bias = 0.014), invasion depth (P bias = 0.000), histo-differentiation (P bias = 0.000), and TNM stage (P bias = 0.041) (Table 2).
Discussion
The recurrence and metastasis in gastric cancer remain a formidable obstacle for therapy and one of the main causes of high mortality. Prognostic factors such as clinicopathological features cannot fully predict individual clinical outcome, especially in patients receiving curative resection and/or with node negativity [90-92]. Therefore, identification of new prognostic markers may be useful in guiding surveillance and explaining survival variability for personalized therapy [93]. In the present report, we introduced two potential biomarkers, STAT3 and MMP9, and precisely estimated their prognostic and clinicopathological significances in Chinese patients with gastric cancer.
STAT3 and STAT3-regulated MMP9 overexpression has been implicated in the etiology of most solid tumors in many studies. They are thought to play key roles in the signaling of tumor proliferation, metastasis, and angiogenesis. Therapeutic agents targeting these factors are currently under development. In this study, we meta-analyzed published data on the expression of STAT3 and MMP9 between gastric cancer and normal gastric mucosa. We also investigated their association with survival and other clinical features in gastric cancer using information from studies. Only studies with IHC evaluation of STAT3 and MMP9 expression were selected to maintain the consistency in the evaluation process among different studies.
Our results demonstrated that STAT3 overexpression occurred at a median frequency of 54.1% in gastric cancer. Patients with high levels of STAT3 often experienced worse outcomes, with a meta-risk for OS (RR = 1.845). Subgroup analysis confirmed that the reduced survival was strikingly correlated with increased dedifferentiation, large tumor size, tumor invasion, lymph node spread, distant metastasis, and advanced TNM stage, which suggested an increased biological aggressiveness and a greater possibility of systemic diffusion. Tumor metastasis is a complex multi-step process, which may allow cancer cells to detach from their lattice to become migratory and invasive. STAT3, a latent self-signaling transcription factor, has been implicated to be the hallmark of tumor invasion and metastasis in a wide variety of human malignancies. Yadav et al. reported that interleukin-6 promoted head and neck tumor metastasis by inducing epithelial-mesenchymal transition via the activation of STAT3 signaling [94]. Additionally, we also confirmed that increased MMP9 expression by IHC studies was linked to poor 5-year OS in gastric cancer patients. The higher odds of death at 5 years was 1.515, with the magnitude of effect OR being > 2 for the main stratified meta-analyses of clinical factors. MMP9, one of the STAT3-regulated responsive genes, not only contributes directly to epithelial-mesenchymal transition through ECM and BM degradation but also regulates tumor angiogenesis, which may offer a possible explanation for the observed strong statistical association of STAT3/MMP9 overexpression with advanced tumor invasion, lymph node spread, distant metastasis, and TNM stage. Our findings therefore suggested that these two markers might have potential prognostic and clinical values, and that they could be included in the routine clinical practice to predict the outcome of individual patient with gastric carcinoma.
Our analyses presented two critical findings. First, STAT3 and MMP9 overexpression was associated with worse outcomes, suggesting that each protein may be a potential therapeutic target. In fact, multiple studies evaluating anti-STAT3 and anti-MMP9 therapeutic strategies are ongoing (Table 3, http://www.clinicaltrials.gov). Second, STAT3/MMP9 expression was significantly different between gastric carcinomas and non-neoplastic mucosa, and such expression was associated with prognostic and clinical factors. Our findings emphasize the values in identifying surrogate markers. We also believe that STAT3 and MMP9 act synergistically in gastric tumor proliferation, metastasis, and angiogenesis. Detection of STAT3 and MMP9 in gastric cancer biopsies may be important to determine an optimal clinical treatment option and achieve a reasonable prognosis assessment.
Table 3.
Ongoing studies evaluating anti-STAT3 and anti–MMP9 therapeutic strategies
| Study | sponsor | Phase/setting | Experimental arm (s) | |
|---|---|---|---|---|
| MMP9 | NCT00783523 | University of California, San Francisco | Arteriovenous Malformations; Cavernous Angiomas; Brain Aneurysms; complete | Doxycycline or Placebo |
| NCT00695851 | Ambrilia Biopharma, Inc | Phase 1; Prostate Cancer; complete | PCK3145 | |
| NCT00538967 | Leiden University Medical Center | Aortic Aneurysm, Abdominal; Phase 2 | Doxycycline | |
| NCT00126204 | Barnes-Jewish Hospital | Aortic Aneurysm; completed | Doxycycline | |
| NCT00001683 | National Cancer Institute (NCI) | Lymphoma Melanoma Neoplasm Metastasis Renal Cell Carcinoma: Phase 1 | COL-3 | |
| STAT3 | NCT01563302 | Isis Pharmaceuticals | Advanced Cancers, DLBCL and Lymphoma; Phase 1/2 | ISIS-STAT3Rx |
| NCT01663571 | New York University School of Medicine | Cutaneous T Cell Lymphoma | - | |
| NCT01839604 | AstraZeneca | Advanced Adult Hepatocellular, Carcinoma Hepatocellular Carcinoma Metastatic; Phase 1 | AZD9150 | |
| NCT01066663 | Dana-Farber Cancer Institute | Chronic Lymphocytic Leukemia Small Lymphocytic Leukemia; Phase 1/2 | Pyrimethamine | |
| NCT01009437 | Masonic Cancer Center, University of Minnesota | Breast Cancer; Phase 1/2 | Ritonavir + therapeutic conventional surgery | |
| NCT01445405 | National Cancer Institute (NCI) | Carcinoma, Squamous Head and Neck Cancer Oral Cancer Laryngeal Cancer Pharyngeal Cancer; Phase 1 | Bortezomib (Velcade, PS-341), Cetuximab and Cisplatin; Procedure: Radiation Therapy | |
| NCT00735930 | National Cancer Institute (NCI) | relapsed or refractory B-cell chronic lymphocytic leukemia or small lymphocytic lymphoma; Phase 1 | Alvocidib + lenalidomide | |
| NCT00955812 | M.D. Anderson Cancer Center | Advanced Cancer Solid Tumor; phase 1 | OPB-31121 | |
| NCT00105950 | GlaxoSmithKline | Neoplasms, Breast; phase 2 | Lapatinib | |
| NCT00655499 | Groupe Cooperateur Multidisciplinaire en Oncologie (GERCOR) | Colorectal Cancer | - | |
| NCT00113217 | M.D. Anderson Cancer Center | Renal Cell Carcinoma Kidney Cancer; phase 2 | Bevacizumab |
In conclusion, the present investigation revealed that STAT3 and MMP9 overexpression was associated with a worse survival in gastric cancer patients and potentially indicated disease invasion and metastasis, especially in a Chinese population. Our results suggested that the development of targeting strategies against these proteins could be a reasonable therapeutic approach. Otherwise, these markers may be included in the routine clinical practice for a better prognostic prediction.
Acknowledgements
This work is supported by the grants from National Natural Science Foundation of China (81260339, 81301886 and 81160249) and Fundamental Research Funds for Ningxia Medical University (XT2012009 and XQ2012014).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Siegel R, Naishadham D. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Chen WQ, Zeng HM. Cancer incidence and mortality in china, 2007. Chin J Cancer Res. 2012;24:1–8. doi: 10.1007/s11670-012-0001-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jung KW, Won YJ. Prediction of cancer incidence and mortality in Korea, 2013. Cancer Res Treat. 2013;45:15–21. doi: 10.4143/crt.2013.45.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katanoda K, Matsuda T. An updated report of the trends in cancer incidence and mortality in Japan. Jpn J Clin Oncol. 2013;43:492–507. doi: 10.1093/jjco/hyt038. [DOI] [PubMed] [Google Scholar]
- 5.Jackson CB, Judd LM. Augmented gp130-mediated cytokine signalling accompanies human gastric cancer progression. J Pathol. 2007;213:140–151. doi: 10.1002/path.2218. [DOI] [PubMed] [Google Scholar]
- 6.Darnell JE Jr. STATs and gene regulation. Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 7.Kamran MZ, Patil P. Role of STAT3 in cancer metastasis and translational advances. Biomed Res Int. 2013;2013:421821. doi: 10.1155/2013/421821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nkansah E, Shah R. Observation of unphosphorylated STAT3 core protein binding to target dsDNA by PEMSA and X-ray crystallography. FEBS Lett. 2013;587:833–839. doi: 10.1016/j.febslet.2013.01.065. [DOI] [PubMed] [Google Scholar]
- 9.Dragutinovic V, Izrael-Zivkovic L. Relation of matrix metalloproteinase-9 to different stages of tumors in the serum of gastric cancer. Dig Dis Sci. 2009;54:1203–1207. doi: 10.1007/s10620-008-0472-y. [DOI] [PubMed] [Google Scholar]
- 10.Roy R, Yang J. Matrix metalloproteinases as novel biomarkers and potential therapeutic targets in human cancer. J. Clin. Oncol. 2009;27:5287–5297. doi: 10.1200/JCO.2009.23.5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng J, Liang H. STAT3 is associated with lymph node metastasis in gastric cancer. Tumour Biol. 2013;34:2791–800. doi: 10.1007/s13277-013-0837-5. [DOI] [PubMed] [Google Scholar]
- 12.Xiong H, Du W. Constitutive activation of STAT3 is predictive of poor prognosis in human gastric cancer. J Mol Med (Berl) 2012;90:1037–1046. doi: 10.1007/s00109-012-0869-0. [DOI] [PubMed] [Google Scholar]
- 13.Hao J CW. Chinese Cancer Registry Annual Report. Military Science Press; 2002. [Google Scholar]
- 14.Liberati A, Altman DG. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ocana A, Vera-Badillo F. HER3 overexpression and survival in solid tumors: a meta-analysis. J Natl Cancer Inst. 2013;105:266–273. doi: 10.1093/jnci/djs501. [DOI] [PubMed] [Google Scholar]
- 16.Chen J, Li T. Prognostic significance of vascular endothelial growth factor expression in gastric carcinoma: a meta-analysis. J Cancer Res Clin Oncol. 2011;137:1799–1812. doi: 10.1007/s00432-011-1057-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cai W, Sun W. Expression of STAT3, Survivin and VEGF in Gastric Cancer Tissues. Clinical Misdiagnosis & Mistherapy. 2010;23:214–216. [Google Scholar]
- 18.Chen J, Guo JZ. Expression of synucleinγ in gastric carcinoma and its relationship with MMP-2 and MMP-9. Shijie Huarenxiaohua Zazhi. 2008;16:3400–3405. [Google Scholar]
- 19.Chen J, Yang QX. Correlation Between Expression of MMP-9 and CD34 and Biological Behavior in Gastric Cancinoma. Chinese JournaI of MedicinaI Guide. 2009;11:632–634. [Google Scholar]
- 20.Chen TY, Meng M. Expression and significance of nm23 and MMP-9 in gastric cancer. Anhui Medical and Pharmaceutical Journal. 2006;10:347–348. [Google Scholar]
- 21.Chen XJ, Zheng JS. Relationship between the expression of E-cadherin, matrix metalloproteinase-9 and the invasion and metastasis of gastric carcinoma. J Shanxi Med Univ. 2004;36:243–246. [Google Scholar]
- 22.Chu D, Zhang Z. Matrix metalloproteinase-9 is associated with disease-free survival and overall survival in patients with gastric cancer. Int J Cancer. 2011;129:887–895. doi: 10.1002/ijc.25734. [DOI] [PubMed] [Google Scholar]
- 23.Deng LL, Zhao HB. The expression of STAT3 and p38 in gastric cancer. Zhongguo Zhongliu Linchuan. 2008;35:202–205. [Google Scholar]
- 24.Deng LL, Zhao YY. The Expression of STAT3 and p38MAPK in Gastric Cancer and its Clinical Significance. China Cancer. 2012;21:383–386. [Google Scholar]
- 25.Du ZC, Ma L. The expression and clinical significance of HIF-1α and STAT3 in gastric cancer. China Prac Med. 2013;8:6–8. [Google Scholar]
- 26.Feng ZY, Shao CG. Expression of MMP9 and PEDF and their correlation with tumor invasion and lymph node metastasis in gastric carcinoma. Guangdong Medical Journal. 2006;27:1468–1470. [Google Scholar]
- 27.Gao F, Huang YX. Expressions of Matrix Metalloproteinase - 9 and Tissue Inhibitor of Metalloproteinase - 1 in Progressive Gastric Cancer. Progress in Modern Biomedicine. 2006;6:25–27. [Google Scholar]
- 28.Gao F, Zhou WX. C-Met, MMP9 and TIMP-1 expressions in gastric carcinoma and their relations to invasiveness and metastasis. J of Harbin medical University. 2004;38:259–262. [Google Scholar]
- 29.Gao P, Guo JB. Relationship between lymph node metastasis and the expression of E-cadherin, matrix metallproteinase, basic fibroblast growth factor and CD44v6 in gastric cancer. Chinese Clinical Oncology. 2005;10:627–631. [Google Scholar]
- 30.Gao ZL, Zhang C. Cilnicalsi gnificanceo fex celularm atrix, M W -9, an dV EGFi np atientsw ithg astircc arcinoma. Clinical Medicine of China. 2006;22:1000–1002. [Google Scholar]
- 31.Guan XQ, Wang CJ. Effects of Ezrin on differentiation and adhesion of hepatocellular carcinoma. Ai Zheng. 2002;21:281–284. [PubMed] [Google Scholar]
- 32.Guo GH, Chen SZ. Alteration of MMP-9 gene expression and serum MMP-9 protein detection in gastric carcinoma. Clin J Gastroenterol Hepatol. 2008;17:220–222. [Google Scholar]
- 33.Han JC, Zhang KL. Expression of seven gastric cancer-associated genes and its relevance for Wnt, NF-kappaB and Stat3 signaling. APMIS. 2007;115:1331–1343. doi: 10.1111/j.1600-0643.2007.00695.x. [DOI] [PubMed] [Google Scholar]
- 34.Hu CY, Wang LF. Study on the correlation between expression of focal adhesion kinase and metalloproteinase in gastric cancerours tissues. J of Harbin Medical University. 2007;41:253–256. [Google Scholar]
- 35.Hu ZL, Wen JF. Expressions of TGIF, MMP9 and VEGF proteins and their clinicopathological relationship in gastric cancer. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2006;31:70–74. [PubMed] [Google Scholar]
- 36.Hu ZQ, Zhang LM. Expression of STAT3 and Survivin in Gastric Cancer and Its Significance. China Cancer. 2009;18:238–241. [Google Scholar]
- 37.Jia Y, Liu D. Expression of AFP and STAT3 is involved in arsenic trioxide-induced apoptosis and inhibition of proliferation in AFP-producing gastric cancer cells. PLoS One. 2013;8:e54774. doi: 10.1371/journal.pone.0054774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang HG, Tang WP. The effect of the expressions of MMP2, MMP9 on MVD (microvessel density) and metasasis in gastric cancer adenocarcinoma. The Practical Journal of Cancer. 2003;18:354–356. [Google Scholar]
- 39.Lee LY, Wu CM. Expression of matrix metalloproteinases MMP-2 and MMP-9 in gastric cancer and their relation to claudin-4 expression. Histol Histopathol. 2008;23:515–521. doi: 10.14670/HH-23.515. [DOI] [PubMed] [Google Scholar]
- 40.Li BB, Zhang Y. Expressions of DEC 1 and STAT3 in gastric cancer tissues. Clin J Curr Adv Gen Surg. 2009;12:95–98. [Google Scholar]
- 41.Li L, Zhang S. Relationship of expression unbalance of matrix metalloproteinase and tissue inhibitor of metalloproteinase to invasiveness and metastasis in gastric carcinomas. Ai Zheng. 2002;21:305–310. [PubMed] [Google Scholar]
- 42.Li P, Feng MH. The expression of VEGF and MMP9 in gastric carcinoma and their roles in incasion and metastasis. J Clin Surg. 2004;12:670–672. [Google Scholar]
- 43.Li T. PTEN and MMP-9 in gastric carcinoma and its significance. Shangdong Yiyao. 2008;48:70–72. [Google Scholar]
- 44.Liang YL, Zhao T. The relationship Matrix metalloproteinases MMP9 And MMP2 With metastasis of gastric cancer. Chin J Gen Surg. 2000;15:119. [Google Scholar]
- 45.Liu YX, Zhao CG. Expression and signigicance of MMP-9 and EGFR in Lauren’s classification of gastric carcanoma. Zhongguo Zhongliu Linchuan. 2006;33:632–635. [Google Scholar]
- 46.Lu D, Chen YL. The expression of CD44v6 and MMP-9 in gastric carcinoma and precancerous lesions. Chin J Gastro Hepa. 2005;14:597–600. [Google Scholar]
- 47.Lv L, Cao W. Gastric matrix metalloproteinases and their inhibitors and their clinical significance. J Diagn Concepts Pract. 2006;5:355–357. [Google Scholar]
- 48.Ni ZJ, Kou YW. MMP-9, CD34 and invasion and metastasis of gastric carcinoma and their correlation. China Healthcare Innovation. 2008;3:10–12. [Google Scholar]
- 49.Pan LL, Liu BC. The signigicance of MMP-7 and MMP-9 protein expression in human gastric carcinoma. Chin J Lab Diagin. 2007;11:865–867. [Google Scholar]
- 50.Peng CW, Liu XL. Co-evolution of cancer microenvironment reveals distinctive patterns of gastric cancer invasion: laboratory evidence and clinical significance. J Transl Med. 2010;8:101. doi: 10.1186/1479-5876-8-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shang CX, Wang XH. The expression of STAT 3 and PCNA in gastric cancer and clinical significa. J of Qinghaimedical College. 2010;31:24–28. [Google Scholar]
- 52.Shen ZX, Dong WG. The serum levle of the MMP9 and the expression of MMP-9 and VEGF in gastric carcinoma. Zhongguo Zhongliu Linchuan. 2003;30:689–693. [Google Scholar]
- 53.Song GQ, Wang Q. Expression of recersion inducing cysteine rich protein with Kazal motifs, matrix metalloproteinase-9 and transforming groeth factor β1 and their correlations in gastric carcinomas. Shijie Huarenxiaohua Zazhi. 2007;15:1731–1737. [Google Scholar]
- 54.Song YH, Ning AB. AFP, VEGF and STAT3 in gastric carcinoma and its significance. J Of Oncology. 2008;14:827–830. [Google Scholar]
- 55.Sun XJ, Jiang XH. Expression of osteopontin and matrix metalloproteinase-9 in gastric cancer and their corrlation with clinicopathologic features. China J Cancer Prev Treat. 2003;10:370–372. [Google Scholar]
- 56.Sun Y, Wu H. Expression of Stat3, Survivin and Bcl-2 in Gastric cancer. Chin J Lab Diagin. 2010;14:73–75. [Google Scholar]
- 57.Sun YL, Sun WH. Expression of Matrix metalloproteinase-9 in gastric carcinoma and its relationship with angiogenesis. Zhongguo Zhongliu Linchuan. 2006;33:408–411. [Google Scholar]
- 58.Tang Y, Zhu J. Associations of matrix metalloproteinase-9 protein polymorphisms with lymph node metastasis but not invasion of gastric cancer. Clin Cancer Res. 2008;14:2870–2877. doi: 10.1158/1078-0432.CCR-07-4042. [DOI] [PubMed] [Google Scholar]
- 59.Tang ZH, Chi ZZ. Corrlation between expression of CD44v6, MMP9 and CD43 and biological behacior in gastric cancinoma. J shanxi Med Univ. 2006;37:1003–1007. [Google Scholar]
- 60.Wang AD, Jia JC. Expression and Significance of Metalloproteinase-9 in Human Primary Gastric Carcinoma. The Practical Journal of Cancer. 2001;16:573–574. [Google Scholar]
- 61.Wang L, Zhang LH. Expression of MMP9 and MMP9 mRNA in gastric carcinoma and its correlation with angiogenesis. Natl Med J China. 2003;83:782–786. [PubMed] [Google Scholar]
- 62.Wang LG, Lin F. Relationship between Expression of Matrix Metalloproteinase-9 and Angiogenesis, Metastasis in Gastric Cancer. Journal of Oncology. 2005;11:33–35. [Google Scholar]
- 63.Wang P, Yang JF. Expression and significance ofRhoC and MMP- 9 in gastric cancer. Chinese Journal of Histochemistry and Cytochemistry. 2007;16:686–690. [Google Scholar]
- 64.Wang XJ, Lin ZB. Expression and significance of MMP- 9 and p27 in gastric cancer. J North China Coal Medical College. 2007;9:774–775. [Google Scholar]
- 65.Wu JD, Zhuang YZ. Relationship between Expression of MMPs in Invasiveness and Metastasis of Gastric Carcinoma as well as Patients Survival. The Practical Journal of Cancer. 2005;20:487–489. [Google Scholar]
- 66.Wu XY, Ying MG. The clinical significance of MMP-9 expression and angiogenesis in gastric cancer. Fujian Med J. 2006;28:103–104. [Google Scholar]
- 67.Xie Z, Lin X. A study of expression an relation between matrix metalloprotrinase 9 mRNA and vasular endothelial growth factror in gastric carcinoma. Chin J Prim Med Pharm. 2005;12:1670–1672. [Google Scholar]
- 68.Xue ZL, Li YM. Expression of matrix metalloptoreinase-9 (MMP-9) in gastric cancer. J Lanzhou Med Coll. 2002;28:4–5. [Google Scholar]
- 69.Yan BB, Zhu YJ. Relationship between expression of SOCS2 and STAT3 and biologic behaviors of gastric carcinoma. Shijie Huarenxiaohua Zazhi. 2012;20:563–567. [Google Scholar]
- 70.Yan QH, Wang T. Expression and significance of Syndecan-1 and MMP-9 in gastric cancer. Shandong Yiyao. 2006;46:27–28. [Google Scholar]
- 71.Yang S, Zhao Z. Expression and biological relationship of vascular endothelial growth factor-A and matrix metalloproteinase-9 in gastric carcinoma. J Int Med Res. 2011;39:2076–2085. doi: 10.1177/147323001103900603. [DOI] [PubMed] [Google Scholar]
- 72.Ye XA, Chen YY. Correlation Between the Expression of CD44v6, MMP-9 and VEGF and the Biological Behavior in Gastric Carcinoma. J Med Theor & Prac. 2006;19:505–508. [Google Scholar]
- 73.Yu WY. Detection of Tissue and Plasma Matrix Metalloprotein-9 in Patients with Gastric Carcinoma and Its Significance. Chin J Hemorh. 2006;16:599–602. [Google Scholar]
- 74.Zhang DT, Yuan J. Osteopontin expression and its relation to invasion and metastases in gastric cancer. Zhonghua Zhong Liu Za Zhi. 2005;27:167–169. [PubMed] [Google Scholar]
- 75.Zhang H, Huang JM. Expression and significance of HIF-α and MMP-9 in gastric cancer. Shangdong Yiyao. 2008;48:81–82. [Google Scholar]
- 76.Zhang JF, Zhang YP. Analysis of DNA ploidy and expression of TIMP-2 and MMP-9 in patients with gastric carcinoma. Med J Qilu. 2007;22:41–45. [Google Scholar]
- 77.Zhang JG, Zhao J. Expression and Clinical Significance of STAT3, p-STAT3 and Survivin in Gastric Carcinoma and Precancerous Lesions. Journal of China Medical University. 2009;38:907–912. [Google Scholar]
- 78.Zhang T, Xu HM. Expression of VEGF, MMP-9 and PCNA in Tissues from Gastric Carcinoma and Its Relationship with the Biological Behacior of Gastric Carcinoma. The Practical Journal of Cancer. 2008;23:242–251. [Google Scholar]
- 79.Zhao D, Xu H. Prognostic factors for patients after curative resection for proximal gastric cancer. J Huazhong Univ Sci Technolog Med Sci. 2010;30:530–535. doi: 10.1007/s11596-010-0463-z. [DOI] [PubMed] [Google Scholar]
- 80.Zhao FJ, Kang CS. The relationship of MMP-9, VEGF and PCNA expressions and their clinical significance in gastric adenocarcinoma. Zhonghua Nei Ke Za Zhi. 2009;48:114–117. [PubMed] [Google Scholar]
- 81.Zhao Z, Zhang M. Expression of MMP-2 and MMP-9 in non-small-cell lung cancer and their prognostic value. Zhongguo Fei Ai Za Zhi. 2000;3:107–110. doi: 10.3779/j.issn.1009-3419.2000.02.09. [DOI] [PubMed] [Google Scholar]
- 82.Zhao ZS, Wang YY. Prognostic value of tumor-related molecular expression in gastric carcinoma. Pathol Oncol Res. 2009;15:589–596. doi: 10.1007/s12253-009-9158-9. [DOI] [PubMed] [Google Scholar]
- 83.Zheng BJ, Wang HY. Signal transducer and activator of transcription and cyclin D expression in gastric carcinoma and its significance. Chin J Gen Surg. 2007;22:629–630. [Google Scholar]
- 84.Zheng WM, Xu QW. The Expression and Significance of PTEN and MMP-9 in Gastric Cancer. Zhejiang Yixue. 2009;31:778–779. [Google Scholar]
- 85.Zhou YM, Li YM. Expression of matrix metalloproteinase-9 (MMP-9) and tissue inhibitor of metalloproteinase-1 (TIMP-1) in gastric carcinoma. Zhonggong Xianzaiputongwaikejinzhan. 2001;4:92–94. [Google Scholar]
- 86.Zhou ZH. The orrelation between the expression of matrix metallorproteniase (MMP-9) and degree of incasion and lymoph node metastasis. China Medical Equipment. 2008;5:52–53. [Google Scholar]
- 87.Zhu BH, Zan WH. Expression of PTEN and matrix metalloproteinase and their clinicopathlolgic and prognostic significance in human gastric carcinoma. Chin J Curr Adv Gen Surg. 2006;9:33–36. [Google Scholar]
- 88.Zhu YH, Zhang SH. The Expression and Significance of COX-2, CD44v6 and MMP-9 in Gastric cancer. Shandong Yiyao. 2009;49:57–59. [Google Scholar]
- 89.Zuo YR, Wang JM. Study on the Exoresssion and Correlation of MMP-9 and uPA in Gastric Cancer. Chin J Hemorh. 2003;13:352–354. [Google Scholar]
- 90.Chen XZ, Zhang WK. Correlation between serum CA724 and gastric cancer: multiple analyses based on Chinese population. Mol Biol Rep. 2012;39:9031–9039. doi: 10.1007/s11033-012-1774-x. [DOI] [PubMed] [Google Scholar]
- 91.Kim BS, Cho SW. Differences in prognostic factors between early and advanced gastric cancer. Hepatogastroenterology. 2011;58:1032–1040. [PubMed] [Google Scholar]
- 92.Seshadri RA, Jayanand SB. Prognostic factors in patients with node-negative gastric cancer: an Indian experience. World J Surg Oncol. 2011;9:48. doi: 10.1186/1477-7819-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;411:355–365. doi: 10.1038/35077225. [DOI] [PubMed] [Google Scholar]
- 94.Yadav A, Kumar B. IL-6 promotes head and neck tumor metastasis by inducing epithelial-mesenchymal transition via the JAK-STAT3-SNAIL signaling pathway. Mol Cancer Res. 2011;9:1658–1667. doi: 10.1158/1541-7786.MCR-11-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


