Abstract
Objective: Aortoesophageal fistula (AEF) is a life-threatening complication of foreign body ingestion. The primary objective of this study was to describe a new management protocol for infected AEFs, which combines endovascular stent grafting and mediastinal drainage using video-assisted thoracoscopic surgery (VATS). Methods: The authors analyzed the clinical data of 22 patients with ingested foreign bodies retrospectively, developed a classification system based on multidetector computed tomography (MDCT) findings for esophageal injuries induced by foreign body ingestion, and used this system and the clinical presentation to guide treatment. Results: Depending on the MDCT findings, the esophageal injuries were divided into four grades: Grade I, non-penetrating injury (six patients); Grade II, penetrating injury with minimal infection (five patients); Grade III, potential AEF (five patients); and Grade IV, definite AEF (six patients). When a foreign body was visible on MDCT, a distance of ≤ 2 mm between the foreign body and aortic wall indicated potential or definite AEF. When no foreign body was visible, a typical clinical presentation, especially sentinel hemorrhage, and MDCT findings were used to establish the diagnosis. Only three Grade IV patients who underwent open surgery died of severe hemorrhage within 24 h postoperatively. The others patients had a good outcome with different treatment. Conclusions: The authors’ experience indicates that MDCT was useful to classify esophageal injuries caused by foreign body ingestion which predicted the risk of AEF; endovascular stent grafting and VATS-guided mediastinal drainage would be a safe and minimally invasive method for treating patients with AEF and has the potential for improved treatment options for AEFs.
Keywords: Aortoesophageal fistula, esophageal foreign body, endovascular stenting, video-assisted thoracoscopic surgery
Introduction
Aortoesophageal fistula (AEF) is a life-threatening condition, with a fatality rate of 40%-60% [1]. In 1914, Chiari first described the AEF symptom triad of retrosternal pain, sentinel arterial hemorrhage and a symptomless period followed by exsanguination [2]. The main causes of AEF are thoracic aneurysms (51%), esophageal foreign bodies (19%), esophageal ulcers and iatrogenic factors such as arterial stent placement [3]. AEFs caused by esophageal foreign bodies are typically not diagnosed and treated at an early stage, due to the negligence of the patient and medical staff, leading to fatal consequences [4]. The first case of a patient surviving an AEF was reported by Ctercteko and Mok in 1980 [5]. Since then, only a few cases of successful treatment of AEF have been reported [4,6-8]. There is no consensus about the standard treatment for AEFs resulting from a foreign body. Some authors recommend aortic replacement and primary esophageal repair [6]. Others recommend thoracic endovascular aortic repair and delayed esophageal repair [9], which has become a widely accepted treatment for AEF today. Still other authors emphasize the importance of early diagnosis and prevention of infections such as mediastinitis for the successful treatment of AEF [10].
In this retrospective study, we describe the early clinical and imaging findings in patients with esophageal foreign bodies; propose a system to classify the resulting foreign body-related damage by using the initial multidetector computed tomography (MDCT) findings in order to predict risk of AEF. Last, we report the outcomes of AEF management with video-assisted thoracic surgery (VATS)-guided mediastinal drainage combined with endovascular stent grafting.
Patients and methods
Between May 2004 and May 2013, 22 patients with ingested foreign bodies in the thoracic esophagus were admitted to the Thoracic Surgery Department. We retrospectively collected their clinical data, including demographics, clinical presentation, interval between ingestion of foreign body and treatment, type of treatment and outcome (Table 1).
Table 1.
Clinical information
| Grade | Age/Sex | Foreign body | Hematemesis | Interval | Space (mm) | HD (d) | PT |
|---|---|---|---|---|---|---|---|
| I | 46/F | Fish bone | < 50 ml | 30 d | 6.25 | 6 | -- |
| I | 67/M | bone | -- | 10 h | 5.8 | 7 | -- |
| I | 60/W | Fish bone | -- | 12 h | 6.58 | 8 | -- |
| I | 52/M | Fish bone | -- | 6 d | 5.21 | 3 | -- |
| I | 17/M | Key | < 50 ml | 1 d | 8.36 | 7 | -- |
| I | 48/F | Fish bone | < 50 ml | 3 d | 7.63 | 4 | -- |
| II | 73/F | Teeth prosthesis | -- | 2 d | 2.32 | 12 | -- |
| II | 65/M | duck bone | 50 ml | 11 d | 4.2 | 9 | -- |
| II | 56/M | bone | 150 ml | 5 d | 2.11 | 7 | -- |
| II | 14/F | Eraser | -- | 9 d | 3.36 | 13 | -- |
| II | 62/M | Chicken bone | 200 ml | 2 d | 3.87 | 9 | -- |
| III | 71/M | Fish bone | -- | 2 d | 0 | 18 | -- |
| III | 52/M | Fish bone | 500 ml | 9 h | 1.36 | 16 | -- |
| III | 67/M | Fish bone | 400 ml | 8 d | 1.59 | 15 | -- |
| III | 70/F | Fish bone | 300 ml | 3 d | 0 | 18 | -- |
| III | 56/M | Fish bone | 200 ml | 4 d | 0 | 28 | -- |
| IV | 31/M | Chicken bone | 1000 ml | 8 d | -- | 24 | FP |
| IV | 66/M | Teeth prosthesis | 700 ml | 5 d | -- | 23 | PA |
| IV | 74/M | Fish bone | 200 ml | 7 h | -- | Died | FP |
| IV | 70/M | Chicken bone | 600 ml | 4 d | -- | Died | MA |
| IV | 75/M | Fish bone | 450 ml | 8 h | -- | 30 | PA |
| IV | 78/F | Fish bone | 500 ml | 13 d | -- | Died | MA |
Note: M, male; F, female; Interval, interval between ingestion of foreign body and hospitalization; d, days; h, hours; Space, distance between the foreign body and aortic wall; HD, duration of hospitalization; PT, pathological type; FP, fibrous encapsulation; PA, pseudoaneurysm; MA, mediastinal abscess; --, minimal hemorrhage or local infection.
All patients underwent 64-slice MDCT (General Electric Company, USA) soon after hospitalization. The region from the oropharynx to the inferior edge of the second lumbar vertebra was scanned using the following parameters: scan voltage, 120 kV; 150-600 mA; pitch, 0.984 mm; slice thickness, 0.625 mm; and layer interval, 0.625 mm. If a diagnosis could not be reached with these parameters, scanning was repeated with thinner slices. Iopromide, a non-ionic contrast agent, was injected using venipuncture of the antecubital vein with high-pressure syringe. Each patient received 70-90 ml of 300 mg/ml iopromide at a rate of 3.5-4.5 ml/s. The delayed arterial phase occurred after 30-40 s, and the delayed venous phase, after 50-60 s. These specifications were selected on the basis of previous reports [11]. Image post-processing was performed with an AW4.4 workstation and digital angiography equipment (INNOVA 2000, General Electric Company, USA) and included volume reconstruction, maximum intensity projection and multiplanar reformation.
To enable the prevention and early treatment of potential AEFs, damage caused by ingested foreign bodies in the esophagus was divided into the following four Grades based on the MDCT findings.
Grade I: Non-penetrating esophageal injury, damage to the esophageal mucosa and submucosal muscles with minimal hemorrhage or local infection.
Grade II: Esophageal perforation with mild mediastinitis, penetrating esophageal injury with limited esophageal and mediastinal inflammation, > 2 mm distance between the foreign body and aortic wall.
Grade III: Esophageal perforation with severe intrathoracic infections, such as mediastinitis, chest empyema, abscess involving the nearby organs and sepsis, ≤ 2 mm distance between the foreign body and aortic wall.
Grade IV: AEF caused by esophageal perforation and infection, with fibrous encapsulation, pseudoaneurysm or mediastinal abscess.
All patients received treatments based on the grade of esophageal injury, as determined using the above MDCT-based classification. Management strategies included esophagoscopic removal of the foreign body, esophageal repair, thoracotomy and mediastinal drainage, VATS-guided mediastinal drainage, endovascular stenting, aortic fistula repair and aortic reconstruction. The institutional review board of our hospital approved this study, and granted an exemption from informed consent on May 10th, 2013.
Results
Patients with severe symptoms such as hematemesis, dysphagia and retrosternal pain were treated in the Department of Thoracic Surgery. Asymptomatic patients were managed in the Ear Nose and Throat Department or Gastroenterology Department. Of the 22 patients with ingested foreign bodies in the thoracic esophagus in our study, six (27.3%) developed AEFs. Only three of these patients survived. The clinical information of the 22 patients is summarized in Table 1.
Grade I
Six of our patients had Grade I injuries. MDCT revealed details of the foreign body such as size, location and distance from the aorta, and showed that all six patients had non-penetrating esophageal injury. The average distance between the foreign body and aorta was 6.64 mm (Figure 1A, 1B). The hematemesis volume was < 50 ml or could not be precisely determined. Endoscopy was used to both retrieve the foreign body and control hematemesis. Enteral nutrition via a nasal tube was administered for 3-7 days after the procedure. Esophageal drainage and fasting were administered postoperatively. Antibiotic treatment was prescribed for 1 day after endoscopic management. All six patients recovered smoothly and were discharged within 1 week after admission, when repeat MDCT and blood tests showed no abnormality. These patients were doing well in 3-months follow-up.
Figure 1.
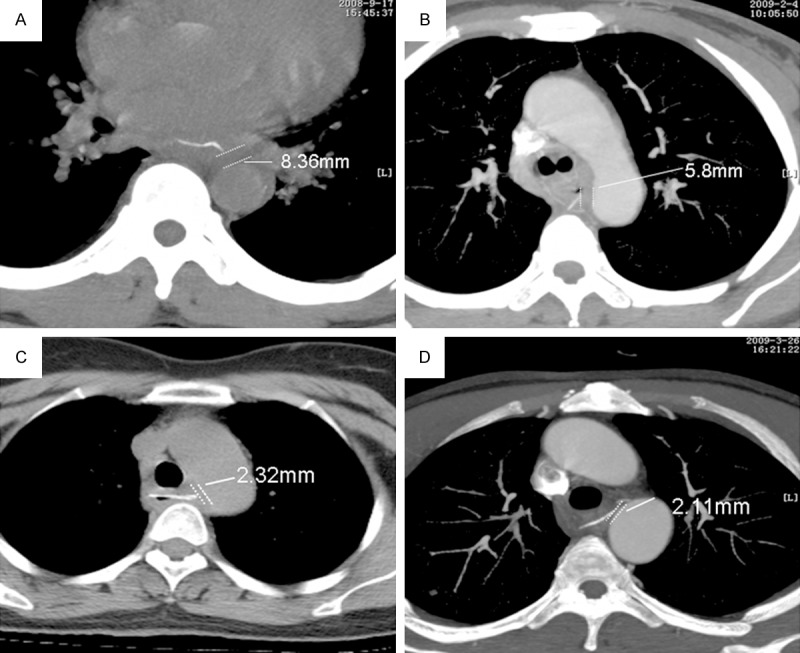
MDCT showing space between the foreign body and aortic wall. A and B: in patients with Grade I, non-penetrating esophageal injury. C, D: Grade II injuries, penetrating esophageal injury, > 2 mm distance between the foreign body and aortic wall.
Grade II
Five patients had esophageal perforation without obvious inflammation and a visible hematoma on MDCT, indicating a Grade II injury. The average distance between the foreign body and aorta was 3.17 mm (Figure 1C, 1D). The hematemesis volume was ≤ 200 ml or could not be determined precisely. These patients did not have any signs of infection, such as fever and abnormal blood cytology, and were therefore confirmed to have Grade II damage. Endoscopic foreign body removal was attempted under general anesthesia. Control of the bleeding and endoscopic esophageal repair were undertaken at the same time. Antibiotic treatment, enteral nutrition via a nasal tube, esophageal drainage and fasting were administered for 7 days postoperatively. When repeat MDCT and blood tests showed no abnormality, these patients were discharged from the hospital. The average duration of hospitalization was 10 days (range, 7-13 days). All patients were doing well at the 3-month follow-up examination.
Grade III
Five patients had Grade III lesions. Most of these patients had a persistent fever, retrosternal pain, hematemesis, dysphagia, low blood pressure or sepsis. The foreign body was stuck to the aortic wall (Figure 2A, 2B) in two patients, which indicated a possible AEF and necessitated emergency treatment. In three patients, the foreign bodies were < 2 mm away from the aorta. In all five patients, the MDCT findings were suggestive of esophageal perforation and severe intrathoracic infections such as mediastinitis, chest empyema and abscess (Figure 2C, 2D). The average hematemesis volume was 280 ml, but this information was not acquirable in all patients. The management of these patients was much more complicated than that of patients with Grade I or II injuries. Two patients underwent left thoracotomy, esophageal repair, mediastinal drainage and esophageal reconstruction with an omental flap. Another two patients underwent thoracoscopic removal of the foreign body, mediastinal and esophageal drainage. The fifth patient underwent esophageal diversion surgery, mediastinal and esophageal drainage and jejunostomy Antibiotic treatment, enteral nutrition via a nasal tube; esophageal drainage and fasting were prescribed for all five patients until blood tests and MDCT examination showed no abnormalities. The average duration of hospitalization was 19 (15~28) days. The 3-month follow-up examination showed that the treatments had been successful in all five patients.
Figure 2.
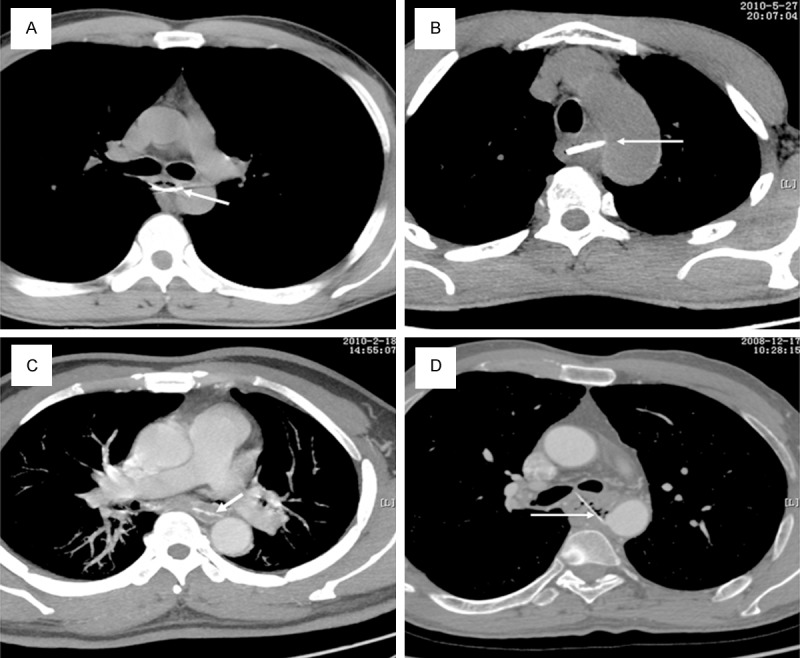
Foreign bodies stuck to the aortic wall (A, B) and severe mediastinitis (C, D; white arrows) in patients with Grade III injuries.
Grade IV
Six patients were diagnosed with Grade IV injuries, namely, AEFs, on the basis of their MDCT findings and clinical characteristics. All of them presented with the Chiari triad, and aortic wall perforation, pseudoaneurysm and mediastinal abscess were found on MDCT (Figure 3). Evidence of hemorrhage could be seen around the perforations in both the esophagus and aorta on MDCT. The hematemesis volume varied among these patients, and ranged from 200 ml to > 1000 ml (average, 575 ml). These patients must be managed carefully because AEF can be fatal. All six patients were transferred to the operation room within 2 h after hospitalization. Three of them underwent left thoracotomy, esophageal repair and reconstruction with an omental flap, along with aortic repair (two patients) or replacement (one patient). The other three patients underwent initial endovascular stenting (Figure 4). Then, they underwent second-stage left VATS exploration for foreign body removal, esophageal repair, and mediastinal and thoracic drainage immediately. Antibiotic treatment, enteral nutrition via a nasal tube, esophageal drainage and fasting were administered postoperatively until blood tests showed no abnormalities. The three patients who did not undergo endovascular stenting died of hemorrhage within 24 h after the operation. The duration of hospitalization in the three surviving patients was 23-30 days, and all three were doing well at the 3-month follow-up examination. We noted that the longer the interval between foreign body ingestion and hospitalization, the worse the prognosis.
Figure 3.
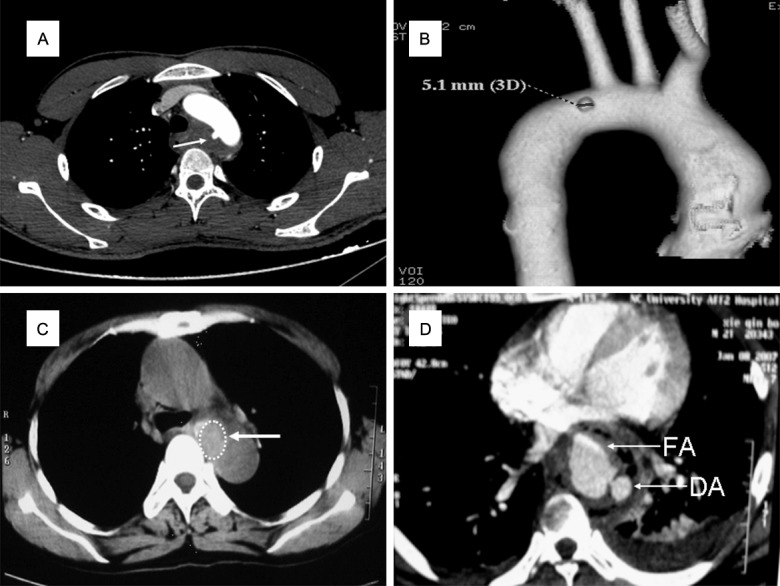
MDCT subtypes of AEFs Grade IV injuries. A, B: Pseudoaneurysm and AEF, with a diverticulum (white arrow) on the medial wall of the aortic arch, surrounded with inflammatory tissue. C: Fibrous encapsulation and AEF, with a swollen soft-tissue mass (white arrow) between the esophagus and descending aortic wall, whose boundary on the right side is unclear. D: Mediastinal abscess and AEF in the plane of the inferior pulmonary vein. A contrast-enhanced scan shows the fistula on the descending aortic (DA) wall, and the contrast agent flows into the false aneurysm (FA). Inflammatory and necrotic tissue is seen around the esophagus and the descending aorta, with gas formation and left pleural effusion.
Figure 4.
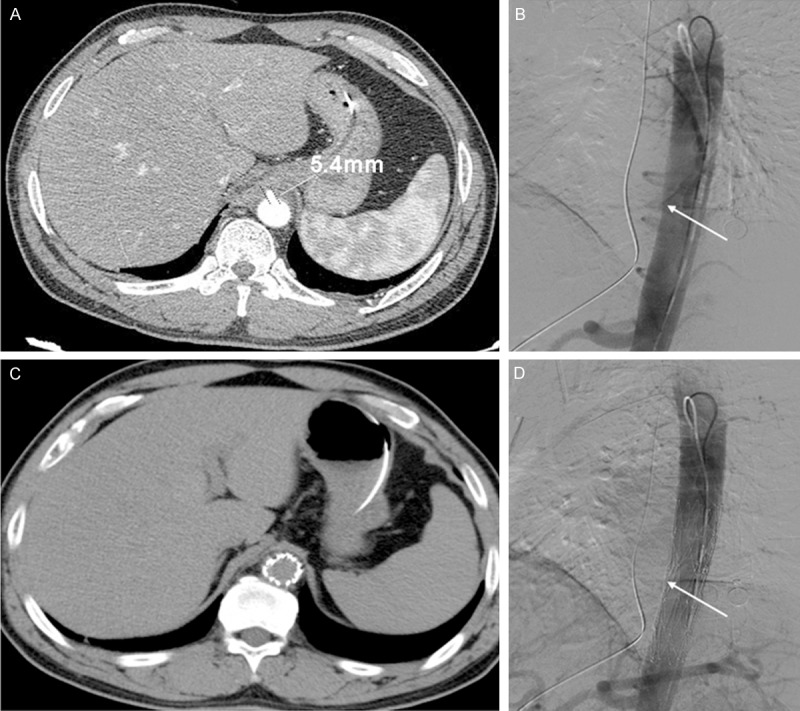
A: CT angiography images. AEF with inflammatory exudate surrounding the descending aorta (diameter 5.4 mm). B: An aortic pseudoaneurysm at the anterior wall of the descending aorta at the lower edge of thoracic vertebrae 10 (arrow). C, D: Aortic endovascular stent graft implantation to close the AEF (arrow).
Discussion
Because of a lack of awareness among doctors that ingested foreign bodies in the thoracic esophagus can cause AEFs, most patients with this condition are not successfully rescued. The main reasons for the poor prognosis of infected AEFs caused by the ingestion of a foreign body are as follows: First, the history of foreign body ingestion may not always be available, as the interval between ingestion and the appearance of clinical symptoms can vary from several days to > 1 week; in addition, some patients may completely forget about the ingested foreign body. Second, the clinical symptoms may not be proportional to the aortic damage. Some patients with severe mediastinal infection have no obvious systemic manifestations, and the typical AEF triad of retrosternal pain, dysphagia and sentinel hemorrhage is not present in every patient. In fact, fatal hematemesis is occasionally the only clinical manifestation. Third, there is a lack of effective auxiliary examinations and a staging system for AEF. Accurate examination is essential to guide treatment. The most common methods used to diagnose foreign body-related damage to the thoracic esophagus are X-ray and CT examinations, which are useful to determine the position of the foreign body, but cannot accurately demonstrate the severity and extent of mediastinal infection and aortic damage. Gastrointestinal endoscopy can be used to accurately localize and remove foreign bodies and perform esophageal repair, but carries the risk of further aortic damage and even fatal bleeding in the absence of accurate staging assessment. Fourth, the conventional surgical treatment of AEF, aortic repair and aortic replacement are often associated with uncontrollable bleeding and high mortality rates in patients with infected AEFs. Fifth, it is difficult to provide adequate drainage of the surgical site, and mediastinal infection is very difficult to control without effective drainage. Due to this, the fistula can recur and result in fatal bleeding [12].
To overcome the above problems, we developed a new management protocol for AEF, which included (1) pre- and post-treatment MSCT to accurately classify the esophageal injury and detect potential AEFs, (2) endovascular stenting instead of conventional thoracotomy and aortic repair, (3) because infection is invariably present after foreign body erosion of the esophageal, we suggest endovascular thoracic aorta stenting should be used as a stop-gap before definitive surgery to ultimately reduce the risk of aortic rupture, followed by treatment of mediastinal infections and esophageal defect in patients with AEFs. VATS exploration sufficient to remove the foreign body, repair the perforated esophagus and provide complete mediastinal and pleural drainage, thereby decreasing operative injury and complications and (4) rational use of effective antibiotics and adequate nutrition support therapy.
Because of the rarity of AEFs, standardized diagnosis and treatment protocols have not been established. Although several classification methods have been proposed for the early diagnosis of AEF [13,14], these methods are not practicable or widely accepted. In this study, we evaluated patients using MDCT and established an MDCT-based system to classify injuries resulting from ingested foreign bodies in the esophagus, in order to facilitate the early detection and treatment of AEFs. As demonstrated in this study, our classification system could detect AEFs and was used to treat five patients with Grade III injuries, i.e., potential AEFs, and six patients with Grade IV injuries, i.e., definite AEFs.
The diagnosis of esophageal foreign body greatly depends on the clinical manifestations and endoscopic or radiographic findings [6,10,15,16]. However, the pathological type cannot be determined using chest roentgenography [17], owing to its low resolution and inability to distinguish between adjacent structures. When the foreign body is at the aortic arch Grade, endoscopic examination may lead to iatrogenic injury, as the foreign body may shift and penetrate the adjacent aortic wall [6]. Endoscopy can reveal damage inside the esophagus, but cannot detect foreign bodies that have pierced through the esophagus and into the surrounding tissue. Magnetic resonance imaging cannot detect bones and is less effective than CT [17]. MDCT has a short scanning time, reduced contrast agent usage and importantly, a high resolution when combined with 3D reconstruction. Enhanced MDCT can detect whether the flow within the lesions is blood, and diagnose aortic and esophageal injuries [17-19]. Moreover, when plain MDCT images are suggestive of esophageal foreign body and AEF, enhanced MDCT scanning with 3D reconstruction or MDCT angiography can be undertaken [11,18].
Foreign bodies in the thoracic esophagus tend to be lodged at the Grade of the left primary bronchus, where the aortic arch presses against the esophagus and creates a relatively narrow lumen. Esophageal foreign bodies can cause severe complications such as esophageal perforation, mediastinal abscess, tracheoesophageal fistula and AEF [20,21]. The mechanism of AEF formation after foreign body ingestion involves the gradual corrosion and subsequent piercing of the aortic wall by the foreign body. This is followed by pseudoaneurysm formation, extensive necrosis and secondary infection [14].
To select the most appropriate treatment, we used the following protocol. We divided the MDCT findings into two groups, namely, injuries with a visible foreign body and those without a foreign body; these were further subdivided into injuries with or without aortic damage.
When a foreign body was visible on MDCT, the duration of symptoms was usually short, and the pathological changes were relatively limited. In the case of injuries with no aortic damage and a visible foreign body, if the distance between the foreign body and aortic wall was ≥ 5 mm, direct endoscopic removal of the foreign body was performed. If the distance between the foreign body and aortic wall was < 5 mm, endoscopic removal was performed under general anesthesia, with immediate conversion to thoracotomy or VATS, if required.
In the case of injuries with aortic damage and a visible foreign body, which was adjacent or stuck to the aorta, AEF was definitively diagnosed if the patient had a history of a single episode of hematemesis of volume > 500 ml or presented with the Chiari triad. If the hematemesis volume was > 300 ml but less than 500 ml, this episode was regarded as sentinel hemorrhage. These patients require emergency endovascular aortic stent grafting. Extracorporeal circulation should be prepared, and second-stage VATS-guided mediastinal drainage should be performed if mediastinal infection is present.
When a foreign body is not found on MDCT, the patient might generally have endured a long medical course, and the pathological changes are much more complex. If there is no aortic damage, simple drainage or conservative treatment should be given. However, these patients can develop hematemesis because of local inflammation. Enhanced MDCT might reveal local inflammatory reaction without aortic fistula. Close attention should be paid during the course of treatment for perforation resulting from necrosis.
If aortic injury is present, the situation becomes rather complicated. Enhanced MDCT should be used to determine the pathological type of this injury, and the following treatments can be undertaken.
AEF with pseudoaneurysm (Figure 3A, 3B): Enhanced MDCT with 3D reconstruction can detect this type of injury. The optimal treatment is initial endovascular stent graft placement to control bleeding, followed by thoracic exploration, aortic repair, partial esophageal resection and esophageal reconstruction with stomach and omental flaps, along with other symptomatic treatments [22].
AEF with fibrous encapsulation (Figure 3C): MDCT alone is sufficient to diagnose AEF in this scenario, if a history of sentinel hemorrhage is present. Treatment options include endovascular stent graft placement, left thoracotomy with aortic fistula repair, esophageal repair, mediastinal drainage and esophageal reconstruction with an omental flap.
AEF with mediastinal abscess (Figure 3D): Enhanced MDCT can detect this condition, which carries a high risk of mortality. Two of our patients with this type of injury died. Both of them had undergone aortic repair/replacement and esophageal resection and reconstruction, accompanied with mediastinal drainage and omental flap reconstruction.
In conclusion, our MDCT-based classification system combined with endovascular stent graft placement and VATS drainage for infected AEFs caused by the ingestion of a foreign body enabled the early management of AEFs and yielded relatively good outcomes.
Acknowledgements
This work was supported by grants from the National Instrumentation Program of China (Project no. 2011YQ170067), the Major Scientific Technological Innovation Projects of Jiangxi Province (Project no. 20124ACB00700).
Disclosure of conflict of interest
None.
References
- 1.Gobolos L, Miskolczi S, Pousios D, Tsang GM, Livesey SA, Barlow CW, Kaarne M, Shambrook J, Lipnevicius A, Ohri SK. Management options for aorto-oesophageal fistula: case histories and review of the literature. Perfusion. 2013;28:286–290. doi: 10.1177/0267659113476329. [DOI] [PubMed] [Google Scholar]
- 2.Chiari H. Uber Pemdkorperve rletzung des Oesophagus mit Aorten-perforation. Berl Klin Wochenschr. 1914;51:7–9. [Google Scholar]
- 3.Sager HB, Wellhoner P, Wermelt JA, Schunkert H, Kurowski V. Lethal Hemorrhage Caused by Aortoesophageal Fistula Secondary to Stent-Graft Repair of the Thoracic Aorta. Cardiovasc Intervent Radiol. 2011 doi: 10.1007/s00270-010-9844-8. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.Venara A, Hamdi S, Desolneux G, Papon X, Lermite E, Arnaud JP. Long-term successful management of an aortoesophageal fistula secondary to the ingestion of a bone. Presse Med. 2012;41:543–546. doi: 10.1016/j.lpm.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 5.Ctercteko G, Mok CK. Aorta-esophageal fistula induced by a foreign body: the first recorded survival. J Thorac Cardiovasc Surg. 1980;80:233–235. [PubMed] [Google Scholar]
- 6.Lai H, Ge D, Zheng YJ, Li J, Wang C. Surgical management of aortoesophageal fistula caused by foreign bodies. Eur J Cardiothorac Surg. 2011;40:13–16. doi: 10.1016/j.ejcts.2010.10.034. [DOI] [PubMed] [Google Scholar]
- 7.Xi EP, Zhu J, Zhu SB, Liu Y, Yin GL, Zhang Y, Zhang XM, Dong YQ. Surgical treatment of aortoesophageal fistula induced by a foreign body in the esophagus: 40 years of experience at a single hospital. Surg Endosc. 2013;27:3412–6. doi: 10.1007/s00464-013-2926-3. [DOI] [PubMed] [Google Scholar]
- 8.Canaud L, Ozdemir BA, Bee WW, Bahia S, Holt P, Thompson M. Thoracic endovascular aortic repair in management of aortoesophageal fistulas. J Vasc Surg. 2014;59:248–54. doi: 10.1016/j.jvs.2013.07.117. [DOI] [PubMed] [Google Scholar]
- 9.Zuber-Jerger I, Hempel U, Rockmann F, Klebl F. Temporary stent placement in 2 cases of aortoesophageal fistula. Gastrointestinal endoscopy. 2008;68:599–602. doi: 10.1016/j.gie.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 10.Marone EM, Coppi G, Kahlberg A, Tshomba Y, Chiesa R. Combined endovascular and surgical treatment of primary aortoesophageal fistula. Tex Heart Inst J. 2010;37:722–724. [PMC free article] [PubMed] [Google Scholar]
- 11.Bae KT. Optimization of contrast enhancement in thoracic MDCT. Radiol Clin North Am. 2010;48:9–29. doi: 10.1016/j.rcl.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 12.Patel HJ, Williams DM, Upchurch GR Jr, Dasika NL, Eliason JL, Deeb GM. Late outcomes of endovascular aortic repair for the infected thoracic aorta. Ann Thorac Surg. 2009;87:1366–1371. doi: 10.1016/j.athoracsur.2009.02.030. [DOI] [PubMed] [Google Scholar]
- 13.Chang S, Cheng BC, Huang J, Mao ZF, Wang TS, Xia J. [Classification and surgical treatment of intrathoracic esophageal injury caused by foreign body] . Zhonghua Wai Ke Za Zhi. 2006;44:409–411. [PubMed] [Google Scholar]
- 14.Zhang X, Liu J, Li J, Hu J, Yu F, Li S, Yang X. Diagnosis and treatment of 32 cases with aortoesophageal fistula due to esophageal foreign body. The Laryngoscope. 2011;121:267–272. doi: 10.1002/lary.21366. [DOI] [PubMed] [Google Scholar]
- 15.Tseng KC, Lin CW, Tan JW. Successful management of aortoesophageal fistula by combining endoscopic cyanoacrylate injection and endovascular stent grafting. Endoscopy. 2011;43(Suppl 2 UCTN):E135–136. doi: 10.1055/s-0030-1256167. [DOI] [PubMed] [Google Scholar]
- 16.Sica GS, Djapardy V, Westaby S, Maynard ND. Diagnosis and management of aortoesophageal fistula caused by a foreign body. Ann Thorac Surg. 2004;77:2217–2218. doi: 10.1016/j.athoracsur.2003.06.031. [DOI] [PubMed] [Google Scholar]
- 17.Mirvis SE. Imaging of acute thoracic injury: the advent of MDCT screening. Semin Ultrasound CT MR. 2005;26:305–331. doi: 10.1053/j.sult.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Maldjian PD, Partyka L. Intimal tears in thoracic aortic dissection: appearance on MDCT with virtual angioscopy. Ajr. 2012;198:955–961. doi: 10.2214/AJR.11.7327. [DOI] [PubMed] [Google Scholar]
- 19.Steenburg SD, Ravenel JG. Acute traumatic thoracic aortic injuries: experience with 64-MDCT. Ajr. 2008;191:1564–1569. doi: 10.2214/AJR.07.3349. [DOI] [PubMed] [Google Scholar]
- 20.Ahn D, Heo SJ, Park JH, Sohn JH. Tracheoesophageal fistula with tracheal stenosis resulting from retained esophageal foreign body. Auris Nasus Larynx. 2011;38:753–756. doi: 10.1016/j.anl.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 21.Kikuchi K, Tsurumaru D, Hiraka K, Komori M, Fujita N, Honda H. Unusual presentation of an esophageal foreign body granulosma caused by a fish bone: usefulness of multidetector computed tomography. Jpn J Radiol. 2011;29:63–66. doi: 10.1007/s11604-010-0495-0. [DOI] [PubMed] [Google Scholar]
- 22.Chen X, Li J, Chen J, Zhou Y, Zhang Y, Ding H, Huang S, Zhang Z. A combined minimally invasive approach for the treatment of aortoesophageal fistula caused by the ingestion of a chicken bone: case report and literature review. Clinics. 2012;67:195–197. doi: 10.6061/clinics/2012(02)19. [DOI] [PMC free article] [PubMed] [Google Scholar]


