Abstract
One hundred mice were randomly divided into five groups. The mice in one group were injected with physiological saline as the normal control group. The mice in the other four groups were injected with physiological saline, sulfated Chuanminshen violaceum polysaccharides (SCVP), Chuanminshen violaceum polysaccharide (CVP) and astragalus polysaccharide (AP) once daily for 7 d and then with cyclophosphamide (CY) in the last 3 d. The serum cytokine level, apoptosis protein expressions, spleen lymphocyte proliferation, changes in peripheral blood T-cell subsets, and immune organ index were then measured. Results showed that SCVP and CVP can overcome CY-induced immunosuppression by promoting spleen lymphocyte proliferation, raising serum IFN-γ and IL-2 levels, enlarging immune organ indexes, and decreasing excessive apoptosis. Moreover, SCVP and CVP showed the potential to treat autoimmune diseases based on CD4+/CD8+ ratios. Results suggested that SCVP and CVP exhibited the potential to treat autoimmune and immunosuppression diseases.
Keywords: Chuanminshen violaceum polysaccharides, immunosuppression, lymphocyte proliferation, cytokine, immunohistochemical, T-cell subsets
Introduction
Immunosuppression caused by infection, stress, abuse of antibiotics and chemicals, and so on, commonly occurs in humans and animals. Several immunosuppressive diseases are usually ignored because of their subclinical signs [1]. An immunosuppressed organism may exhibit an increasing incidence of secondary infections and immunodeficiency, which can increase the occurrence of sickness [2]. Therefore, an effective immunopotentiator must be identified.
Polysaccharides isolated from natural plants have recently been studied as a new immunopotentiator source for their unique bioactivities and chemical structures [3]. Botanical polysaccharides reportedly possess a wide range of pharmacological properties, such as anti-inflammatory, anti-oxidant, anti-tumor, immunomodulatory, and anti-diabetic activities [4-6]. The biological activities of polysaccharides can be obviously increased by chemical modification, such as sulfation [7-11].
Chuanminshen violaceum (CV) is a traditional Chinese medicinal herb used as a tonic drink. The polysaccharides (CVP) are the main components of CV that comprise 28% of CVP content [12]. CVP is composed of D-carubinose and D-glucose with a ratio of 1:16.2 [13]. The weight average molecular weight and the number average molecular weight of CVP are 9.7632 × 105 and 5.2270 × 104 Da, respectively [14].
Our previous studies demonstrated that sulfated Chuanminshen violaceum polysaccharides (SCVP) exhibited inhibitory effects on the duck enteritis virus and Newcastle disease virus [14,15]. Moreover, SCVP and CVP can significantly enhance the proliferation of mice spleen lymphocytes in vitro [16].
In this study, the effects of SCVP and CVP on immunosuppression were evaluated in immunosuppressed mice. The immunosuppression was induced by cyclophosphamide (CY). The potency was measured based on serum cytokine levels, expressions of Bax, Bcl-2, and Caspase-3 in the immune organ, spleen lymphocyte proliferation, changes in peripheral blood T-cell subsets, and immune organ index. This research aimed to validate the immune enhancement effect of SCVP and CVP against immunosuppression, as well as to investigate the potential of SCVP and CVP in the prevention and treatment of immunosuppressive diseases.
Material and methods
Extraction and purification of polysaccharide
CVP was prepared in the laboratory. Briefly, CV was defatted for 6 h with ether and alcohol (1:1, V/V) and then decocted with distilled water. The decoction was collected and purified by using Sevag’s method to remove proteins. The solutions were dialyzed to remove small molecules and then lyophilized to yield CVP. The polysaccharide content (w/w) of CVP was 93.85%, which was measured using phenol-sulfuric acid method [17].
Sulfated modification of polysaccharide
SCVP was prepared using chlorosulfonic acid-pyridine method. Briefly, chlorosulfonic acid was added dropwise to pyridine (1:4) in an ice-water bath with stirring. CVP (300 mg) dispersed in dry N, N-dimethylformamide (20 mL) was then added. The mixtures were stirred in a water bath at 60°C for 2 h and subsequently neutralized with NaOH (15%). Finally, the solutions were dialyzed and lyophilized to yield SCVP [14]. The degree of substitution was 1.37, which was determined using Antonopoulos’ method [18].
Reagents
D-Hanks (HyClone) was used to wash the cells and dissolve 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT, Sigma). RPMI-1640 (HyClone) supplemented with 10% fetal bovine serum (HyClone) was used for resupending, diluting, and culturing the cells. Lipopolysaccharides (LPS, Sigma), as the B-cell mitogen, were dissolved to 15 μg mL-1 with RPMI-1640. Concanavalin A (ConA, Sigma), as the T-cell mitogen, was dissolved to 30 μg mL-1 with RPMI-1640 (containing 10% fetal bovine serum). MTT was dissolved to 5 mg mL-1 with D-Hanks. These reagents were filtered through a 0.22 μm syringe filter. ConA solution was stored at -20°C, MTT solution was stored at 4°C in a dark bottle, and LPS solution was stored at 4°C. Red blood cell cracking liquid (TIANGEN Biotech Co., Ltd.) was used to remove the red cells in cell suspensions.
Animals and experimental design
One hundred mice (kunming mice), five to six weeks of age and weighing 18 g to 22 g, were purchased from Sichuan University laboratory animal center (Chengdu, China). The mice were provided with sufficient water and complete formula feed and housed in a rodent facility at 20 ± 2°C with a 12 h light-dark cycle for acclimatization. All procedures involving animals and their care used were approved by the Ethics Committee of Sichuan Agricultural University. After being given 7 d to adapt to their environment, the mice were randomly divided into five groups.
The mice in the normal control group were given an intraperitoneal injection of similar volume of physiological saline solution (0.9%, w/v) at the same time as other groups. The mice in the other four groups were given intraperitoneal injections of physiological saline, SCVP (8 mg/kg of bodyweight), CVP (8 mg/kg of bodyweight), and astragalus polysaccharide (AP, 8 mg/kg of bodyweight; Shanghai kayon Biological Technology Co., Ltd.) once daily for 7 d. When the drugs have been administered for 5 d, the mice in the four groups were injected with CY at a dose of 70 mg/kg once daily for the last 3 d. The mice injected with physiological saline and CY served as the negative control, whereas those injected with AP served as the positive control. The drug dose was designed according to the clinical dose of AP in China and our experiment in vitro; AP as an immunopotentiator has been confirmed in clinical, so we choose AP as comparative control. Twenty-four hours after the last drug administration, the animals were weighed and then sacrificed.
Relative weight of spleen and thymus assay
After the body weight was measured, the spleen and thymus of 15 mice in each group were dissected and weighed. The relative weight of the spleen and thymus were calculated using the following formula:
Related weight = organ weight (mg)/body weight (g)
Spleen lymphocyte proliferation assay
Five mice from each group were sacrificed and placed in 75% alcohol for 3 min for sterilization. The spleens were then collected under a sterile environment and cut into 1 mm3 pieces in D-Hanks. The single-cell suspensions were prepared after being filtered through a nylon mesh. The suspensions were centrifuged at 1000 g for 10 min, and the supernatant was removed. Red blood cell cracking liquids (3 mL) were then added. After the liquid became transparent, 6 mL D-Hanks was added to stop the reaction. The liquid was then centrifuged at 1000 g for 10 min. The cells were re-suspended with RPMI-1640 after being washed thrice with DHanks. Finally, the cell concentration was adjusted to 5 × 106 cells mL-1. The cell suspension was placed into 96-well culture plates (100 μL per well) with 50 μL of ConA, LPS, or RPMI-1640. The cells from each mouse were seeded in six wells. The plates were incubated at 37°C for 48 h in a humid atmosphere of 5% CO2. After 44 h of incubation, 20 μL of MTT (5 mg mL-1) was added into each well. The sample was then incubated for 4 h. The plates were centrifuged at 2000 g for 6 min at room temperature with a tablet centrifuge. The supernatant was carefully removed, and 150 μL of dimethylsulfoxide was added into each well to dissolve the formazan crystals. The absorbance at a wavelength of 490 nm (A490 value) of lymphocyte cells in each well was measured using a microplate reader. The mean A490 values were used as indicators of spleen lymphocyte proliferation [18-21].
Serum IFN-γ and IL-2 assay
The sera of 10 blood samples from each group were collected, and the concentrations of IFN-γ and IL-2 were assayed by using an ELISA kit according to manufacturer’s instructions (Shanghai Lengton Biological Technology Co. Ltd., China).
Determination of the peripheral blood T-cell subsets
The peripheral lymphocytes were separated from anticoagulant peripheral blood of three mice in each group. The cells were stained with 2 μL of FITC Hamster Anti-mouse, PE Rat Anti-mouse CD4 (GK1.5), and PerCP Rat Anti-mouse CD8a for 30 min at room temperature. Then, 1.5 mL of Lysing Solution was added, and the resulting mixture was centrifuged at 1000 g for 5 min. The supernatant was discarded, and 2 mL PBS was added to wash the cells. The cells were blended with 0.5 mL PBS, and then flow cytometry was conducted for 1 h. Cell Quest software was used to analyze the percentages of CD3+, CD3+CD4+, and CD3+CD8+ T-cells in peripheral blood.
Bax, Bcl-2, and Caspase-3 detected by using the immunohistochemical method
The spleens and thymus were placed in 4% paraformaldehyde and routinely processed in paraffin. Thin sections (5 μm) of each tissue were sliced from each block and mounted on glass. Immunohistochemical staining against Bax, Bcl-2, and Caspase-3 was performed. Briefly, a HRP/DAB detection IHC kit was used according to manufacturer’s protocol. Then sections were deparaffinized, and the paraformaldehyde-fixed and paraffin-embedded tissue sections were rehydrated. Hydrogen peroxide block was added to cover the sections, which were then incubated for 10 min. After antigen retrieval for 20 min in a domestic pressure cooker and blocking non-specific binding sites with a protein block, the sections were incubated with 10 μg/mL of primary antibodies against Bax, Bcl-2, and Caspase-3 overnight at 4°C. For the negative controls, the sections were immersed in PBS instead of the specific antibody. Mouse-specific HRP was then applied to the conjugate and incubated for 15 min at room temperature. DAB was applied to the tissue sections counterstained with hematoxylin.
For the immunohistochemical quantification, two slices were selected from one integration receptor sample. Five 400 × microscopic views were randomly selected and photographed using a Nikon ECLIPSE80i microscope equipped with a Nikon DS-Ri1 camera. The immunopositive reactions in the photographs were analyzed using the software Image Pro-Plus 6.0 (Media Cybernetics, Silver Spring, MD, USA) according to a previously presented method [22,23]. All photographs were taken and measured using the same parameter settings to ensure that the data were comparable. The area of positive staining was measured in pixels using Image-Pro Software, which detected brown staining in the spleen and thymus. The sum optical density (IOD) ratio was defined as the IOD sum of every group, divided by the average IOD sum of normal control group.
Statistical analysis
Data were expressed as the mean ± S.D and Duncan’s multiple range test was used to analyze the difference among groups with the software SPSS 17.0. P-values of less than 0.05 were considered to be statistically significant.
Results
Changes in immune organ index
Figure 1 shows that the spleen and thymus indexes of the animals treated with CY at dose of 70 mg/kg bodyweight significantly decreased compared with the normal control group, suggesting that immunosuppressed modeling was successfully established. The spleen index of the mice treated with SCVP, CVP, or AP increased compared with that of the mice in the negative control group, and the spleen index was significantly enhanced (P < 0.05) in the SCVP and CVP groups. The thymus index of the mice treated with SCVP, CVP, or AP increased compared with the negative control group, and CVP can significantly improve the thymus index (P < 0.05).
Figure 1.
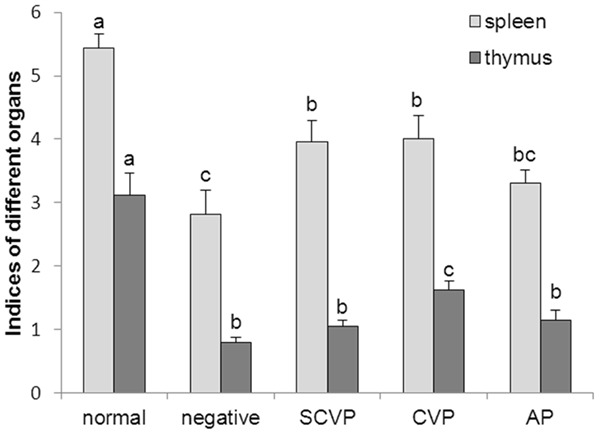
Effect of SCVP and CVP on spleen and thymus index in CY-treated mice. The data are expressed as the mean ± S.D. Significant differences were considered at P < 0.05. a,bBars in the same organ without the same superscripts differ significantly (P < 0.05).
Changes in proliferation
The changes in the A490 values are listed in Table 1. In the negative control group, the A490 values were significantly lower than those in the normal control group (P < 0.05). In the SCVP, CVP, and AP groups, proliferation of resting lymphocytes, T lymphocytes, and B lymphocytes was significantly higher than that of the negative control group (P < 0.05). No significant differences were observed in the proliferation of resting lymphocytes among the groups, except for the negative control group. The proliferation of T lymphocytes in the SCVP, CVP, and AP groups exhibited no significant changes compared with the normal control group. In the CVP group, the proliferation of B lymphocytes was significantly higher than that in the normal control, SCVP, and AP groups (P < 0.05).
Table 1.
Lymphocyte proliferation in each group (A490 value)
| Group | A490 | ||
|---|---|---|---|
|
| |||
| Resting cells | T cells treated with ConA | B cells treated with LPS | |
| Normal | 0.141 ± 0.004a | 0.277 ± 0.003a,b | 0.285 ± 0.003a |
| Negative | 0.128 ± 0.009b | 0.198 ± 0.009c | 0.201 ± 0.007b |
| SCVP | 0.148 ± 0.003a | 0.275 ± 0.003a,b | 0.287 ± 0.002a |
| CVP | 0.151 ± 0.002a | 0.285 ± 0.005b | 0.299 ± 0.003c |
| AP | 0.149 ± 0.005a | 0.271 ± 0.003a | 0.281 ± 0.005a |
Data within a column without the same superscripts differ significantly (P < 0.05);
Data within a column without the same superscripts differ significantly (P < 0.05);
Data within a column without the same superscripts differ significantly (P < 0.05).
Changes in IFN-γ and IL-2 concentration
The changes in IFN-γ and IL-2 concentrations are illustrated in Figure 2. The IFN-γ and IL-2 concentrations in the negative control group were significantly lower than those in the normal control group (P < 0.05). In the CVP, SCVP, and AP groups, the IL-2 concentrations were significantly higher than those in the negative control group (P < 0.05). The IFN-γ concentrations in the CVP and SCVP groups were significantly higher than those in the negative control group (P < 0.05). However, no significant difference was observed between the negative control and AP groups in terms of IFN-γ concentration.
Figure 2.
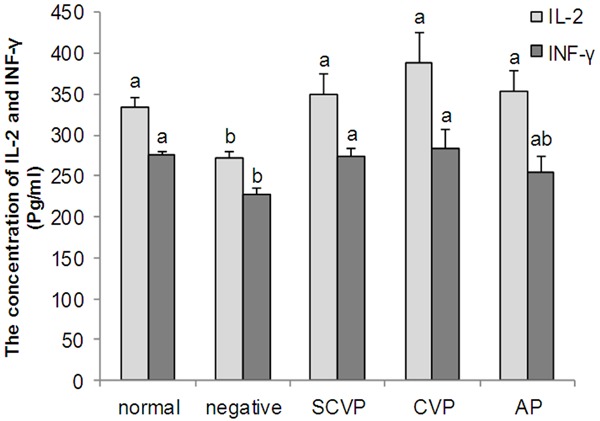
The serum IFN-γ and IL-2 concentration (pg/mL). The data are expressed as the mean ± S.D. Significant differences were considered at P < 0.05. a,bBars in the same cytokine without the same superscripts differ significantly (P < 0.05).
Determination of Peripheral blood T-cell subsets
The effects of CVP and SCVP on CD3+, CD4+, and CD8+ counts were studied based on CY-induced immunosuppressed mice (Table 2). CD3+ and CD4+ levels in the negative control, SCVP, CVP, and AP groups were significantly higher than that in the normal control group (P < 0.05). CD8+ levels in the negative control and AP groups were significantly lower (P < 0.05) than that in the normal control group. The CD8+ levels exhibited no significant changes among the normal control, CVP and SCVP groups. When compared with the normal control group, the negative control and AP groups exhibited a significantly higher ratio of CD4+/CD8+ (P < 0.05). In the CVP and SCVP groups, the ratio of CD4+/CD8+ was close to that of the normal control group.
Table 2.
Peripheral blood T-cell subsets
| Items | Normal | Negative | SCVP | CVP | AP |
|---|---|---|---|---|---|
| CD3+ (%) | 76.51 ± 1.87a | 97.04 ± 0.68b | 96.21 ± 0.94b | 95.36 ± 0.64b | 95.24 ± 0.89b |
| CD3+CD4+ (%) | 54.26 ± 2.58a | 83.99 ± 0.86b | 79.67 ± 0.74b | 74.12 ± 0.56c | 81.26 ± 4.23b |
| CD3+CD8+ (%) | 21.4 ± 2.90a | 13.07 ± 0.62b | 16.44 ± 1.55a,b | 21.15 ± 1.03a | 13.87 ± 3.27b |
| CD4+/CD8+ | 2.61 ± 0.41a | 6.45 ± 0.36c | 4.90 ± 0.55b,c | 3.52 ± 0.19a,b | 6.30 ± 1.54c |
Data within a row without the same superscripts differ significantly (P < 0.05);
Data within a row without the same superscripts differ significantly (P < 0.05);
Data within a row without the same superscripts differ significantly (P < 0.05).
Bax, Bcl-2, and Caspase-3 expression using immunohistochemical method
In the thymus (Table 3), the IOD sum ratio of Bax expression in the negative control group was higher (P < 0.05) than that in the normal control group. The IOD sum ratio of Bax expression in the CVP group was significantly decreased (P < 0.05) compared with that of the negative control group. The IOD sum ratio of Bcl-2 expression in the negative control group was significantly lower (P < 0.05) than that in normal control group. The IOD sum ratio of Bcl-2 expression in the CVP group was significantly higher (P < 0.05) than that in the negative control group. The IOD sum ratio of Caspase-3 expression in the negative control group was significantly higher (P < 0.05) than that in the normal control group. The IOD sum ratio of Caspase-3 expression in the SCVP, CVP, and AP groups was significantly lower (P < 0.05) than that in the negative control group. The histological images (Figure 3) are clearly expressed (Table 3).
Table 3.
Bax, Bcl-2 and Caspase-3 expression in thymus and spleen
| Items | Normal | Negative | SCVP | CVP | AP | |
|---|---|---|---|---|---|---|
| thymus | Bax | 1.00 ± 0.18a | 1.59 ± 0.12c | 1.32 ± 0.10a,b,c | 1.09 ± 0.05a,b | 1.36 ± 0.11b,c |
| Bcl-2 | 1.00 ± 0.04a | 0.77 ± 0.04c | 0.89 ± 0.05a,b,c | 0.96 ± 0.04a,b | 0.85 ± 0.06b,c | |
| Caspase-3 | 1.00 ± 0.07a | 1.47 ± 0.07b | 1.21 ± 0.04c | 1.11 ± 0.04a,c | 1.26 ± 0.04c | |
| spleen | Bax | 1.00 ± 0.07a | 1.49 ± 0.08c | 1.17 ± 0.08a,b | 1.09 ± 0.07a,b | 1.34 ± 0.20b,c |
| Bcl-2 | 1.00 ± 0.19a | 0.75 ± 0.03b | 0.87 ± 0.07a,b | 0.83 ± 0.07a,b | 0.89 ± 0.04a,b | |
| Caspase-3 | 1.00 ± 0.10a | 1.47 ± 0.11b | 1.10 ± 0.10a | 1.01 ± 0.10a | 1.18 ± 0.15a,b | |
Data within a line without the same superscripts differ significantly (P < 0.05);
Data within a line without the same superscripts differ significantly (P < 0.05);
Data within a line without the same superscripts differ significantly (P < 0.05).
Figure 3.
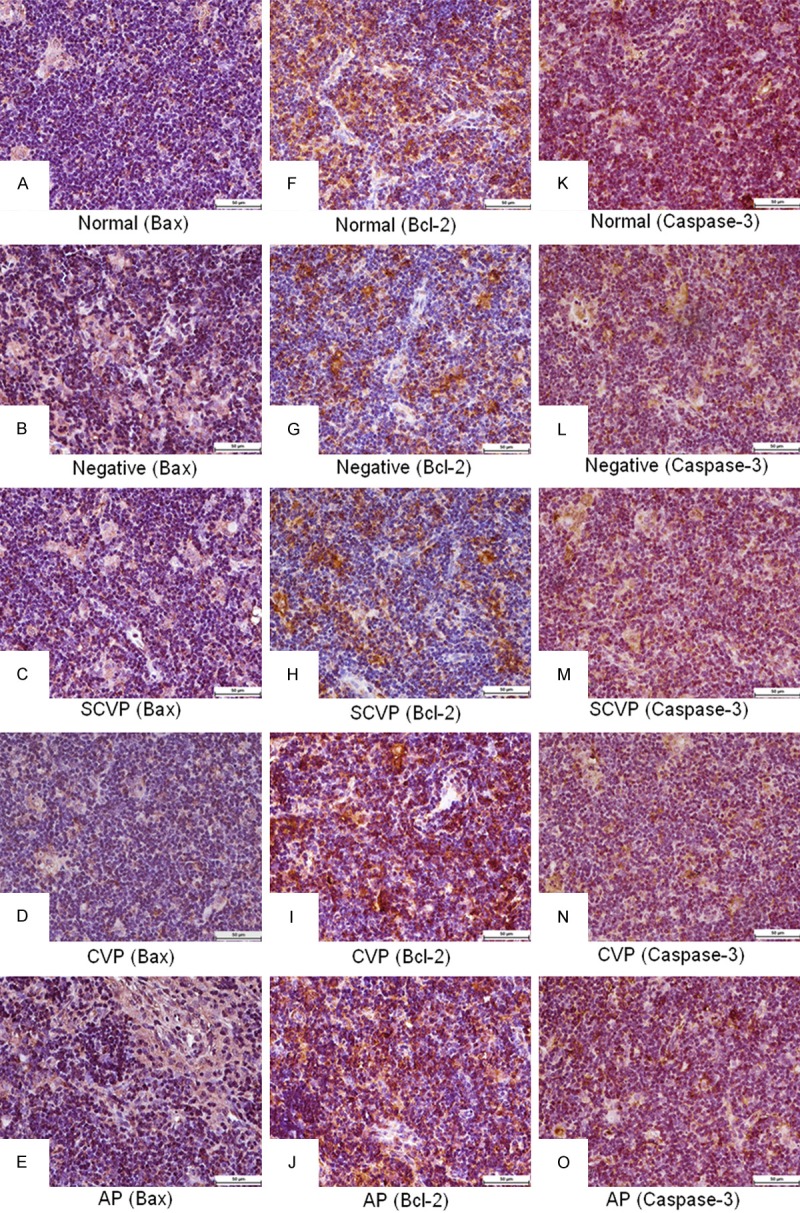
Bax, Bcl-2, and Caspase-3 expression in thymus. A-E: Bax expression; F-J: Bcl-2 expression; K-O: Caspase-3 expression. Bars = 50 μm.
In the spleen (Table 3), the IOD sum ratio of Bax expression in the negative control group was significantly higher (P < 0.05) than that in the normal control group. The IOD sum ratio of Bax expression in the SCVP and CVP groups were significantly decreased (P < 0.05) compared with that in negative control group. The IOD sum ratio of Bcl-2 expression in the negative control group was significantly lower (P < 0.05) than that in the normal control group. No significant differences were observed among the negative, SCVP, CVP, and AP groups. The IOD sum ratio of Caspase-3 expression in the negative control group was significantly higher (P < 0.05) than that in the normal control group. The IOD sum ratio of Caspase-3 expression in the SCVP and CVP groups was significantly decreased (P < 0.05) compared with that in the negative control group. The histological images (Figure 4) clearly express the results (Table 3).
Figure 4.
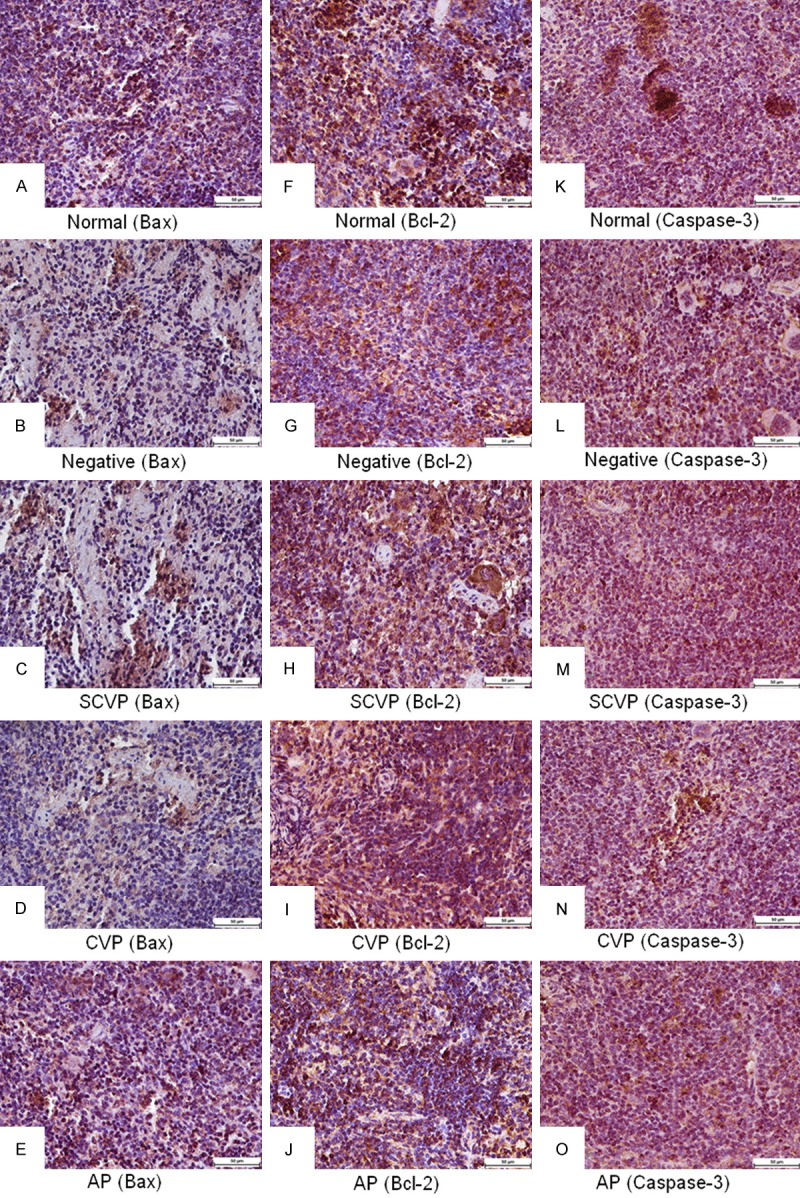
Bax, Bcl-2, and Caspase-3 expression in spleen. A-E: Bax expression; F-J: Bcl-2 expression; K-O: Caspase-3 expression. Bars = 50 μm.
Discussion
Virus infections are the main causes of immunosuppression or immune dysfunction in humans and animals [24]. The mechanism is attributed to the infection of lymphocytes by viruses in important immune organs. This condition causes cell degeneration or necrosis, cytokine dysregulation, and apoptosis resulting in immunosuppression and immune dysfunction [2,24]. CY, a cytotoxic chemotherapeutic drug, aids in the treatment of tumors, organ transplantation, and autoimmune diseases [25]. The mechanism of immunosuppression caused by CY is similar to that caused by a viral infection [26,27]. Therefore, CY was used to establish the mouse immunosuppressive model to measure the immune-enhancement of SCVP and CVP in this experiment. The results showed that various immune indexes were significantly different between the CY and normal control groups, indicating that the immunosuppressive model was successfully established.
Thymus is closely linked with T lymphocyte development, differentiation, and maturation. Thymus does not only serve as the location of T lymphocytes formation, but also secretes hormones, including thymopoietin and thymosin [28,29]. Spleen is the organ that can create lymphocytes, purify blood, and store white cells [11,29]. In this study, the effects of SCVP and CVP on the thymus and spleen indexes were measured. The immune organ indexes indicated that SCVP and CVP improved the immune level induced by CY in vitro. In addition, the curative effect of SCVP and CVP were superior to that of AP at a dose of 8 mg/kg (Figure 1). Rhizoma atractylodis polysaccharides and cordyceps militaris polysaccharides can also improve the immunosuppression induced by CY in vivo [1,30].
The lymphocyte proliferation index is widely used as a method to evaluate the immune-enhancing activity of drugs [20,21,31,32]. The vitality of the lymphocyte can be reflected by the A490 values [21]. The results of our research indicated that SCVP and CVP can significantly promote the activation potential of lymphocytes in CY-induced immunosuppressed mice and enhance the immune response. Moreover, the vitality of the lymphocyte can be normally recovered when treated with SCVP and CVP (Table 1). Similar results have confirmed that sulfated ophiopogon polysaccharide, sulfated jujube polysaccharide, Strongylocentrotus nudus polysaccharide, and Sargassum fusiforme polysaccharide can increase the lymphocyte proliferation index [24,33,34].
As a cytokine, IFN-γ widely exhibits biological activities and is a major immunoregulatory molecule inducing immune response to resist bacteria and exogenous infectious agents [34]. IL-2 has a wide spectrum of immune-promoting activities, such as inducing the differentiation of lymphocytes, promoting the function of NK cells, and releasing interferon [31]. These results show that SCVP and CVP significantly increase cytokine (IL-2 and IFN-γ) expression at the a dose of 8 mg/kg, thus indicating that SCVP and CVP not only reverse the splenocyte function reduced by CY, but also markedly improve such function in CY-inhibited mice (Figures 1 and 2). Similar results have reported that astragalus polysaccharide, sulfated epimedium polysaccharide, and sargassum fusiforme polysaccharide can increase the cytokine content in serum [1,34].
T lymphocytes serve an important function in the pathogenesis of T cell-mediated autoimmune diseases [35]. The ratio of CD4+/CD8+ is high in cases with autoimmune diseases [35,36]. In our study, the ratio of CD4+/CD8+ in mice challenged with CY increased, which indicates that CY may result in autoimmune and atopic diseases. After treatment with CVP and SCVP, the ratio of CD4+/CD8+ became close to the normal value, suggesting that CVP and SCVP can adjust an imbalanced CD4+/CD8+ ratio. However, AP did not possess this function. These results indicate that CVP and SCVP can be used to treat autoimmune diseases.
Caspases mainly mediate apoptosis, and Caspase-3 is the critical factor in the execution of the apoptotic process [37,38]. Bax also facilitates the release of apoptogenic molecules from mitochondria to the cytosol, which results in the promotion of apoptosis by competing with Bcl-2 [39]. In this study, the results showed that Bax and Caspase-3 expressions increased while Bcl-2 expression decreased in the thymus and spleen of the mice in the CY group compared with those in the normal control group. However, the expressions of Bax, Caspase-3, and Bcl-2 recovered to the normal level when the immunosuppressive mice were treated with SCVP and CVP. These results indicate that SCVP and CVP can control excessive apoptosis.
Conclusion
SCVP and CVP can enhance the resistance to immunosuppression by promoting lymphocyte proliferation, increasing the contents of IFN-γ and IL-2, promoting immune organ development, and decreasing excessive immune organ apoptosis in immunosuppressive mice induced by CY. The immune enhancement effects of SCVP and CVP are superior to those of AP at a dose of 8 mg/kg. Therefore, SCVP and CVP have the potential to treat autoimmune diseases and can be used as immunopotentiators.
Acknowledgements
This study was supported by the International cooperation projects of Sichuan Province (2014HH0058, 2013HH0042), the Sichuan Youth Science and Technology Innovation Research Team for waterfowl disease prevention and control (2013TD0015) and the National Natural Science Foundation of China (Grant No. 31372477). A Project Supported by Scientific Research Fund of Sichuan Provincial Education Department (No. 14TD0031).
Disclosure of conflict of interest
None.
References
- 1.Guo L, Liu J, Hu Y, Wang D, Li Z, Zhang J, Qin T, Liu X, Liu C, Zhao X, Fan YP, Han G, Nguyen TL. Astragalus polysaccharide and sulfated epimedium polysaccharide synergistically resist the immunosuppression. Carbohydr Polym. 2012;90:1055–1060. doi: 10.1016/j.carbpol.2012.06.042. [DOI] [PubMed] [Google Scholar]
- 2.Tompkins MB, Tompkins WA. Lentivirus-induced immune dysregulation. Vet Immunol Immunopathol. 2008;123:45–55. doi: 10.1016/j.vetimm.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang L, Zhang LM. Chemical structural and chain conformational characterization of some bioactive polysaccharides isolated from natural sources. Carbohydr Polym. 2009;76:349–361. [Google Scholar]
- 4.Ananthi S, Raghavendran HR, Sunil AG, Gayathri V, Ramakrishnan G, Vasanthi HR. In vitro antioxidant and in vivo anti-inflammatory potential of crude polysaccharide from Turbinaria ornate (Marine Brown Alga) Food Chem Toxicol. 2010;48:187–192. doi: 10.1016/j.fct.2009.09.036. [DOI] [PubMed] [Google Scholar]
- 5.Ding X, Zhu F, Gao S. Purification, antitumour and immunomodulatory activity of water-extractable and alkali-extractable polysaccharides from Solanum nigrum L. Food Chem. 2012;131:677–684. [Google Scholar]
- 6.Schepetkin IA, Quinn MT. Botanical polysaccharides: macrophage immunomodulation and therapeutic potential. Int Immunopharmacol. 2006;6:317–333. doi: 10.1016/j.intimp.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Xing R, Liu S, Yu H, Zhang Q, Li Z, Li P. Preparation of low-molecular-weight and high-sulfate-content chitosans and their potential antioxidant activity in vitro. Carbohydr Res. 2005;61:148–154. doi: 10.1016/j.carres.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y, Liu C, Tan H, Zhao T, Cao J, Wang F. Sulfation of a polysaccharide obtained from Phellinus ribis and potential biological activities of the sulfated derivatives. Carbohydr Polym. 2009;77:370–375. [Google Scholar]
- 9.Qian XP, Zha XQ, Xiao JJ, Zhang HL, Pan LH, Luo JP. Sulfated modification can enhance antiglycation abilities of polysaccharides from Dendrobium huoshanense. Carbohydr Polym. 2014;101:982–989. doi: 10.1016/j.carbpol.2013.10.035. [DOI] [PubMed] [Google Scholar]
- 10.Liu C, Chen H, Chen K, Gao Y, Gao S, Liu X, Li J. Sulfated modification can enhance antiviral activities of Achyranthes bidentata polysaccharide against porcine reproductive and respiratory syndrome virus (PRRSV) in vitro. Int J Biol Macromol. 2013;52:21–24. doi: 10.1016/j.ijbiomac.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 11.Li JE, Nie SP, Xie MY, Li C. Isolation and partial characterization of a neutral polysaccharide from Mosla chinensis Maxim. cv. Jiangxiangru and its antioxidant and immunomodulatory activities. J Funct Foods. 2014;6:410–418. [Google Scholar]
- 12.Lei XL, Zhang M. Study on the impact of different processing methods on the polysaecharide content and peulustrin content in Chuanminshen. Pharm Clin Chin Materia Medica. 2012;3:34–38. [Google Scholar]
- 13.Zhang M, Yu T, Su X, Zhang HB. Physiochemical properties and the immunological activity of the Chuanminshen polysaccharide. West China J Pharm Sci. 2007;4:396–398. [Google Scholar]
- 14.Song X, Yin Z, Li L, Cheng A, Jia R, Xu J, Wang Y, Yao X, Lv C, Zhao X. Antiviral activity of sulfated Chuanminshen violaceum polysaccharide against duck enteritis virus in vitro. Antiviral Res. 2013;98:344–351. doi: 10.1016/j.antiviral.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 15.Song X, Yin Z, Zhao X, Cheng A, Jia R, Yuan G, Xu J, Fan Q, Dai S, Lu H, Lv C, Liang X, He C, Su G, Zhao L, Ye G, Shi F. Antiviral activity of sulfated Chuanminshen violaceum polysaccharide against Newcastle disease virus. J Gen Virol. 2013;94:2164–2174. doi: 10.1099/vir.0.054270-0. [DOI] [PubMed] [Google Scholar]
- 16.Zhao X, Yin Z, Jia R, Song X, Dai SJ, Li L, Xu J, Li ZW, Kang S, Chen ZZ. Effect of Chuanminshen violaceum polysaccharides and its solfated derivatives on proliferation of mice spleen lymphocytes in vitro. Chin J Immunol. 2014;02:213–215. [Google Scholar]
- 17.Li F, Yuan Q, Rashid F. Isolation, purifi cation and immunobiological activity of a new water-soluble bee pollen polysaccharide from Crataegus pinnatifi da Bge. Carbohydr Polym. 2009;78:80–88. [Google Scholar]
- 18.Huifen Z, Baocai L, Jiaheng F. Study on gravimetric analysis of hydrochloric acid hydrolysis and barium sulfate precipitation in sulfated poly saccharides. Food Science. 2002;5:027. [Google Scholar]
- 19.Wang D, Hu Y, Fan Y, Nguyen TL, Wang J, Abula S, Guo L, Zhang J, khakame SK, Dang BK. Immuno-enhancing activity of sulfated Auricularia auricular polysaccharides. Carbohydr Polym. 2012;89:1117–1122. doi: 10.1016/j.carbpol.2012.03.082. [DOI] [PubMed] [Google Scholar]
- 20.Wang J, Hu Y, Wang D, Liu J, Zhang J, Abula S, Zhao B, Ruan S. Sulfated modification can enhance the immune-enhancing activity of Lycium barbarum polysaccharides. Cell Immunol. 2010;263:219–223. doi: 10.1016/j.cellimm.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 21.Zhang J, Chen J, Wang D, Hu Y, Zhang C, Qin T, Liu C, Sheng X, Nguyen TL. Immune-enhancing activity comparison of sulfated ophiopogonpolysaccharide and sulfated jujube polysaccharide. Int J Biol Macromol. 2013;52:212–217. doi: 10.1016/j.ijbiomac.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 22.Dai S, Yin Z, Yuan G, Lu H, Jia R, Xu J, Song X, Li L, Shu Y, Liang X, He C, Lv C, Zhang W. Quantification of metallothionein on the liver and kidney of rats by subchronic lead and cadmium in combination. Environ Toxicol Pharmacol. 2013;36:1207–1216. doi: 10.1016/j.etap.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 23.JiangFeng F, Jiu YS, Wen ZZ, Ben L. The expression of Fas/FasL and apoptosis in yak placentomes. Anim Reprod Sci. 2011;128:107–116. doi: 10.1016/j.anireprosci.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 24.Fan Y, Lu Y, Wang D, Liu J, Song X, Zhang W, Zhao X, Nguyen TL, Hu Y. Effect of epimedium polysaccharide-propolis flavone immunopotentiator on immunosuppression induced by cyclophosphamide in chickens. Cell Immunol. 2013;281:37–43. doi: 10.1016/j.cellimm.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 25.Hong F, Yan J, Baran JT, Allendorf DJ, Hansen RD, Ostroff GR, Xing PX, Cheung NK, Ross GD. Mechanism by which orally administered β-1, 3-glucans enhance the tumoricidal activity of antitumor monoclonal antibodies in murine tumor models. J Immunol. 2004;173:797–806. doi: 10.4049/jimmunol.173.2.797. [DOI] [PubMed] [Google Scholar]
- 26.Cruz-Chamorro L, Puertollano MA, Puertollano E, Alvarez de Cienfuegos G, de Pablo MA. Examination of host immune resistance against Listeria monocytogenes infection in cyclophosphamide- treated mice after dietary lipid administration. Clin Nutr. 2007;26:631–639. doi: 10.1016/j.clnu.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 27.El-Abasy M, Motobu M, Nakamura K, Koge K, Onodera T, Vainio O, Toivanen P, Hirota Y. Preventive and therapeutic effects of sugar cane extract on cyclophosphamide-induced immunosuppression in chickens. Int Immunopharmacol. 2004;4:983–990. doi: 10.1016/j.intimp.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 28.Markert ML, Devlin BH, McCarthy EA. Thymus transplantation. Clin Immunol. 2010;135:236–246. doi: 10.1016/j.clim.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo L, Le Sun Y, Wang AH, Xu CE, Zhang MY. Effect of polysaccharides extract of rhizoma atractylodis macrocephalae on thymus, spleen and cardiac indexes, caspase-3 activity ratio, Smac/DIABLO and HtrA2/Omi protein and mRNA expression levels in aged rats. Mol Biol Rep. 2012;39:9285–9290. doi: 10.1007/s11033-012-1677-x. [DOI] [PubMed] [Google Scholar]
- 30.Wang M, Meng XY, Yang RL, Qin T, Wang XY, Zhang KY, Fei CZ, Li Y, Hu YI, Xue FQ. Cordyceps militaris polysaccharides can enhance the immunity and antioxidation activity in immunosuppressed mice. Carbohydr Polym. 2012;89:461–466. doi: 10.1016/j.carbpol.2012.03.029. [DOI] [PubMed] [Google Scholar]
- 31.Li LL, Yin FG, Zhang B, Peng HZ, Li FN, Zhu NS, Hou DX, Yinemail YL, Luo JJ, Tang ZR, Liu G. Dietary supplementation with Atractylodes Macrophala Koidz polysaccharides ameliorate metabolic status and improve immune function in early-weaned pigs. Livestock Science. 2011;142:33–41. [Google Scholar]
- 32.Nguyen TL, Wang D, Hu Y, Fan Y, Wang J, Abula S, Guo L, Zhang J, khakame SK, Dang BK. Immuno-enhancing activity of sulfated Auricularia auricular polysaccharides. Carbohydr Polym. 2012;89:1117–1122. doi: 10.1016/j.carbpol.2012.03.082. [DOI] [PubMed] [Google Scholar]
- 33.Wang H, Wang M, Chen J, Tang Y, Dou J, Yu J, Xi T, Zhou C. A polysaccharide from Strongylocentrotus nudus eggs protects against myelosuppression and immunosuppression in cyclophosphamide-treated mice. Int Immunopharmacol. 2011;11:1946–1953. doi: 10.1016/j.intimp.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 34.Chen X, Nie W, Fan S, Zhang J, Wang Y, Lu J, Jin L. A polysaccharide from Sargassum fusiforme protects against immunosuppression in cyclophosphamide-treated mice. Carbohydr polym. 2012;90:1114–1119. doi: 10.1016/j.carbpol.2012.06.052. [DOI] [PubMed] [Google Scholar]
- 35.Kuang H, Xia Y, Liang J, Yang B, Wang Q, Wang X. Structural characteristics of a hyperbranched acidic polysaccharide from the stems of Ephedra sinica and its effect on T-cell subsets and their cytokines in DTH mice. Carbohydr Polym. 2011;86:1705–1711. [Google Scholar]
- 36.Kang H, Choi TW, Ahn KS, Lee JY, Ham IH, Choi HY, Shim ES, Sohn NW. Upregulation of interferon-γ and interleukin-4, Th cell-derived cytokines by So-Shi-Ho-Tang (Sho-Saiko-To) occurs at the level of antigen presenting cells, but not CD4 T cells. J Ethnopharmacol. 2009;123:6–14. doi: 10.1016/j.jep.2009.02.045. [DOI] [PubMed] [Google Scholar]
- 37.Denault JB, Salvesen GS. Apoptotic caspase activation and activity. Methods Mol Biol. 2008;414:191–220. doi: 10.1007/978-1-59745-339-4_15. [DOI] [PubMed] [Google Scholar]
- 38.Chen TA, Yang F, Cole GM, Chan SO. Inhibition of caspase-3-like activity reduces glutamate induced cell death in adult rat retina. Brain Res. 2001;904:177–188. doi: 10.1016/s0006-8993(01)02485-4. [DOI] [PubMed] [Google Scholar]
- 39.Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]


