Abstract
Beta1-adrenoreceptor (β1-AR) predominantly exists in the heart and β1-AR reduction is closely related to severity of heart failure (HF). In this study, our research focused on the miRNAs that may repress β1-AR directly, and aim to find out new markers and target molecules for HF. We first did Argonaute2 AGO2 knock down experiments and confirmed that endogenous adrenoceptor beta 1 (ADRB1) expression was suppressed by miRNAs. To further identify which miRNA suppress ADRB1 expression directly, we constructed the ADRB1 3’UTR reporter plasmid and selected sixteen candidate miRNAs. Confirmed by dual-luciferase assay and western blot, we found that miR-19a suppressed ADRB1 expression by directly targeting 3’UTR. Further expressions detection the levels of miR-19a, BNP and cAMP in 32 plasma samples of HF patients helped us to construct positive correlations between the expression levels of miR-19a and BNP or cAMP, hints miR-19a may be used as a biomarker in HF patients indicating cardiac function. In conclusion, this study confirmed miR-19a suppressed ADRB1 expression by directly targeting 3’UTR of ADRB1 and found an positive correlation between plasma miR-19a and BNP or cAMP levels in HF patients, which may contributes to fully understand the HF pathogenesis and develops new therapy for HF.
Keywords: Heart failure, miRNA, ADRB1, 3’ untranslated region
Introduction
Heart failure (HF) has been classified as an epidemic of the 21st century and is now one of the major cause of morbidity in the elderly in China. The etiology of heart failure includes coronary artery disease including a previous myocardial infarction, high blood pressure, atrial fibrillation, valvular heart disease, and cardiomyopathy. These cause heart failure by changing either the structure or the functioning of the heart. Although with heavy economic burden worldwide, the mechanisms underlying HF remain largely undetermined.
MicroRNAs (miRNAs) are short, noncoding RNA sequences that regulate gene expression at the posttranscriptional level by targeting the 3’-untranslated region of mRNA sequences. Bioinformatical study indicated that more than 60% of human genes may be regulated by miRNAs [1]. Loss-of-function studies in mice firmly established that miRNAs control a variety of cellular processes essential to the heart [2,3]. MiRNAs play very important roles in maintaining normal human body physiology conditions, and abnormal miRNA expressions have been found related to many human diseases spanning from psychiatric disorders to malignant cancers [4-6].
Initial studies of miRNAs in animal models of hypertrophy and heart failure showed specific signature patterns suggesting that they could be used as valuable biomarkers for disease [7]. Recently, more and more studies have documented the regulatory role of miRNAs in HF. Studies from Olson and co-workers [8] and Condorelli and co-workers [9] have shown in mice that miRNAs play a critical role in development and/or progression toward heart failure. Meanwhile, altered miRNAs expression has also been found in human HF patients. Van Rooij E and co-workers reported that overexpressed miR-195 is related to HF in mouse model and human [7]. Disturbed circulating miRNAs expressions were also suggested to be used as biomarker for HF [10].
β-adrenoreceptors (β-ARs) and their associated guanine nucleotide regulatory protein (G protein)/adenylyl cyclase signal transduction pathways, are central to the regulation of cardiac function. β-ARs display peculiar tissue distribution and pharmacological properties: β1 is the “cardiac” receptor, while β2 is expressed predominantly in smooth muscle cells, and β3 in the adipose tissue. It is well known thatβ1-AR subtype is down-regulated in heart failure. In this study, our research focused on the miRNAs that may repress β1-AR directly aim to find out new therapy for HF.
Materials and methods
Clinical samples
Peripheral blood samples were collected from 32 patients (age: 53±5; 20 males and 12 females) at the Emergency Department, Beijing Friendship Hospital with a diagnosis of dilated cardiomyopathy (DCM). The non-failing controls were obtained from 9 age and sex matched patients (age: 51±4 years old; 5 males and 4 females) with unexplained ventricular tachy-arrhythmias but with a normal ejection fraction. To get the cell free plasma, samples were centrifuges at 4°C 3,000 g for 10 min, and the supernatant plasma was quickly removed and stored immediately at -80°C until analysis. All protocols for this research were in compliance with institutional guidelines for human research and approved by the Beijing Friendship Hospital Review Board.
Plasmids construction
To generate 3’-UTR luciferase reporter, full length of 1342 bp 3’-UTR of ADRB1 were cloned into the downstream of the firefly luciferase coding region in pGL3 control vector (Promega). Plasmid with 4 nucleotides mutation in the putative miR-19a target site was constructed to confirm the binding region of miR-19a.
Dual-luciferase assay
Using Lipofectamine 2000 (Invitrogen), cells were transfected with 0.8 μg of pGL3-ADRB1 plasmid, 20 pmol of either a stability-enhanced 2’-O-methyl nontargeting RNA control or miR-19a oligonucleotides (GenePharma). pRL-TK vector expressing renilla luciferase was used as transfection control. At 48 h after transfection, cells were lysed and luciferase activity was measured. Each treatment was performed in triplicate in three independent experiments. The results were expressed as relative luciferase activity (Firefly Luc/Renilla Luc).
RNA isolation
Total RNA was extracted from plasma by using TRIzol LS reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Briefly, about 250 μl plasma sample was added into 750 μl TRIzol reagent, and were incubated at room temperature for 5 min. Chloroform was added to the samples, vigorously mixed, and incubated at room temperature for 5 min. Following incubation, the samples were centrifuged at 12,000 g for 15 min at 4°C. RNA was precipitated from the aqueous phase by addition of isopropyl alcohol to a fresh tube containing the supernatant aqueous phase. The integrity of the RNA was tested by spectroscopic analysis.
miRNA real-time RT-qPCR
Quantitive RT-PCR analysis was used to determine the relative expression level of candidate miRNAs. The expression level of miRNAs was detected by TaqMan miRNA RT-Real Time PCR. Single-stranded cDNA was synthesized by using TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA) and then amplified by using TaqMan Universal PCR Master Mix (Applied Biosystems, Foster City, CA, USA) together with miRNA-specific TaqMan MGB probes (Applied Biosystems, Foster City, CA, USA). The U6 snRNA was used for normalization. Each sample in each group was measured in triplicate and the experiment was repeated at least three times for the detection of miRNAs.
Western blotting
Protein extracts from cells were boiled in SDS/β-mercaptoethanol sample buffer, and 20 μg samples were loaded into each lane of 10% polyacrylamide gels. The proteins were separated by electrophoresis, and the proteins in the gels were blotted onto PVDF membranes (Amersham Pharmacia Biotech, St. Albans, Herts, UK) by electrophoretic transfer. The membrane was incubated with rabbit anti-ADRB1 or AGO2 polyclonal antibody (Abcam, Cambridge, MA, USA) or mouse anti-β-actin monoclonal antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) for 2 h at 37°C. The specific protein-antibody complex was detected by using horseradish peroxidase conjugated goat anti-rabbit or rabbit anti-mouse IgG. Detection by the chemiluminescence reaction was carried using the ECL kit (Pierce, Appleton, WI, USA). The β-actin signal was used as a loading control.
Plasma BNP and cAMP detection
The plasma BNP and cAMP levels were detected by enzyme-linked immunosorbent assay (ELISA) kit (eBioscience Inc., San Diego, CA, USA) following the manufacturers’ instructions.
Statistical analysis
The relationship between the expression of miR-19a, the BNP level, and the patient clinical characteristics was analyzed using Student’s t-test or a χ2-analysis. The findings were considered to be significant at a P-value <0.05. All statistical analyses were performed using the SPSS v. 16.0 software program (SPSS, Inc., Chicago, IL, USA).
Results
β1-AR predominantly exists in the heart and the chronic activation of β1-AR signaling has been confirmed to be related to many kinds of heart diseases like DCM and HF. The name of β1-AR coding gene is ADRB1.To understand whether the expression of ADRB1 is regulated by miRNAs in the heart, we first knockdown the expression of AGO2 in HEK293T cells and mouse cardiomyocytes, which is a key component of miRISC. As shown in Figure 1A, the expression of hAGO2 and mAGO2 was reduced by in the Si-AGO2 groups. Meanwhile, the expression of ADRB1 was overexpressed by Si-AGO2 transfection. These results indicated that the expression of ADRB1 was suppressed by miRNAs in HEK293T and mouse cardiomyocytes.
Figure 1.
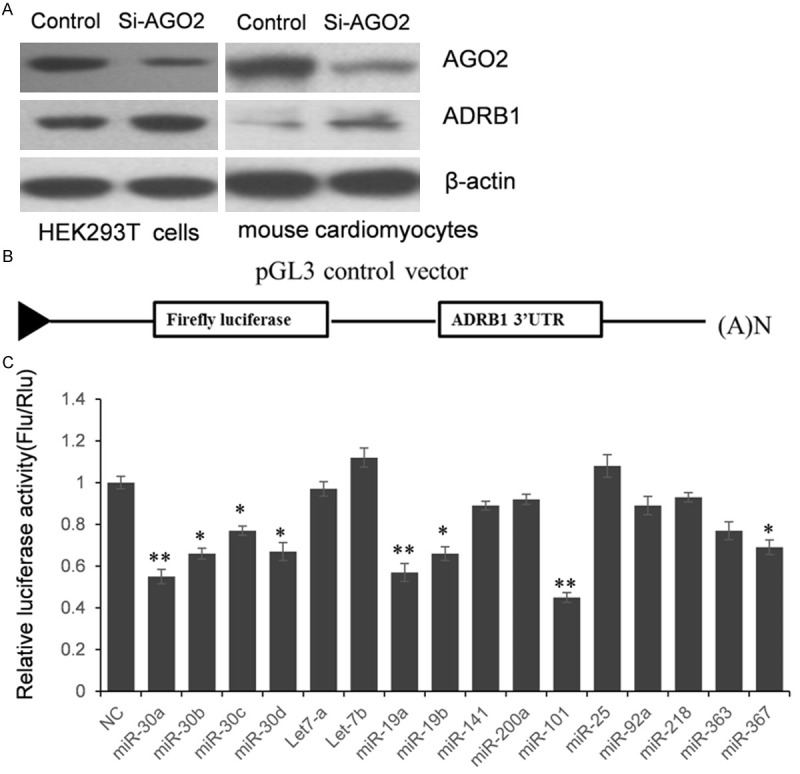
The expression of ADRB1 was regulated by miRNAs. A. The expression of ADRB1 was up-regulated when AGO2 was knocked down, which means ADRB1 expression was repressed by endogenous microRNAs. B. The schematic diagram of constructing ADRB1 3’UTR reporter vector. C. Dual-luciferase assay to screen miRNAs which may target ADRB1 3’UTR. The results were analyzed by student t-test, and P<0.05 was considered statistically significant *P<0.05, compared with control; **P<0.01, compared with control.
miRNAs suppress post-transcriptional gene expression through targeting the 3’UTR of targeting genes. To identify which microRNA suppresses ADRB1 expression by targeting 3’UTR directly, we first constructed ADRB1 3’UTR luciferase reporter vector. A shown in the schematic diagram (Figure 1B), the full length of 1342 bp human ADRB1 3’UTR was cloned into the pGL3 control vector, downstreaming the coding region of firefly luciferase gene. According to the prediction of TargetScan (http://www.targetscan.org/), an online bioinformatics tool, sixteen candidate miRNAs were selected. HEK293T cells were co-transfected with pGL3-ADRB1 and miRNAs mimics or inhibitors (Figure 1C). 24 h after transfection, cells were lysed and luciferase activities were detected. As shown in Figure 1C, compared with the miRNA control, the luciferase activity was significantly suppressed by miR-19a, miR-19b, miR-30a, miR-30b, miR-30c, miR-30d, miR-101 and miR-367. These results indicate that these eight miRNAs target the 3’-UTR of ADRB1, leading to the change of firefly luciferase translation.
In order to understand the clinical presentations of these miRNAs, we detected their expression in plasma samples of nine HF patients and paired controls. As shown in Figure 2, the expression of miR-19a has a significant up-regulation in HF patients compared with control (P=0.029).
Figure 2.
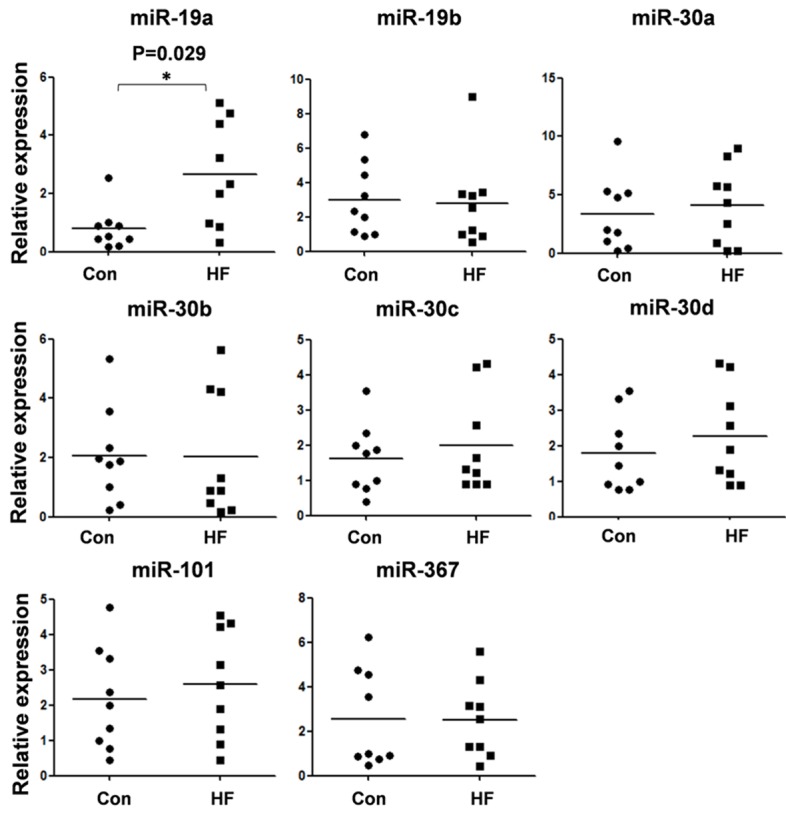
The expression of eight candidate miRNAs in HF plasma samples. The expression of candidate miRNAs in plasma samples from HF patients and non-failing controls were detected by quantitative RT-PCR. The results were analyzed by student t-test, and P<0.05 was considered statistically significant. The expression of miR-19a exhibited a significant up-regulation compared with control. *P<0.05, compared with control.
According to the prediction of TargetScan, three miR-19a target sites existed in the 3’UTR of ADRB1. We divided them into three independent segments and cloned into pGL3 vector separately, to identify the functional pattern of miR-19a. As shown in Figure 3A-C, miR-19a only inhibit the luciferase activity of the first vector which use the ADRB1 3’UTR first segment containing 143 bp of ADRB1 3’UTR. Furthermore, the luciferase activity was significantly up-regulated by the miR-19a inhibitor compared with the anti-miR control, about 33.3% (P<0.05). These results indicate that miR-19a targets the 3’-UTR of ADRB1, leading to the change of firefly luciferase translation, and the target site exist in the region between 602-745 bp of ADRB1 3’UTR.
Figure 3.
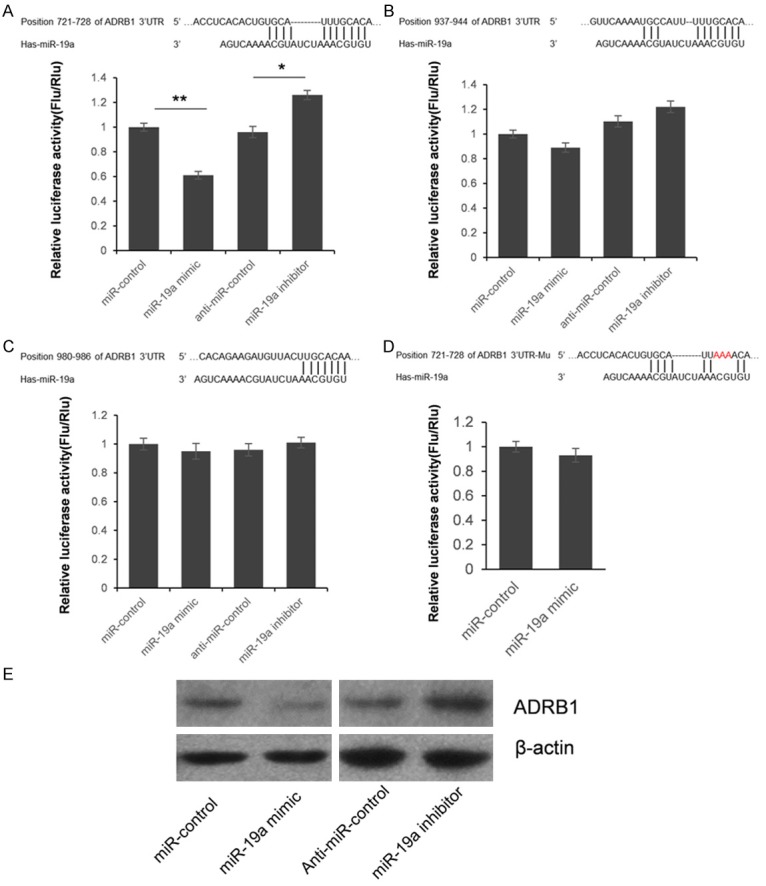
ADRB1 is a target gene of miR-19a. A-C. Since the 3’UTR of ADRB1 contains three putative miR-19a target sites, we divided them into three segments and subcloned into pGL3 vector independently. HEK293T cells were co-transfected with miRNA control, miR-19a mimic, anti-miR control or miR-19a inhibitor and pGL3-ADRB1 for dual-luciferase assay. PRL-TK containing Renilla luciferase was co-transfected for data normalization. D. M utation analysis of the miR-19a binding site. When 3 nucleotides of the binding site of miR-19a in the 3’-UTR of ADRB1 was mutated, the luciferase activity was not significantly changed by miR-19a mimic. E. ADRB1 protein level in miR-19a mimic or inhibitor-treated HEK293T cells was detected by western blot. The results were analyzed by student t-test, and P<0.05 was considered statistically significant. *P<0.05, compared with control. **P<0.01, compared with control.
Seed sequence mutation clone was also used to further confirm the binding site for miR-19a (Figure 3D). Putative miR-19a binding region in the 3’-UTR of ADRB1 with 3 mutant nucleotides was constructed and co-transfected with miR-19a mimic into HEK293T cells, with pRL-TK as transfection control. The histogram in Figure 2D showed that the enzyme activity was not significantly changed in cells transfected miR-19a mimics and miRNA control (P>0.05). These data indicate that miR-19a may suppress gene expression through binding to seed sequence at the 3’-UTR of ADRB1, and the target site is at the position 721-728 bp of ADRB1 3’UTR.
Although ADRB1 was identified as a target gene for miR-19a, it was unknown whether miR-19a could regulate endogenous ADRB1 expression. HEK293T cells were transfected with miR-19a mimics or inhibitor to see whether the dysregulation of miR-19a expression affected endogenous ADRB1 expression. Compared with corresponding control, the level of ADRB1 protein was significantly suppressed by miR-19a mimics and up-regulated by miR-19a inhibitor (Figure 3E). To further understand the clinical roles of overexpressed miR-19a, we detected the plasma BNP level which has been recognized to have a significant application value in the early diagnosis and assessment of severity, prognosis, and therapeutic outcome in patients with heart failure. As shown in Figure 4A, the plasma BNP level has a positive correlation between miR-19a (r=0.42, P=0.017). Meanwhile, we also detected the plasma cAMP level, which was found to have a negative correlation with cardiac function [11]. As shown in Figure 4B, a significant positive correlation (r=0.46, P=0.008) was constructed between plasma miR-19a and cAMP levels in 32 HF patients.
Figure 4.
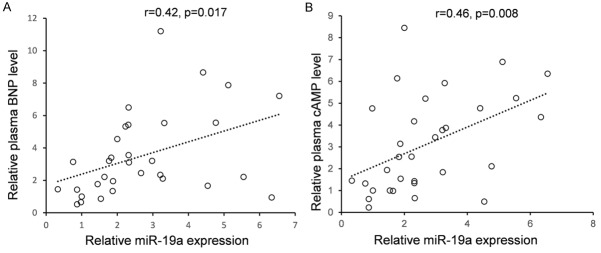
The correlation between the expression of miR-19a and the plasma BNP or cAMP level in HF patients. The plasma BNP and cAMP levels were detected by enzyme-linked immunosorbent assay (ELISA). The plasma miR-19a expression was detected by using stem-loop qRT-PCR.
Discussion
β1-AR predominantly exists in the heart and β1-AR reduction is closely related to severity of HF. In this study, our research focused on the miRNAs that may repress β1-AR directly aim to find out new markers and target molecules for HF. The AGO2 knock down experiment confirmed that endogenous ADRB1 expression is suppressed by miRNAs. To further identify which miRNA suppress ADRB1 expression directly, we construct the ADRB1 3’UTR reporter plasmid and selected sixteen candidate miRNAs. Confirmed by dual-luciferase assay and western blot, we found that miR-19a suppressed ADRB1 expression by directly targeting 3’UTR. Further expressions detection the levels of miR-19a, BNP and cAMP in 32 plasma samples of HF patients helped us to construct positive correlations between the expression levels of miR-19a and BNP or miR-19a and cAMP, hints miR-19a may be used as a biomarker in HF patients indicating cardiac function.
Normal human heart is composed of predominately β1-AR that selectively decrease during HF compared to β2-AR and the severity of the loss of β1-AR also correlates with the severity of the HF [12,13]. Recently, there are reports indicate that β1-AR participate the miRNAs processing progress [14]. However, whether the expression of β1-AR is regulated by miRNA is still unknown. Herein, we first confirmed that the expression of ADRB1 is regulated by miRNAs in HEK293T cells and mouse cardiomyocytes. Consequently, we identified that miR-19a, which had an up-regulated expression in HF patients, suppressed ADRB1 expression by directly targeting 3’UTR. Finally, we found an inverse correlation was existed between miR-19a and BNP or cAMP level in 32 HF patients. To our knowledge, this is the first report that ADRB1 expression is suppressed by miRNA and helping shed light on the mechanism of ADRB1 reduction in HF patients.
In conclusion, this study confirmed miR-19a suppressed ADRB1 expression by directly targeting 3’UTR of ADRB1 and found an positive correlation between miR-19a and BNP or cAMP level in HF patients’ plasma, which may contributes to fully understand the HF pathogenesis and develops new diagnostic method for HF.
Disclosure of conflict of interest
None.
References
- 1.Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Rosa S, Curcio A, Indolfi C. Emerging role of micrornas in cardiovascular diseases. Circ J. 2004;78:567–575. doi: 10.1253/circj.cj-14-0086. [DOI] [PubMed] [Google Scholar]
- 3.Corsten MF, Papageorgiou A, Verhesen W, Carai P, Lindow M, Obad S, Summer G, Coort SL, Hazebroek M, van Leeuwen R, Gijbels MJ, Wijnands E, Biessen EA, De Winther MP, Stassen FR, Carmeliet P, Kauppinen S, Schroen B, Heymans S. MicroRNA profiling identifies microRNA-155 as an adverse mediator of cardiac injury and dysfunction during acute viral myocarditis. Circ Res. 2012;111:415–425. doi: 10.1161/CIRCRESAHA.112.267443. [DOI] [PubMed] [Google Scholar]
- 4.Maes OC, Chertkow HM, Wang E, Schipper HM. MicroRNA: Implications for Alzheimer Disease and other Human CNS Disorders. Curr Genomics. 2009;10:154–168. doi: 10.2174/138920209788185252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu J, Li Y, Wang F, Wang X, Cheng B, Ye F, Xie X, Zhou C, Lu W. Suppressed miR-424 expression via upregulation of target gene Chk1 contributes to the progression of cervical cancer. Oncogene. 2013;32:976–987. doi: 10.1038/onc.2012.121. [DOI] [PubMed] [Google Scholar]
- 6.Farazi TA, Hoell JI, Morozov P, Tuschl T. MicroRNAs in human cancer. Adv Exp Med Biol. 2013;774:1–20. doi: 10.1007/978-94-007-5590-1_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Rooij E, Sutherland LB, Liu N, Williams AH, McAnally J, Gerard RD, Richardson JA, Olson EN. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc Natl Acad Sci U S A. 2006;103:18255–18260. doi: 10.1073/pnas.0608791103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Rooij E, Sutherland LB, Qi X, Richardson JA, Hill J, Olson EN. Control of stress-dependent cardiac growth and gene expression by a microRNA. Science. 2007;316:575–579. doi: 10.1126/science.1139089. [DOI] [PubMed] [Google Scholar]
- 9.Care A, Catalucci D, Felicetti F, Bonci D, Addario A, Gallo P, Bang ML, Segnalini P, Gu Y, Dalton ND, Elia L, Latronico MV, HɈydal M, Autore C, Russo MA, Dorn GW 2nd, Ellingsen O, Ruiz-Lozano P, Peterson KL, Croce CM, Peschle C, Condorelli G. MicroRNA-133 controls cardiac hypertrophy. Nat Med. 2007;13:613–618. doi: 10.1038/nm1582. [DOI] [PubMed] [Google Scholar]
- 10.Tijsen AJ, Pinto YM, Creemers EE. Circulating microRNAs as diagnostic biomarkers for cardiovascular diseases. Am J Physiol Heart Circ Physiol. 2012;303:H1085–1095. doi: 10.1152/ajpheart.00191.2012. [DOI] [PubMed] [Google Scholar]
- 11.Francis GS, Cohn JN, Johnson G, Rector TS, Goldman S, Simon A. Plasma norepinephrine, plasma renin activity, and congestive heart failure. Relations to survival and the effects of therapy in V-HeFT II. The V-HeFT VA Cooperative Studies Group. Circulation. 1993;87:VI40–48. [PubMed] [Google Scholar]
- 12.Bristow MR, Ginsburg R, Umans V, Fowler M, Minobe W, Rasmussen R, Zera P, Menlove R, Shah P, Jamieson S, et al. Beta 1- and beta 2-adrenergic-receptor subpopulations in nonfailing and failing human ventricular myocardium: coupling of both receptor subtypes to muscle contraction and selective beta 1-receptor down-regulation in heart failure. Circ Res. 1986;59:297–309. doi: 10.1161/01.res.59.3.297. [DOI] [PubMed] [Google Scholar]
- 13.Iwaoka M, Obata JE, Abe M, Nakamura T, Kitta Y, Kodama Y, Kawabata K, Takano H, Fujioka D, Saito Y, Kobayashi T, Hasebe H, Kugiyama K. Association of low serum levels of apolipoprotein A-I with adverse outcomes in patients with nonischemic heart failure. J Card Fail. 2007;13:247–253. doi: 10.1016/j.cardfail.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 14.Kim IM, Wang Y, Park KM, Tang Y, Teoh JP, Vinson J, Traynham CJ, Pironti G, Mao L, Su H, Johnson JA, Koch WJ, Rockman HA. beta-arrestin1-biased beta1-adrenergic receptor signaling regulates microRNA processing. Circ Res. 2014;114:833–844. doi: 10.1161/CIRCRESAHA.114.302766. [DOI] [PMC free article] [PubMed] [Google Scholar]


