Abstract
Aim: Ghrelin, a gastric peptide, is involved in several metabolic and cardiovascular processes. Emerging evidence indicates the potential involvement of ghrelin in low-grade inflammatory diseases such as atherosclerosis and hypertension. Cytokine-induced inflammation is critical in these pathological states. The growth hormone secretagogue receptor (GHSR) has been identified in blood vessels, so we predict that ghrelin might inhibit proinflammatory responses in human umbilical vein endothelial cells (HUVECs). The aim of this study is to examine the effect of ghrelin on angiotension II (AngII)-induced expression of TNF-α, MCP-1, IL-8 in HUVECs. Method: HUVECs were pretreated with ghrelin, with or without the specific antagonist of GHSR [D-Lys3]-GHRP-6, the selective inhibitor of nuclear factor-kappaB (NF-κB) PDTC, and the selective inhibitor of extracellular signal-regulated kinase (ERK1/2) PD98059. The cells were finally treated with AngII. The expression of TNF-α, MCP-1, IL-8 was examined by reverse transcription-polymerase chain reaction (RT-PCR) and enzyme-linked immunosorbent assay (ELISA). The activity of ERK1/2 and NF-κB was analyzed by Western blot. Result: our study showed that ghrelin inhibited AngII -induced expression of IL-8, TNF-α and MCP-1 in the HUVECs via GHSR pathway in concentration- and time-dependent manners. We also found that ghrelin inhibited AngII -induced activation of ERK1/2 and NF-κB. Conclusions: these results suggest that Ghrelin may play novel antiinflammatory and immunoregulatory roles in HUVECs.
Keywords: Ghrelin, inflammation, atherosclerosis, tumor necrosis factor-α, interleukin-8, monocyte chemoattractant protein-1, NF-κB and MAPK pathways
Introduction
Ghrelin, a 28-amino acid peptide secreted by ghrelin cells in the gastric mucosa, increases appetite and the release of cortisol, anti-diuretic hormone, growth hormone and other homeostatic hormones [1]. Previous studies demonstrated that low plasma ghrelin levels are associated with coronary heart disease [2]. The expression of ghrelin receptor is higher in the cardiovascular system of atherosclerotic mice than that in normal control [3], indicating that ghrelin and its receptor are potential targets for treatment of cardiovascular disease. Ghrelin is shown to inhibit pro-atherogenic changes in the experimental models of atherosclerosis. Low plasma ghrelin level is closely related to angiographically-detected severity and the complex lesion morphology in Chinese diabetic patients with Coronary artery disease [4]. Ghrelin exhibits protective effects on the development of atherosclerosis via multiple pathways, including increasing coronary blood flow [5], improving endothelial function [6], inhibiting endothelial injury [7], inducing vasodilation, enhancing cholesterol efflux in macrophages [8], inhibiting smooth muscle cell (SMC) proliferation and apoptosis [9,10] and exerting anti-inflammatory effect [11-13]. Acylated ghrelin levels are decreased in patients with coronary atherosclerosis, which is independent of body weight and the presence of type 2 diabetes mellitus [14]. Ghrelin vaccination decreases plasma MCP-1 levels even though no effects on atherosclerosis development and weight gain in mice were observed. Ghrelin has emerged as a novel cardiovascular risk factor, but serum ghrelin level in atherosclerosis yields contradictory result. In some previous studies, plasma ghrelin level is positively related to early atherosclerosis measured by carotid artery intima media thickness. Therefore, the precise regulatory mechanism of ghrelin on atherosclerosis is not clear. Angiotensin II (AngII)-induced endothelial injury is associated with atherosclerosis. It has been demonstrated that AngII increases the expression of TNF-α, MCP-1 and IL-8 in human umbilical vein endothelial cells (HUVECs) [15-17]. However, it is unclear whether ghrelin can inhibit AngII -induced expression of TNF-α, MCP-1 and IL-8 in HUVECs. The present study was to study the effect of ghrelin on AngII -induced expression of TNF-α, MCP-1 and IL-8 in HUVECs and the possible signaling pathways.
Materials and methods
Materials
Ghrelin and ghrelin receptor selective antagonist [d-Lys3]-GHRP-6 were obtained from Phoenix Pharmaceuticals (Burlingame, CA). HUVEC cell line was obtained from American Type Culture Collection (Manassas VA). Complete Dulbecco modified Eagle medium (DMEM), RPMI 1640 medium, recombinant human AngII, pyrrolidine dithiocarbamate (PDTC) and mitogen activated protein kinase (MAPK) inhibitor PD98059 were purchased from Sigma (St. Louis, MO). Fetal bovine serum (FBS), Trizol and diethyl pyrocarbonate (DEPC) were purchased from Gibco-BRL (Life Technologies, Grand Island, NY). Rabbit anti-human-ERK1/2, rabbit anti-human-phospho-ERK1/2, rabbit anti-human NF-κB p65 and rabbit anti-human-phospho-NF-κB p65 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). 3-N-Morpholino-propane sulfonic acid (MOPS), Moloney murine leukemia virus (M-MLV) reverse transcriptase, random primers, dNTP and DNA marker (50-1000 bp) were purchased from Promega (Fitchburg, WI). Taq enz-yme was from Biostar International (Toronto, Canada). Western blotting reagent kit and enhanced chemiluminescence reagent kit were from Hoffman-La Roche (Vienna, Austria). Horseradish peroxidase (HRP)-conjugated goat anti-rabbit immunoglobulin G (IgG, H + L) was purchased from KPL (Gaithersburg, MD).
Culture and treatment of HUVEC cells
HUVEC cells were seeded in 6-well plates and cultured for 24-48 h in RPMI 1640 medium containing 10% FBS at 37°C, in 5% CO2. For cell treatments with ghrelin and AngII, complete culture medium was replaced with RPMI 1640 medium without FBS and further incubated in 5% CO2 at 37°C overnight. Cells were pretreated with ghrelin (10-6 mol/L) in RPMI 1640 medium without FBS for 0 min, 3 min, 10 min and 30 min, then treated with AngII (10-6 mol/L) for 24 h. Cells were collected for the subsequent analyses. For cell treatments with inhibitors, the cells were replaced with serum-free RPMI 1640 medium, then treated with control medium, AngII (10-6 mol/L), ghrelin (10-6 mol/L), PDTC (1 μmol/L), PD98059 (25 μmol/L), [d-Lys]-GHRP-6 (25 μmol/L) for 24 h. In combined treatments, the cells were preteated with ghrelin (10-6 mol/L) for 30 min, then treated with AngII (10-6 mol/L) for 24 h; or pretreated with [d-Lys]-GHRP-6 (25 μmol/L) + ghrelin (10-6 mol/L) for 30 min, then treated with AngII (10-6 mol/L) for 24 h; or pretreated with PDTC (1 μmol/L) + ghrelin (10-6 mol/L) for 30 min, then treated with AngII (10-6 mol/L) for 24 h, or pretreated with PD98059 (25 μmol/L) + ghrelin (10-6 mol/L) for 30 min, then treated with AngII for 24 h, or pretreated with PD98059 (25 μmol/L) for 30 min, then treated with AngII (10-6 mol/L) for 24 h, or pretreated with [d-Lys3]-GHRP-6 (25 μmol/L) for 30 min, then treated with AngII (10-6 mol/L) for 24 h. Cells were collected for the subsequent analyses.
Reverse transcription-polymerase chain reaction (RT-PCR)
HUVEC cells with different interventions were washed with PBS. Total RNA was extracted from cells using the single-step guanidinium thiocyanate method [18]. Two micrograms of total RNA was used for RT in a total volume of 10 μl containing 2 μg RNA, 0.5 μl random primer, 1 μl Rnasin and DEPC-treated water. The mixture was incubated at 6°C for 5 min, and then placed on ice. One microliter of M-MLV reverse transcriptase (200 U/μl), 4 μl buffer solution, 1 μl of 10 mM dNTP, and DEPC were added and incubated at 3°C for 1 h and inactivated at 9°C for 5 min. cDNA was used for PCR. Primers of TNF-α, IL-8, MCP-1 and β-actin were designed according to Krzesz et al [19], and synthesized by Shanghai Biology Engineering Inc. (Shanghai, China). The cycling conditions of TNF-α gene (Table 1) were 4°C for 4 min, followed by 28 cycles of 4°C for 30 s, 2°C for 45 s and 72°C for 1 min, and a final extension of 72°C for 10 min. The cycling conditions of β-actin (Table 1) were 95°C for 5 min, followed by 30 cycles of 94°C for 30 s, 55°C for 45 s and 72°C for 1 min, and a final extension of 72°C for 10 min. The cycling conditions of IL-8 gene (Table 1) were 95°C for 5 min, followed by 35 cycles of 94°C for 30 s, 52°C for 30 s and 72°C for 45 s, and a final extension of 72°C for 10 min. The cycling conditions of MCP-1 gene (Table 1) were 95°C for 5 min, followed by 35 cycles of 94°C for 30 s, 56°C for 30 s and 72°C for 45 s, and a final extension of 72°C for 10 min. respectively. The cycling conditions of the second set of β-actin gene (Table 1) were 95°C for 5 min, followed by 35 cycles of 94°C for 30 s, 54°C for 30 s and 72°C for 45 s, and a final extension of 72°C for 10 min. PCR products were separated on a 1.3% agarose gel and examined by ethidium bromide staining. Density quantification of the electrophoresis strip was carried out by gel scanning software (UVP, Upland, CA) and relative expression of genes was normalized to β-actin.
Table 1.
Primer sequences and reaction condition
| Sequence | Product size (bp) | Annealing temperature (°C) | |
|---|---|---|---|
| IL-8 | F 5’-TGGCAGCCTTCCTGATTT-3’ | 246 | 56 |
| R 5’-AACTTCTTCCACAACCCTC-3’ | |||
| MCP-1 | F 5’-GCTCATAGCAGCAGCCACCTT-3’ | 228 | 56 |
| R 5’-GGAATCCTGAACCCACTT-3’ | |||
| TNFα | F 5 ’-GCTCATAGCAGCAGCCACCTT-3’ | 590 | 56 |
| R 5’-GGAATCCTGAACCCACTT-3’ | |||
| β-actin | F 5’-AGCGAGCATCCCCCAAAGT-3’ | 285 | 54 |
| R 5’GGGCACGAAGGCTCATCATT-3’ | |||
| β-actin | F 5’-CTGGGACGACATGGAGAAAA-3’ | 564 | 54 |
| R 5’-AAGGAAGGCTGGAAGAGTGC-3’ |
Detection of TNF-α, IL-8 and MCP-1 in cell culture medium by enzyme-linked immunosorbent assay (ELISA)
Briefly, 96-well Immulon plates (Dynatech Laboratories Inc., Alexandria, VA) were coated with monoclonal antibody against human TNF-α, IL-8 or MCP-1 (4 μg/ml in 100 mM bicarbonate buffer [pH 9.6], MAB208, R&D Systems, Minneapolis, MN) Overnight. Between each step, plates were washed three times with phosphate-buffered saline (PBS) containing 0.05% Tween 20. Plates were blocked for 1 h with PBS containing 1% BSA, 5% sucrose and 0.05% sodium azide. Samples were diluted in the appropriate medium and added to the wells with biotinylated goat anti-human IL-8 secondary polyclonal antibody (40 ng/ml in Tris-buffered saline). Antibody binding was visualized with horseradish peroxidase-conjugated streptavidin (1:1,000 in TBS, Pierce, Rockford IL) and a TMB Peroxidase EIA Substrate kit (Bio-Rad Laboratories, Hercules, CA). Development of the color was stopped by addition of 0.5 M H2SO4, and the absorbance at 450 nm was measured. Values were determined relative to a standard curve.
Detection of NF-κB p65 and ERK1/2 expression by Western blot analysis
Cells were lysed in TBS buffer (pH 7.4) containing 0.6% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 50 mg/ml aprotinin, 100 mg/ml phenylmethylsulfonyl fluoride, 1 mM sodium orthovanadate, and 50 mM sodium fluoride. Cell lysates were centrifuged at 10,000 g for 15 min a 4°C, and the protein concentration was determined using a BCA protein assay kit (Pierce). Samples containing 10 μg protein were boiled for 10 min and subjected to 8% SDS-PAGE, then transferred to the PVDF membranes. After blocking and washing, membranes were incubated with rabbit anti-human phospho ERK1/2 antibody (1100), or phospho-NF-κB p65 antibody (1:100) overnight a 4°C, and further incubated for 1 h with HRP-conjugated goat anti-rabbit IgG (1:5000). The bands were detected with an enhanced chemiluminescence kit. Membranes were then washed in TBS at 50°C for 30 min to remove the antibody. Western blot analysis was carried out to examine the corresponding total ERK1/2 or total NF-κB p65. Density of bands was evaluated by an image analysis system (Bio-Rad) and quantitated as previously described [20].
Statistical analysis
All experiments were repeated for four times. SPSS 16.0 was used for statistical analysis and data were expressed as the mean ± standard deviation (SD). Comparison between measurements was analyzed by one-way ANOVA. If the result of one-way ANOVA was significant, the student-New-man-Keuls was performed. P < 0.05 was considered statistically significant.
Results
Ghrelin inhibits AngII-induced expression of TNF-α, MCP-1 and IL-8 in HUVECs
AngII (10-6 mol/L) stimulated mRNA expression of TNF-α, MCP-1 and IL-8. When we pretreated HUVECs with 10-6 mol/ L ghrelin for 0, 3, 10, 30 min before AngII treatment, we found ghrelin pretreatment significantly decreased AngII-induced mRNA expression of TNF-α, MCP-1, IL-8 in a time-dependent manner, and the maximum effect was observed at 30 min (Figure 1).
Figure 1.
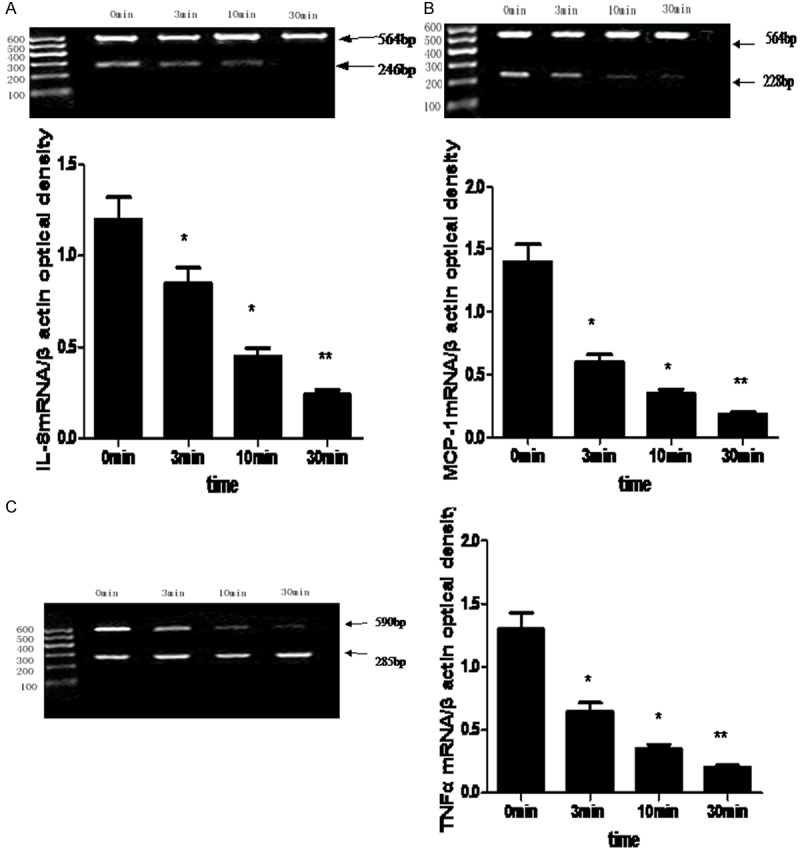
The effect of ghrelin on AngII-induced mRNA expression of IL-8 (A), MCP-1 (B) and TNF-α(C). Difference between groups was analyzed by one-way ANOVA, *compared with control, P < 0.05; **compared with control, P < 0.01.
Effects of different inhibitors on the mRNA expression of IL-8, MCP-1 and TNF-α in HUVECs
To determine whether ERK1/2 and NF-κB signaling pathways are involved in the process that ghrelin inhibited AngII-induced mRNA expression of IL-8, MCP-1 and TNF-α, we treated HUVECs with AngII and ERK1/2 inhibitor PD98059, NF-κB inhibitor PDTC, or GHSR1a receptor antagonist [d-Lys3]-GHRP-6. We found ghrelin significantly inhibited AngII-induced mRNA expression of IL-8, MCP-1 and TNF-α (Figure 1). However, when the cells were treated with both ghrelin (10-6 mol/L) and [d-Lys3]-GHRP-6 (25 μmol/L), the inhibitory effects of ghrelin were completely abolished by [d-Lys3]-GHRP-6, in which the mRNA levels of IL-8, MCP-1 and TNF-α were comparable to those in AngII-treated cells (Figure 2). Interestingly, we observed that both PDTC and PD98059 didn’t affect the mRNA expression of IL-8, MCP-1 and TNF-α compared with untreated cells, but both PDTC and PD98059 completely abolished the promoting effects of AngII on the mRNA expression of IL-8, MCP-1 and TNF-α. Moreover, ghrelin didn’t change the effect of PDTC or PD98059 on the mRNA expression of IL-8, MCP-1 and TNF-α in AngII-treated HUVECs (Figure 2).
Figure 2.
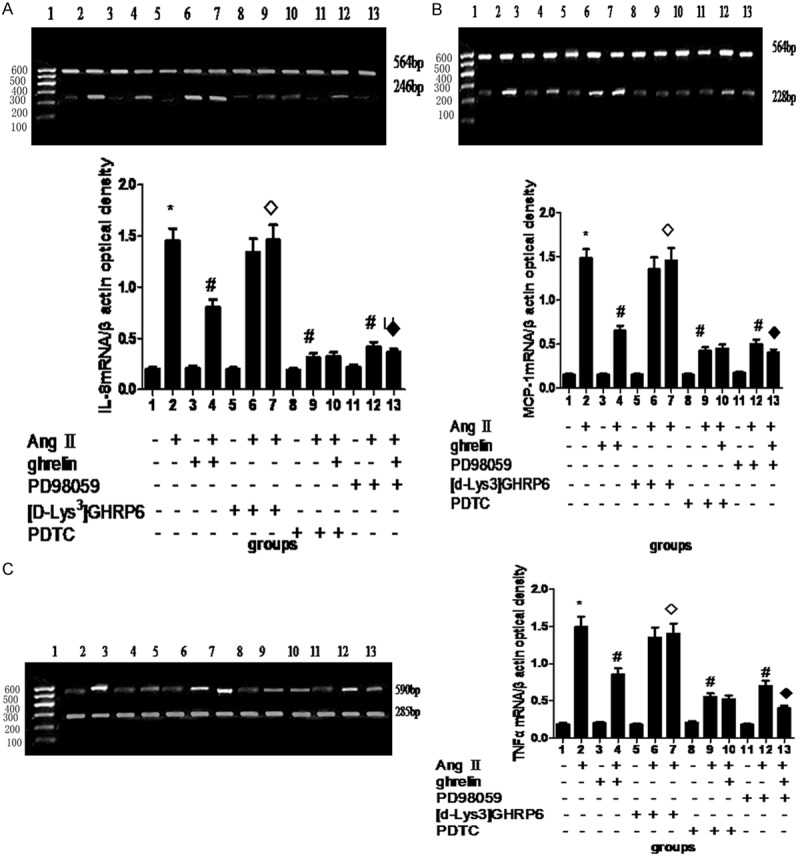
The effect of different inhibitors on the expression of IL-8 (A), MCP-1 (B) and TNF-α (C) mRNAs. AngII: 10-6 mol/L; ghrelin: 10-6 mol/L; [d-Lys3] GHRP: 625 μmol/L; PDTC: 1 μmol/L; PD98059: 25 μmol/L. *Compared with control, P < 0.05; #compared with AngII, P < 0.05; ◊compared with AngII + ghrelin or AngII + PD98059, P < 0.05.
Effect of ghrelin on AngII-induced IL-8, MCP-1 and TNF-α protein expression in the culture medium
Compared with control, AngII (10-6 mol/L) treatment significantly increased the levels of IL-8, MCP-1 and TNF-α in the culture medium (Figure 3A). Ghrelin (10-9-10-6 mol/L) significantly inhibited AngII-induced IL-8, MCP-1 and TNF-α levels in HUVECs (Figure 3A). However, [d-Lys3] GHRP6 completely abolished the inhibitory effect of ghrelin on AngII-induced IL-8, MCP-1 and TNF-α expression (Figure 3A). These data suggest that the anti-inflammatory effect of ghrelin is via GHSR1a.
Figure 3.
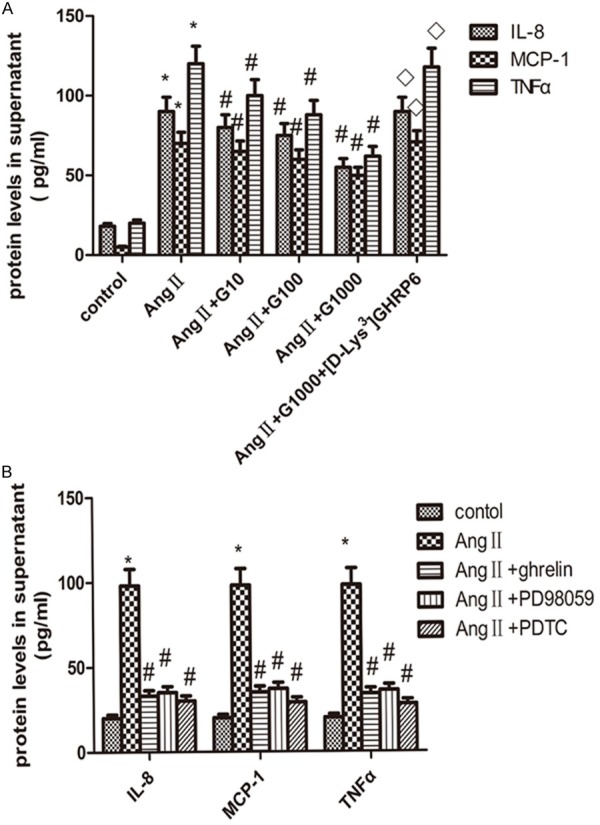
The effect of ghrelin (A) and different inhibitors (B) on AngII-induced IL-8, MCP-1 and TNF-α protein expression in the culture medium. HUVECs were pretreated with 10, 100, 1000 nmol/L ghrelin, or 1000 nmol/L ghrelin + 25 μmol/L [Dys3] GHRP6 for 1 h, then treated with AngII (10-6 mol/L) for 24 h. The concentration of TNF-α, IL-8 and MCP-1 was measured by ELISA. *Compared with control, P < 0.05; #compared with AngII, P < 0.05, ◊compared with AngII + G1000, P < 0.05. Data were presented as mean ± SD (n=4).
Effect of different inhibitors on AngII-induced expression of IL-8, MCP-1 and TNF-α in cell culture medium of HUVECs
To analyze whether ghrelin and signaling pathway inhibitors are involved in AngII-induced IL-8, MCP-1 and TNF-α protein expression in HUVECs, we treated HUVECs with ghrelin, PD98059, or PDTC before AngII treatment. We found ghrelin, PD98059 and PDTC completely abolished AngII-induced IL-8, MCP-1 and TNF-α protein expression in HUVECs cell culture medium, which are comparable to their levels in nontreated cells (Figure 3B).
Effects of ghrelin on ERK and NF-κB signaling pathways
We found AngII significantly activated ERK1/2 and NF-κB p65 in a time- dependent manner, the levels of phosphorylated NF-κB p65 and ERK1/2 phosphorylation were gradually increased (Figure 4A, 4B). However, ghrelin treatment inhibited NF-κB p65 and ERK1/2 activation in a time dependent manner. Pretreatment with ghrelin for 5 min could observe the weak expression of NF-κB p65 and ERK1/2 expresion, and more weaker after in 20 and 30 min and lasted for 1 h (Figure 4C, 4D).
Figure 4.
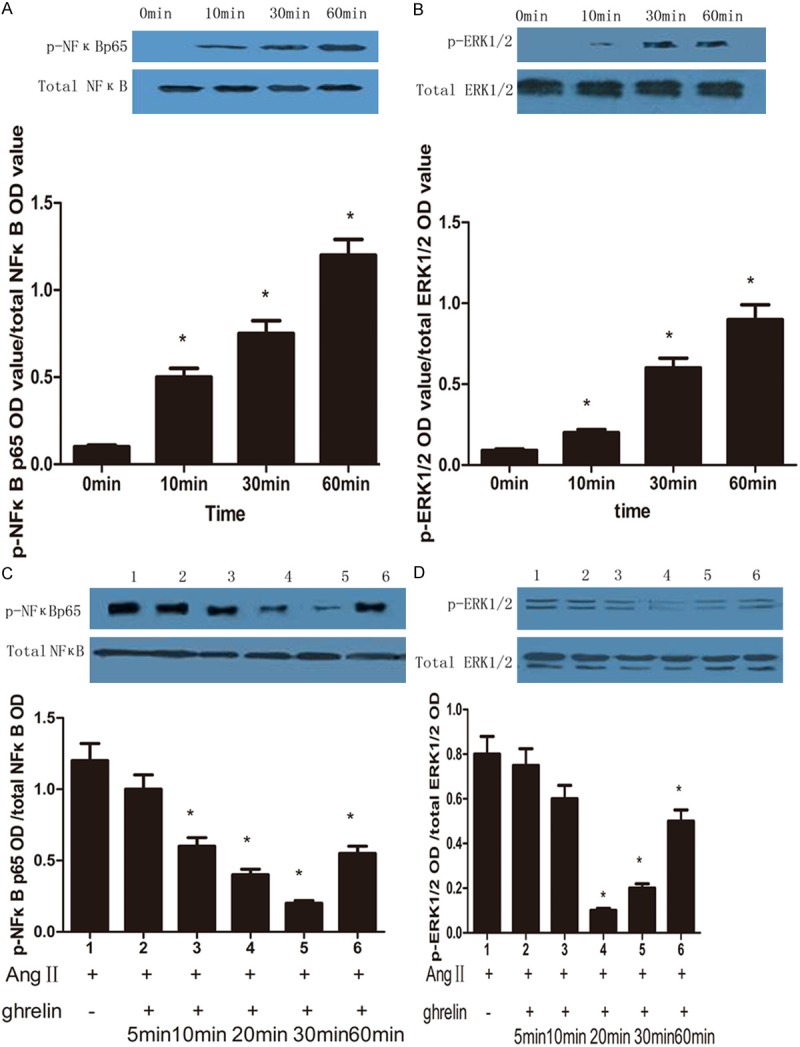
The effect of AngII and ghrelin on NF-κB and ERK1/2 activation. A: AngII activates NF-κB in a time-dependent manner. *Compared with 0 min group, P < 0.05. B: AngII activates ERK1/2 in a time-dependent manner. *compared with 0 min group, P < 0.05. C: Ghrelin inhibits ERK activation in a time-dependent manner. 1, AngII (10-6 mol/L); 2-6, ghrelin (10-6 mol/L) pretreatment for 5, 10, 20, 30 and 60 min, then treated with AngII. *compared with AngII group, P < 0.05. D: Ghrelin inhibits NF-κB activation in a time-dependent manner. 1, AngII (10-6 mol/L); 2-6, ghrelin (10-6 mol/L) pretreatment for 5, 10, 20, 30 and 60 min, then treated with AngII. *Compared with AngII group, P < 0.05.
Discussion
Ghrelin in circulation is produced mainly from X/A like enteroendocrine cells in the oxyntic mucosa of stomach [21]. Some studies [22,23] demonstrate that ghrelin can regulate inflammatory reaction and have anti-inflammatory effect in liver, pancreas, and far distance organs that are damaged by oxidative stress. Ghrelin protects alcohol-induced gastric ulcer [24]. Ghrelin can improve the cachexy condition of heart failure in cancer patients [25], and improve haemodynamics and metabolic disorder in septic shock patients. Inflammation status not only plays important functions in these conditions, but also cannot be ignored in the formation and advance of atherosclerosis and the occurrence of cardiovascular events. Hypertension is related with vascular inflammation. It is the major risk factor of atherosclerotic advancement, but the molecular mechanisms are still not clear. Monocyte-induced chronic vascular wall inflammation is visible in hypertension experiment anima [27,28], atherosclerosis is closely related to inflammation [29]. Therefore, hypertention is easy to develop into atherosclerosis. Recent epidemiological studies found ghrelin is related to hypertension and it has anti-inflammtory effect. Clinical and animal experiments proved the effect of ghrelin on endothelial dysfunction [30,31] and the vasodilation effect of anti-vasoconstrictors [32], however, whether ghrelin can improve AngII-induced inflammatory factor secretion is unknown. Our study demonstrated ghrelin decreased AngII-induced inflammatory factor secretion through GHSR1a pathway, and inhibited MAPK/ERK1/2 and NF-κB signaling pathways. Some reports demonstrate that ghrelin inhibits TNF-α-induced IL-8 and MCP-1 secretion in endothelia cells [13]. Dixit et al [33] proved that ghrelin could be secreted by T cells and peripheral mononuclear cells and inhibited the secretion of IL-1β, IL-6 and TNF-α from peripheral mononuclear cells. Gozalez-Rey [34] and Ceranowicz et al [35] found ghrelin had anti-inflammatory effect in rat colitis and pancreatitis model. However, our study found that ghrelin could not completely block AngII-induced secretion of TNF-α, IL-8 and MCP-1 in endothelia cells, but it could alleviate AngII-induced gene and protein expression of TNF-α, IL-8 and MCP-1 in time- and dose-dependent manners, suggesting ghrelin has anti-inflammatory effect. Moreover, we detected GHSR1a mRNA expression in HUVECs, and GHSR1a antagonist [d-Lys3] GHRP6 completely blocked the inhibitory effect of ghrelin on AngII-induced IL-8, MCP-1 and TNF-α expression in HUVECs, suggesting ghrelin may play anti-inflammatory effect through GHSR1a.
MAPK pathway is related to AngII-activated production of inflammatory factors in HUVECs. Previous study reported that AngII stimulated IL-8 expression in rat smooth muscle cells though MAPK pathway [36]. TNF-α promoter region has MAPK-dependent response element. TNF-α transcriptional activation involves MAPK signaling cascade. It is controversial for ghrelin-activated MAPK signaling pathway in different cells. In adrenal gland cells, ghrelin activates Ras-Raf-MEK-MAPK signaling pathway through activating tyrosine kinase [37], while ghrelin activates Gi/o protein through PI3K and PKC in 3T3-L1 cells and ghrellin increases MAPK activity through growth factor receptor binding protein2 (GRB2) in hepatoma cells [38,39]. It is the first time to report that ghrelin regulates MAPK signaling pathways via GHSR1a receptor. Furthermore, our results demonstrated that both PD98059 and ghrelin attenuated AngII-induced gene and protein expression of IL-8, MCP-1 and TNF-α in HUVECs, and ghrelin inhibited AngII-activated ERK1/2 phosphorylation. These data suggest MAPK signaling pathway is involved in the regulation of ghrelin on the expression of IL-8, MCP-1 and TNF-α in HUVECs.
NF-κB is an important signaling molecule that is stimulated by various factors, including growth factor, lymphokine, ultraviolet irradiation, drug stimulation and oxidative stress. IL-8, MCP-1 and TNF-α belong to the chemotactic factors that have an effect on the migration of neutrophile granulocytes and monocytes to inflammatory area. NF-κB binding domain is verified in the promotor region in IL-8, MCP-1 and TNF-α genes. When NF-κB is activated, the mRNA expression of IL-8, MCP-1 and TNF-α is increased. So we predict that ghrelin may regulate AngII-induced inflammtory reaction via NF-κB activation. Here we proved that ghrelin inhibited AngII-induced expression of IL-8, MCP-1 and TNF-α through inhibiting NF-κB phosphorylation. Both PDTC and ghrelin neutralized the AngII-induced gene and protein expression of IL-8, MCP-1 and TNF-α in HUVECs, suggesting that the anti-inflammatory effect of ghrelin is related to inhibiting NF-κB signaling pathway. However, the combination of ghrelin with GHSR1a inhibited NF-κB phosphorlation, its mechanism needs further study.
In conclusion, our results suggest that ghrelin inhibits AngII-induced expression of IL-8, TNF-α and MCP-1 through inhibiting ERK1/2 MAPK and NF-κB activation in HUVECs. These novel antiinflammatory and immunoregulatory actions of ghrelin may play a certain role in atherosclerosis.
Acknowledgements
This study was supported by the grant from National Nature Science Foundation of China (81301997) and Natural Science Foundation of Hunan province, China (No. 14JJ2020, No. 12JJ5054).
Disclosure of conflict of interest
None.
References
- 1.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–60. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 2.Tang NP, Wang LS, Yang L, Gu HJ, Zhu HJ, Zhou B, Sun QM, Cong RH, Wang B. Preproghrelin Leu72Met polymorphism in Chinese subjects with coronary artery disease and controls. Clin Chim Acta. 2008;387:42–7. doi: 10.1016/j.cca.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 3.Katugampola SD, Pallikaros Z, Davenport AP. [125I-His (9)] -ghrelin, a novel radioligand for localizing GHS orphan receptors in human and rat tissue: up-regulation of receptors with atherosclerosis. Br J Pharmacol. 2001;134:143–9. doi: 10.1038/sj.bjp.0704228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang M, Fang WY, Yuan F, Qu XK, Liu H, Xu YJ, Chen H, Yu YF, Shen Y, Zheng ZC. Plasma ghrelin levels are closely associated with severity and morphology of angiographically-detected coronary atherosclerosis in Chinese patients with diabetes mellitus. Acta Pharmacol Sin. 2012;33:452–8. doi: 10.1038/aps.2011.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang L, Ren Y, Liu X, Li WG, Yang J, Geng B, Weintraub NL, Tang C. Protective effects of ghrelin on ischemia/reperfusion injury in the isolated rat heart. J Cardiovasc Pharmacol. 2004;43:165–70. doi: 10.1097/00005344-200402000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Shimizu Y, Nagaya N, Teranishi Y, Imazu M, Yamamoto H, Shokawa T, Kangawa K, Kohno N, Yoshizumi M. Ghrelin improves endothelial dysfunction through growth hormone-independent mechanisms in rats. Biochem Biophys Res Commun. 2003;310:830–5. doi: 10.1016/j.bbrc.2003.09.085. [DOI] [PubMed] [Google Scholar]
- 7.Caliskan Y, Gorgulu N, Yelken B, Yazici H, Oflaz H, Elitok A, Turkmen A, Bozfakioglu S, Sever MS. Plasma ghrelin levels are associated with coronary microvascular and endothelial dysfunction in peritoneal dialysis patients. Ren Fail. 2009;31:807–13. doi: 10.3109/08860220903151419. [DOI] [PubMed] [Google Scholar]
- 8.Demers A, Caron V, Rodrigue-Way A, Wahli W, Ong H, Tremblay A. A concerted kinase interplay identifies PPARgamma as a molecular target of ghrelin signaling in macrophages. PLoS One. 2009;4:e7728. doi: 10.1371/journal.pone.0007728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang M, Yuan F, Liu H, Chen H, Qiu X, Fang W. Inhibition of proliferation and apoptosis of vascular smooth muscle cells by ghrelin. Acta Biochim Biophys Sin. 2008;40:769–76. [PubMed] [Google Scholar]
- 10.Baldanzi G, Filigheddu N, Cutrupi S, Catapano F, Bonissoni S, Fubini A, Malan D, Baj G, Granata R, Broglio F, Papotti M, Surico N, Bussolino F, Isgaard J, Deghenghi R, Sinigaglia F, Prat M, Muccioli G, Ghigo E, Graziani A. Ghrelin and des-acyl ghrelin inhibit cell death in cardiomyocytes and endothelial cells through ERK1/2 and PI 3-kinase/AKT. J Cell Biol. 2002;159:1029–37. doi: 10.1083/jcb.200207165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang M, Yuan F, Chen H, Qiu X, Fang W. Effect of exogenous ghrelin on cell differentiation antigen 40 expression in endothelial cells. Acta Biochim Biophys Sin. 2007;39:974–81. doi: 10.1111/j.1745-7270.2007.00365.x. [DOI] [PubMed] [Google Scholar]
- 12.Skilton MR, Nakhla S, Sieveking DP, Caterson ID, Celermajer DS. Pathophysiological levels of the obesity related peptides resistin and ghrelin increase adhesion molecule expression on human vascular endothelial cells. Clin Exp Pharmacol Physiol. 2005;32:839–44. doi: 10.1111/j.1440-1681.2005.04274.x. [DOI] [PubMed] [Google Scholar]
- 13.Li WG, Gavrila D, Liu X, Wang L, Gunnlaugsson S, Stoll LL, McCormick ML, Sigmund CD, Tang C, Weintraub NL. Ghrelin inhibits proinflammatory responses and nuclear factor-kappaB activation in human endothelial cells. Circulation. 2004;109:2221–6. doi: 10.1161/01.CIR.0000127956.43874.F2. [DOI] [PubMed] [Google Scholar]
- 14.Vörös K, Prohászka Z, Kaszás E, Alliquander A, Terebesy A, Horváth F, Janik L, Sima A, Forrai J, Cseh K, Kalabay L. Serum ghrelin level and TNF-α/ghrelin ratio in patients with previous myocardial infarction. Arch Med Res. 2012;43:548–54. doi: 10.1016/j.arcmed.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 15.Kim HY, Kang YJ, Song IH, Choi HC, Kim HS. Upregulation of interleukin-8/CXCL8 in vascular smooth muscle cells from spontaneously hypertensive rats. Hypertens Res. 2008;31:515–23. doi: 10.1291/hypres.31.515. [DOI] [PubMed] [Google Scholar]
- 16.Li CH, Zhang XC. The effect of activation peroxidase proliferation activated receptor on AngⅡinduced expression of monocyte chemoattractant protein-1 in Human Umbilical Vein Endothelial Cells. Chinese Journal of Geriatrics. 2006;25:664–7. [Google Scholar]
- 17.Jiang XY, Gao GD, Zhou J, Guo R, Li YX. Role of AT2 receptors on angiotensin II-induced tumor necrosis factor alpha and interleukin 1 beta secretion in adult rat cardiac fibroblasts. Nan Fang Yi Ke Da Xue Xue Bao. 2007;27:1307–9. [PubMed] [Google Scholar]
- 18.Puissant C, Houdebine LM. An improvement of the single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Biotechniques. 1990;8:148–9. [PubMed] [Google Scholar]
- 19.Krzesz R, Wagner AH, Cattaruzza M, Hecker M. Cytokine-inducible CD40 gene expression in vascular smooth muscle cells is mediated by nuclear factor kappa B and signal transducer and activation of transcription-1. FEBS Lett. 1999;453:191–6. doi: 10.1016/s0014-5793(99)00683-3. [DOI] [PubMed] [Google Scholar]
- 20.Tian F, Zang WD, Hou WH, Liu HT, Xue LX. Nuclear factor-kB signaling pathway constitutively activated in esophageal squamous cell carcinoma cell lines and inhibition of growth of cells by small interfering RNA. Acta Biochim Biophys Sin. 2006;38:318–26. doi: 10.1111/j.1745-7270.2006.00166.x. [DOI] [PubMed] [Google Scholar]
- 21.Broglio F, Arvat E, Benso A, Gottero C, Muccioli G, Papotti M, van der Lely AJ, Deqhenqhi R, Ghigo E. Ghrelin, a natural GH secretagogue produced by the stomach, induces hyperglycemia and reduces insulin secretion in humans. J Clin Endocrinol Metab. 2001;86:5083–86. doi: 10.1210/jcem.86.10.8098. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Hai J, Li L, Chen X, Peng H, Cao M, Zhang Q. Administration of ghrelin improves inflammation, oxidative stress, and apoptosis during and after non-alcoholic fatty liver disease development. Endocrine. 2013;43:376–86. doi: 10.1007/s12020-012-9761-5. [DOI] [PubMed] [Google Scholar]
- 23.Kasımay O, Işeri SO, Barlas A, Bangir D, Yeğen C, Arbak S, Yeğen BC. Ghrelin ameliorates pancreaticobiliary inflammation and associated remote organ injury in rats. Hepatol Res. 2006;36:11–9. doi: 10.1016/j.hepres.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 24.Sibilia V, Rindi G, Pagani F, Rapetti D, Locatelli V, Torsello A, Campanini N, Deghenghi R, Netti C. Ghrelin protects against ethanol-induced gastric ulcers in rats: studies on the mechanisms of action. Endocrinology. 2003;144:353–9. doi: 10.1210/en.2002-220756. [DOI] [PubMed] [Google Scholar]
- 25.Nagaya N, Kanqawa K. Ghrelin improves left ventricular dysfunction and cardiac cachexia in heart failure. Curr Opin Pharmacol. 2003;3:146–51. doi: 10.1016/s1471-4892(03)00013-4. [DOI] [PubMed] [Google Scholar]
- 26.Schillaci G, Pirro M, Gemelli F, Pasqualini L, Vaudo G, Marchesi S, Siepi D, Bagaglia F, Mannarino E. Increased C-reactive protein concentrations in never-treated hypertension: the role of systolic and pulse pressure. J Hypertens. 2003;21:1841–6. doi: 10.1097/00004872-200310000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Hilgers KF. Monocytes/macrophages in hypertension. J Hypertens. 2002;20:593–6. doi: 10.1097/00004872-200204000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Gerszten RE. Pleiotropic effects of chemokines in vascular lesion development. Arterioscler Thromb Vasc Biol. 2002;22:528–9. doi: 10.1161/01.atv.0000015099.41408.31. [DOI] [PubMed] [Google Scholar]
- 29.Libby P, Mra AM. Inflammation and atherosclerosis. Circulation. 105:1135–43. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 30.Manfredi Tesauro FS, Micaela Iantorno SR, Domenico Melina DL, Cardillo C. Ghrelin Improves Endothelial Function in Patients With Metabolic Syndrome. Circulation. 2005;112:2986–92. doi: 10.1161/CIRCULATIONAHA.105.553883. [DOI] [PubMed] [Google Scholar]
- 31.Shimizu Y, Nagaya N, Teranishi Y, Imazu M, Yamamoto H, Shokawa T, Kangawa K, Kohno N, Yoshizumi M. Ghrelin improves endothelial dysfunction through growth hormone-independent mechanisms in rats. Biochem Biophys Res Commun. 2003;310:830–5. doi: 10.1016/j.bbrc.2003.09.085. [DOI] [PubMed] [Google Scholar]
- 32.Wiley KE, Davenport AP. Comparison of vasodilators in human internal mammary artery: ghrelin is a potent physiological antagonist of endothelin-1. Br J Pharmacol. 2002;136:1146–52. doi: 10.1038/sj.bjp.0704815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dixit VD, Schaffer EM, Pyle RS, Collins GD, Sakthivel SK, Palaniappan R, Lillard JW Jr, Taub DD. Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. J Clin Invest. 2004;114:57–66. doi: 10.1172/JCI21134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gonzalez-Rey E, Chorny A, Delgado M. Therapeutic action of ghrelin in a mouse model of colitis. Gastroenterology. 2006;130:1707–20. doi: 10.1053/j.gastro.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 35.Dembinski A, Warzecha Z, Ceranowicz P, Tomaszewska R, Stachura J, Konturek SJ, Konturek PC. Ghrelin attenuates the development of acute pancreatitis in rat. J Physiol Pharmacol. 2003;54:561–73. [PubMed] [Google Scholar]
- 36.Kim Y, Kang YJ, Song IH, Choi HC, Kim HS. Upregulation of Interleukin-8/CXCL8 in Vascular Smooth Muscle Cells from Spontaneously Hypertensive Rats. Hypertens Res. 2008;31:515–23. doi: 10.1291/hypres.31.515. [DOI] [PubMed] [Google Scholar]
- 37.Mazzocchi G, Neri G, Rucinski M, Rebuffat P, Spinazzi R, Malendowicz LK, Nussdorfer GG. Ghrelin enhances the growth of cultured human adrenal zona glomerulosa cells by exerting MAPK-mediated proliferogenic and antiapoptotic effects. Peptides. 2004;25:1269–77. doi: 10.1016/j.peptides.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 38.Kim MS, Yoon CY, Jang PG, Park YJ, Shin CS, Park HS, Ryu JW, Pak YK, Park JY, Lee KU, Kim SY, Lee HK, Kim YB, Park KS. The mitogenic and antiapoptotic actions of ghrelin in 3T3-L1 adipocytes. Mol Endocrinol. 2004;18:2291–301. doi: 10.1210/me.2003-0459. [DOI] [PubMed] [Google Scholar]
- 39.Murata M, Okimura Y, Iida K, Matsumoto M, Sowa H, Kaji H, Kojima M, Kanqawa K, Chihara K. Ghrelin modulates the downstream molecules of insulin signaling in hepatoma cells. J Biol Chem. 2002;277:5667–74. doi: 10.1074/jbc.M103898200. [DOI] [PubMed] [Google Scholar]


