Abstract
Background: Chemokine receptor 5 (CCR5) is one of the pro-inflammatory G protein coupled receptors. Many studies have accessed the association between CCR5 gene polymorphism and atherosclerotic disease. However, the results are conflicting and inconclusive. The aim of this study was to evaluate the association more precisely. Research Design and Methods: Trials were retrieved through Pubmed, Embase, Medline, China National Knowledge Infrastructure, Web of Science, and Cochrane database without restrictions on language. The pooled odds ratio (OR) and 95% confidence interval (CI) were used to describe the strength of association with atherosclerotic disease. The subgroup analysis was used to explore the heterogeneity bias among studies. Results: Data were obtained from 13 case-control studies that included 5321 patients with atherosclerotic disease and 4283 control subjects. In the overall analysis, the CCR5-delta32 (Δ32) genetic variants was not associated with increased the risk of atherosclerotic disease (dominant model: OR = 0.93, 95% CI = 0.69-1.24, I2 = 77%, P = 0.60; recessive model: OR = 1.01, 95% CI = 0.61-1.65, I2 = 0%, P = 0.98), even after stratification for the status of CCR5-delta32 allele. However, in subgroup analysis, the association was significant for Asians population (OR: 2.29, 95% CI: 1.44-3.64, P = 0.0004). Conclusions: Our studies add to the evidence that CCR5 Δ32-positive genotype (Δ32/Δ32 or wt/Δ32) increases the risk of atherosclerotic disease in Asian population. Ethnicity difference might contribute to the inconsistency in isolated studies.
Keywords: CCR5, gene polymorphism, atherosclerosis, meta-analysis
Introduction
Atherosclerosis (AS) causes high mortality and morbidity worldwide [1]. The risk factors of AS include obesity, high blood pressure, high cholesterol and genetic factors. Losing weight, antihypertensive and Lipid lowering treatments may prevent the progression of AS in some cases, however, many patients developed AS even their weight, blood pressure and blood lipid reached normal levels [2,3]. These patients may have genetic risk factors associated with AS [2].
It is widely accepted that inflammatory response is a major risk factor for atherosclerotic diseases [4]. CCR5 is one of the pro-inflammatory G protein coupled receptors, and predominantly expressed on the surface of leukocytes, monocytes/macrophages, and endothelial cells [3-6]. CCR5 expression is up-regulated in atherosclerotic plaques during the process of AS, and its increasing expression is associated with the recruitment of leukocytes to atherosclerotic plaques [7]. Given the crucial role of CCR5 in the lymphocytic infiltration and plaques formation, the mutations in CCR5 may play a significant role in the progression of AS.
A number of molecular and epidemiological studies have evaluated the association between the CCR5 gene variants and the risk of AS [8]. The most common gene polymorphism is CCR5Δ32 variant [9-11]. However, studies concerning CCR5 Δ32 variant and AS risk were controversial. Several studies have reported that CCR5 Δ32 genes variants may have a protective role for AS [9-12], genetic inactivation of CCR5 was associated with the reduction of pro-atherogenic cytokines and the accumulation of monocytes/macrophages in atherosclerotic plaques [9,12]. However, these findings have not been verified in other studies [13-18]. On the contrary, some studies found that CCR5Δ32 polymorphism increased the risk of AS [19-21]. The primary aim of our study was to derive a more precise evaluation of the associations between the CCR5 Δ32 genes variants and the risk of AS. The secondary analysis was to identify factors that might affect the association strength between the CCR5 Δ32 gene polymorphism and the risk of AS.
Methods
Search strategy
We searched Pubmed, Embase, Medline, China National Knowledge Infrastructure (CNKI), Web of Science, Cochrane database and the references list of relevant studies. We used the terms “CCR5” in combination with “myocardial infarction”, “coronary artery disease”, “coronary heart disease”, “atherosclerosis”, “stroke”, “ischemic cardiovascular disease”, “ischemic cardiovascular events”, “ischemic cerebrovascular events”, “cerebrovascular disease”, “cerebral infarction”, “cerebral ischemia”, “brain infarction”, “carotid artery stenosis”, “transient ischemic attack”, “peripheral arterial disease”, “peripheral artery occlusive disease”, “aortic aneurysm” “renal artery stenosis”, and “genetic variant” or “polymorphism”, respectively. There was no restriction on language or whether the articles had been published.
Inclusion criteria and information extracted
Eligible articles met the following criteria: (1) Case-control studies; (2) Prospective cohort studies; (3) Studies evaluated the association between the CCR5 Δ32 polymorphism and AS; (4) Studies with sufficient data for calculating ORs and 95% CIs. Exclusion criteria included: (1) Patients with heart transplant; (2) Animal studies.
Data extraction
Two investigators (Z.Z. and L.L.) independently extracted data according to the pre-specified inclusion criteria. The data extracted from articles including the name of first author, year of publication, ethnic origin, numbers of case and control subjects, mean ages, source of controls, methods for assessment of study endpoints, gender component in cases/controls, and Hardy-Weinberg equilibrium in case and control subjects. If more than one study from the same author were available, only one publication with largest sample size and most complete data was included.
Statistical analysis
Hardy-weinberg equilibrium (HWE) was tested using the chi-square test. The strength of CCR5 Δ32 genetic variants and AS association were evaluated by OR and 95% CIs. Heterogeneity across studies was tested using the Cochran’s Q statistic and the I2 statistic. If the association exhibited heterogeneity (I2 > 25%), the random effects models were merged. Otherwise, a fixed effects model was used [22]. Sensitivity analysis was performed to explore the source of heterogeneity. The association between gene mutation and AS was analyzed by the following methods: CCR5-Δ32 (Δ32/Δ32 + wt/Δ32 vs wt/wt, Δ32/Δ32 vs wt/Δ32 + wt/wt). The risk frequency of Δ32 allele (CCR5-Δ32) was also calculated in these case-control groups. Stratified analysis were done for ethnicity (Caucasians vs Asians), type of atherosclerotic disease (coronary artery disease and ischemic stroke), mean age level, status of HWE (yes or no) and source of controls (population-based studies and hospital-based studies). Publication bias was tested with funnel plot and fail-safe number (Nfs). If the calculated Nfs value was smaller than the number of studies, there might be a risk of publication bias. The formula Nfs 0.05 = (ΣZ/1.64) 2 - k (k is the number of articles included in this research). Values of P < 0.05 was considered as significant differences. All data were analyzed by Stata 10.0 (Stata Corp, College Station, TX, USA).
Results
Studies characteristics
The study met the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses). The details of the literature search were presented in Figure 1. The initial electronic and manual searches yielded 982 potential literature citations, of which 935 were excluded after scanning the titles and abstracts. Two authors searched full articles for the remaining 47 citations, and excluded additional 34 studies for various reasons as shown in Figure 1. Finally 13 articles [9-21] were included in this meta-analysis. All studies were full papers published in English. Among them, 9 studies [10-18] investigated Caucasians population, 3 [19-21] for Asians, and 1 [9] for Africans. The most commonly atherosclerotic disease included in this study was myocardial infection (MI). In addition, there were 2 studies [13,16] involving ischemic stroke (IS), 5 studies [11,15,17,18,20] involving coronary heart disease (CAD). For data synthesis, 11 studies [11-21] followed the HWE and 2 studies [9,10] deviated from the HWE. The main characteristics of the included studies were listed in Table 1.
Figure 1.
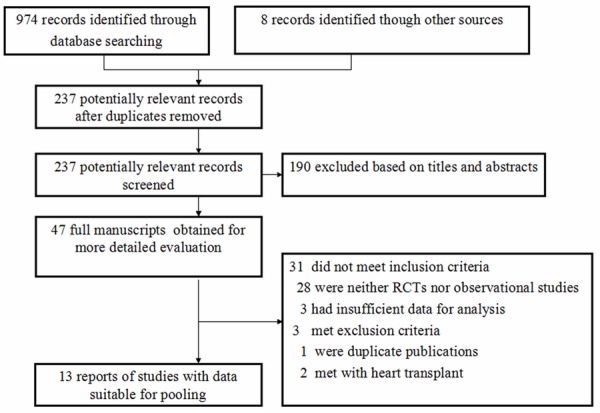
Flow chart of literature search for meta-analysis.
Table 1.
Characteristics of the association studies between CCR5-delta32 and the risk of atherosclerotic disease
| Author and year | Ethnicity | Subjects, n cases/controls | Mean age, y cases/controls | Source of controls | Disease | % male | HWE X2 P |
|---|---|---|---|---|---|---|---|
| Amani kallel etal. (2012) [9] | African | 290/282 | 53 ± 8/52 ± 9 | PB | MI | 100/100 | 14.41 |
| K. Nikolaos etal. (2009) [13] | Caucasian | 478/803 | 68 ± 9/58 ± 6 | HB | IS | 69.6/56 | 0.025 |
| Giorgio Ghilardi etal. (2008) [16] | Caucasian | 112/282 | 68 ± 2/65 ± 7 | HB | IS | 66.9/65.2 | 1.476 |
| Neha Singh etal. (2012) [19] | Asian | 230/300 | 49.9/44.9 | PB | MI | 85.0/85.2 | 0.021 |
| S. Sharda etal. (2008) [20] | Asian | 197/199 | 47.3/44.5 | PB | CAD | 84.2/81.4 | 0.011 |
| Stavros Apostolakis etal. (2007) [18] | Caucasian | 210/165 | 63.7/63.2 | HB | CAD | 77.6/76.4 | 0.196 |
| Jennifer K. Pai etal. (2006) [17] | Caucasian | 232/459 | 60.6/60.3 | HB | CAD | 0/0 | 0.095 |
| J. Petrkova etal. (2005) [14] | Caucasian | 80/247 | < 55/NA | PB | MI | 100/100 | 0.111 |
| Eleonora Simeoni etal. (2004) [15] | Caucasian | 2681/528 | 63.8/56.9 | HB | CAD | 73.9/51.3 | 0.105 |
| Csaba Szalai etal. (2001) [11] | Caucasian | 318/320 | 57.6/58.9 | HB | CAD | 76.1/75.0 | 0.118 |
| González P etal. (2001) [12] | Caucasian | 214/360 | (45/65)/42 | HB | MI | 0.68/0.63 | 0.079 |
| Zeynep Ermis Karaali etal. (2010) [21] | Asian | 146/202 | 56.38/54.2 | PB | MI | 60.4/59.4 | 0.081 |
| Balistreri CR etal. (2008) [10] | Caucasian | 133/136 | < 45/< 45 | NA/NA | MI | NA/NA | 0.073 |
Abbreviations: PB, population-based; HB, hospital-based; NA = not available; Data are mean ± SD; CAD, coronary artery disease; IS, ischemic stroke; MI, myocardial infarction.
Main meta-analysis results
Under the random-effects model, no significant correlation was found between the CCR5Δ32 polymorphism and the risk of AS (dominant model: pooled OR = 0.93, 95% CI = 0.69-1.24, and P = 0.60; recessive model: OR = 1.01, 95% CI = 0.61-1.65, and P = 0.98), neither in the subtype analysis of atherosclerotic diseases (CAD: OR = 1.06, 95% CI = 0.89-1.26, and P = 0.51; MI: OR = 0.77, 95% CI = 0.37-1.61, and P = 0.48; IS: OR = 0.91, 95% CI = 0.71-1.17, and P = 0.48) and source of controls (population-based group: OR = 1.34, 95% CI = 0.57-3.18, and P = 0.50; hospital-based group: OR = 0.99, 95% CI = 0.86-1.13, and P = 0.85). Nevertheless, the subtype analysis of ethnic showed a significant association between CCR5Δ32 polymorphism and increased AS risk in Asians population (OR = 2.29, 95% CI = 1.44-3.64, and P = 0.0004), suggesting that CCR5 Δ32-positive genotype (Δ32/Δ32 or wt/Δ32) is an independent risk factor for AS in Asian population (Figure 2).
Figure 2.
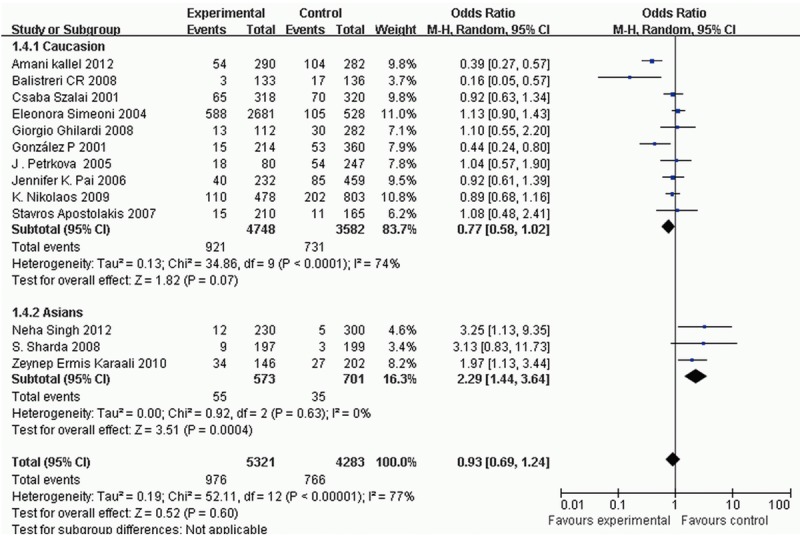
Forest plots of the meta-analysis for CCR5 Δ32 polymorphism associated with ethnic.
Sensitivity/subgroup analysis
Since a significant heterogeneity was found in the dominant model (I2 = 77%), sensitivity analysis was conducted and found that the heterogeneity arose from the trial of Kallel A [9], Balistreri CR [10], González P [12]. Simeoni E [15], and Karaali ZE [21] (Figure 3). The genotype frequencies of CCR5 Δ32 in the control group deviated from HWE (P < 0.05) in studies by Kallel A [9] and Balistreri CR [10]. After excluding two studies, the heterogeneity was removed (I2 = 48%) without any influence on the results of meta-analysis (OR = 1.05, 95% CI = 0.92-1.19). The trails published in González P [12]. Simeoni E [15], and Karaali ZE [21] were available in the stratification analysis by ethnic and type of atherosclerotic diseases. Thus, the ethnic and type of atherosclerotic diseases were selected for subgroup analysis. The results indicated that both of them were correlated with heterogeneity. After excluding Caucasians studies, the heterogeneity disappeared (I2 = 0%) and the result indicated that CCR5 Δ32 genotype was significantly associated with AS in Asians (OR = 2.29, 95% CI = 1.44-3.64, and P = 0.02). No significant association of CCR5 Δ32 genotype and the risk of AS after adjustment for type of atherosclerotic diseases in subgroup analysis, though the heterogeneity disappeared (I2 = 0%). Results of subgroup analyses for the estimates are in Table 2.
Figure 3.
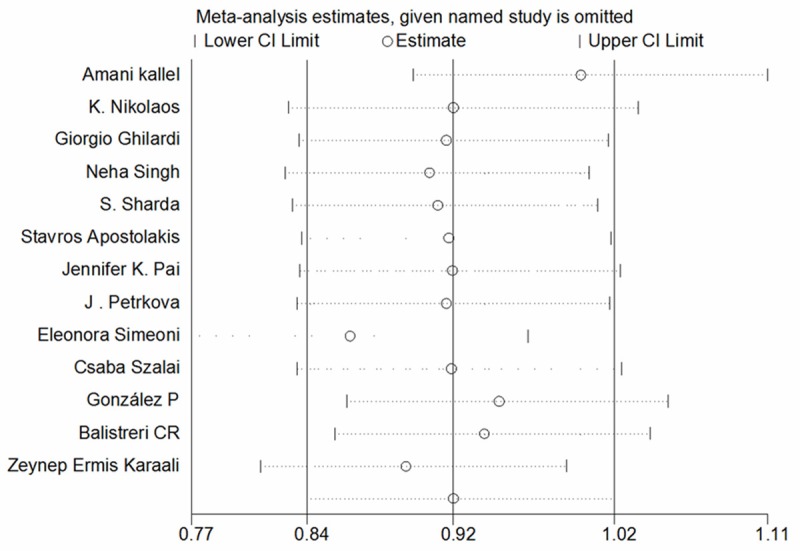
The sensitivity analysis for the association of CCR5 Δ32 polymorphism with risk of atherosclerotic disease.
Table 2.
Meta-analysis of CCR5 polymorphism and the risk of atherosclerotic disease with a dominant model (Δ32/Δ32 or wt/Δ32)
| Category | N | Subjects, n cases/controls | Heterogeneity | OR (95% CI) | Z test | |
|---|---|---|---|---|---|---|
|
| ||||||
| Ph | I2 (%) | |||||
| Overall | 13 | 5321/4283 | < 0.00001 | 77 | 0.93 (0.69- 1.24) | Z = 0.52; PZ = 0.60 |
| Adjustment by ethnicity | ||||||
| Caucasian | 10 | 4748/3582 | < 0.0001 | 74 | 0.77 (0.58-1.02) | Z = 1.82; PZ = 0.07 |
| Asian | 3 | 573/701 | 0.63 | 0 | 2.29 (1.44-3.64)§ | Z = 3.51; PZ = 0.0004 |
| Adjustment by subtypes of AS | ||||||
| CAD | 5 | 3638/1671 | 0.42 | 0 | 1.06 (0.89-1.26)§ | Z = 0.66; PZ = 0.51 |
| MI | 6 | 1093/1527 | < 0.0001 | 87 | 0.77 (0.37-1.61) | Z = 0.70; PZ = 0.48 |
| IS | 2 | 590/1085 | 0.57 | 0 | 0.91 (0.71-1.17)§ | Z = 0.71; PZ = 0.48 |
| Mean age level | ||||||
| > 50 | 8 | 4467/3014 | 0.0003 | 75 | 0.94 (0.70-1.25) | Z = 0.44; PZ = 0.66 |
| < 50 | 5 | 996/1163 | 0.004 | 74 | 1.05 (0.50-2.20) | Z = 0.14; PZ = 0.89 |
| Status of HWE | ||||||
| yes | 11 | 4994/3838 | 0.16 | 0 | 1.05 (0.92-1.19)§ | Z = 0.73; PZ = 0.46 |
| no | 2 | 423/418 | 0.32 | 0 | 0.37 (0.25-0.53)§ | Z = 5.42; PZ < 0.00001 |
| Source of controls | ||||||
| PB | 5 | 943/1203 | < 0.00001 | 87 | 1.34 (0.57-3.18) | Z = 0.67; PZ = 0.50 |
| HB | 7 | 4341/2917 | 0.75 | 0 | 0.99 (0.86-1.13)§ | Z = 0.19; PZ = 0.85 |
Abbreviations: AS, atherosclerosis; CAD, coronary artery disease; MI, myocardial infarction; IS, ischemic stroke; HWE, hardy-weinberg equilibrium; PB, population-based; HB, hospital-based; N, number of invealed studies; Ph, P values for heterogeneity of Q test;
Fixed-effects model;
PZ < 0.05, indicate significant association.
Publication bias
Publication biases were examined for all the outcomes of the included studies. No significant publication bias was found among these studies in funnel plot (Figure 4). We also calculated the Nfs0.05 for CCR5-Δ32, the Nfs0.05 value was greater than the number of studies included in our meta-analysis.
Figure 4.
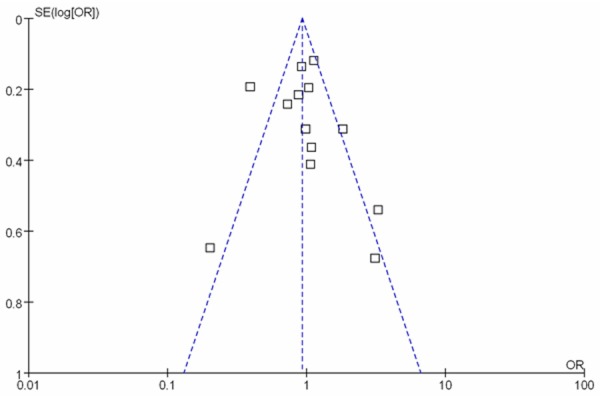
Funnel plot of publication bias for the association of CCR5 Δ32 polymorphism with risk of atherosclerotic disease.
Discussion
To the best of our knowledge, this is the first systematic review investigating the association between the CCR5 polymorphism and the risk of AS. Subtype analysis suggested that ethnic difference might contribute to the inconsistency in isolated studies and CCR5 Δ32-positive genotype (Δ32/Δ32 or wt/Δ32) was associated with increased risk of AS in Asians. Ethnic differences may partly attribute to the interaction between the genetic and geographical environment [23]. Since AS has a complex etiology generated by the combined effects of environmental and genetic factors [24-27], ethnic populations have different frequencies of alleles and genetic backgrounds [8], which may contribute to the risk of AS. Moreover, the ethnic difference might correlate with the curative activities, such as diet, lifestyle, and drug administration [27]. As only a few studies are available, we were not able to explore whether curative activities was the causes of heterogeneity.
Age of onset may be an important factor which leads to the divergent genetic risk of different ethnic [8]. Thus, we performed the subtype analysis by the age of onset (> 50, < 50, respectively) and found that the heterogeneity of CCR5 Δ32 was removed in the group with a mean age over 50 years. The results suggested that age of onset was the source of heterogeneity in CCR5 gene polymorphism. Given the limited numbers of studies are available, the result has to be further confirmed by large population studies.
The quality of articles may affect the overall results of the analysis [27]. Thus, sensitivity analyses was performed by status of HWE. Two articles deviated from the HWE, which evaluated the risk of CCR5 Δ32 genes variants and MI risk. After excluded these studies, the heterogeneity was removed (I2 = 48%), suggesting that the status of HWE may affect the heterogeneity of the overall research. In addition, this study only focused on atherosclerotic disease and did not evaluate other disease for CCR5 polymorphism, such as diabetic nephropathy, multiple sclerosis, and atopic asthma [28-31]. The potential role of CCR5 SNPs may be masked by other gene-gene or gene-environment interactions.
AS has a complex etiology generated by multiple effects of environmental as well as genetic risk factors. A comprehensive research concerning the multiple loci will help us to find a novel biological insight and improve measures of individual aetiological processes of AS [32]. In current study, we have shown that the CCR5 Δ32 gene polymorphism is a significant susceptibility factor for AS in Asians, which implies that Asians lacking CCR5 as a result of homozygous inheritance of the complete loss-of-function allele Δ32 may have increased the risk of AS. Thus, the gene polymorphism of CCR5 may present a new target for the early detection and preventive treatment of AS in Asian population.
Some limitations in this study should be noted. The sample size of CCR5 polymorphism studies is relatively small and additional studies are needed to confirm this conclusion. In addition, we could not retrieve information for various confounding factors which are considered as effective modulators for the development of AS.
In conclusion, our meta-analysis indicated that CCR5 Δ32-positive genotype (Δ32/Δ32 or wt/Δ32) increased the risk of AS in Asian population. This genetic variant may serve as a diagnostic indicator for an individual’s susceptibility to AS in Asians.
Acknowledgements
This work was funded by National Natural Science Foundation of China Grants (81070637, 81273082), Shandong Provincial Natural Science Foundation of China Grants (No. Y2006C76, Y2008C73, ZR2010HM044), Shandong Provincial Science & Technology Development Program, China (2009GGB14001, 2010GSF10228, 2012GGH11862, 2014GSF118118), Fund for the Returned Oversea Scholars Sponsored by National Ministry of Personnel (2008, No. 102), Grant for Excellent Young, and Middle-aged Scientists of Shandong Province (No. 2004BS02016). We are grateful for the support by Shandong Taishan Scholarship (Ju Liu).
Disclosure of conflict of interest
None.
References
- 1.Faxon DP, Creager MA, Smith SC Jr, Pasternak RC, Olin JW, Bettmann MA, Criqui MH, Milani RV, Loscalzo J, Kaufman JA, Jones DW, Pearce WH American Heart Association. Atherosclerotic Vascular Disease Conference: Executive summary: Atherosclerotic Vascular Disease Conference proceeding for healthcare professionals from a special writing group of the American Heart Association. Circulation. 2004;109:2595–2604. doi: 10.1161/01.CIR.0000128517.52533.DB. [DOI] [PubMed] [Google Scholar]
- 2.Chamberlain JC, Galton DJ. Genetic susceptibility to atherosclerosis. Br Med Bull. 1990;46:917–940. doi: 10.1093/oxfordjournals.bmb.a072446. [DOI] [PubMed] [Google Scholar]
- 3.Estrada-Velasco BI, Cruz M, Madrid-Marina V, Martinez-Nava GA, Gomez-Zamudio J, Burguete-Garcia AI. IRS1, TCF7L2, ADRB1, PPARG, and HHEX polymorphisms associated with atherogenic risk in Mexican population. Biomed Res Int. 2013;2013:394523. doi: 10.1155/2013/394523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stampfer MJ, Sacks FM, Salvini S, Willett WC, Hennekens CH. A prospective study of cholesterol, apolipoproteins, and the risk of myocardial infarction. N Engl J Med. 1991;325:373–381. doi: 10.1056/NEJM199108083250601. [DOI] [PubMed] [Google Scholar]
- 5.Jones KL, Maguire JJ, Davenport AP. Chemokine receptor CCR5: from AIDS to atherosclerosis. Br J Pharmacol. 2011;162:1453–1469. doi: 10.1111/j.1476-5381.2010.01147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zernecke A, Shagdarsuren E, Weber C. Chemokines in atherosclerosis: an update. Arterioscler Thromb Vasc Biol. 2008;28:1897–1908. doi: 10.1161/ATVBAHA.107.161174. [DOI] [PubMed] [Google Scholar]
- 7.Bot I, Daissormont IT, Zernecke A, van Puijvelde GH, Kramp B, de Jager SC, Sluimer JC, Manca M, Herias V, Westra MM, Bot M, van Santbrink PJ, van Berkel TJ, Su L, Skjelland M, Gullestad L, Kuiper J, Halvorsen B, Aukrust P, Koenen RR, Weber C, Biessen EA. CXCR4 blockade induces atherosclerosis by affecting neutrophil function. J Mol Cell Cardiol. 2014;74:44–52. doi: 10.1016/j.yjmcc.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang L, Hu X, Zhang S, Xu X, Wang J. Association of the CCR5Delta32 polymorphism and its ligand RANTES-403G/A polymorphism with coronary artery disease: a meta-analysis. Thromb Res. 2013;131:e77–84. doi: 10.1016/j.thromres.2012.07.024. [DOI] [PubMed] [Google Scholar]
- 9.Kallel A, Abdessalem S, Sediri Y, Mourali MS, Feki M, Mechmeche R, Jemaa R, Kaabachi N. Polymorphisms in the CC-chemokine receptor-2 (CCR2) and -5 (CCR5) genes and risk of myocardial infarction among Tunisian male patients. Clin Biochem. 2012;45:420–424. doi: 10.1016/j.clinbiochem.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Balistreri CR, Candore G, Caruso M, Incalcaterra E, Franceschi C, Caruso C. Role of polymorphisms of CC-chemokine receptor-5 gene in acute myocardial infarction and biological implications for longevity. Haematologica. 2008;93:637–638. doi: 10.3324/haematol.12239. [DOI] [PubMed] [Google Scholar]
- 11.Szalai C, Duba J, Prohaszka Z, Kalina A, Szabo T, Nagy B, Horvath L, Csaszar A. Involvement of polymorphisms in the chemokine system in the susceptibility for coronary artery disease (CAD). Coincidence of elevated Lp(a) and MCP-1 -2518 G/G genotype in CAD patients. Atherosclerosis. 2001;158:233–239. doi: 10.1016/s0021-9150(01)00423-3. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez P, Alvarez R, Batalla A, Reguero JR, Alvarez V, Astudillo A, Cubero GI, Cortina A, Coto E. Genetic variation at the chemokine receptors CCR5/CCR2 in myocardial infarction. Genes Immun. 2001;2:191–195. doi: 10.1038/sj.gene.6363760. [DOI] [PubMed] [Google Scholar]
- 13.Kostulas N, Markaki I, Kostulas V, Hillert J, Kostulas K. Common CCR 5 polymorphism in stroke: the CCR 5 delta32 polymorphism differentiates cardioembolism from other aetiologies of ischaemic cerebrovascular diseases. Scand J Immunol. 2009;70:475–480. doi: 10.1111/j.1365-3083.2009.02323.x. [DOI] [PubMed] [Google Scholar]
- 14.Petrkova J, Cermakova Z, Lukl J, Petrek M. CC chemokine receptor 5 (CCR5) deletion polymorphism does not protect Czech males against early myocardial infarction. J Intern Med. 2005;257:564–566. doi: 10.1111/j.1365-2796.2005.01491.x. [DOI] [PubMed] [Google Scholar]
- 15.Simeoni E, Winkelmann BR, Hoffmann MM, Fleury S, Ruiz J, Kappenberger L, Marz W, Vassalli G. Association of RANTES G-403A gene polymorphism with increased risk of coronary arteriosclerosis. Eur Heart J. 2004;25:1438–1446. doi: 10.1016/j.ehj.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Ghilardi G, Biondi ML, Turri O, Pateri F, d’Eril GM, Scorza R. Genetic control of chemokines in severe human internal carotid artery stenosis. Cytokine. 2008;41:24–28. doi: 10.1016/j.cyto.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 17.Pai JK, Kraft P, Cannuscio CC, Manson JE, Rexrode KM, Albert CM, Hunter D, Rimm EB. Polymorphisms in the CC-chemokine receptor-2 (CCR2) and -5 (CCR5) genes and risk of coronary heart disease among US women. Atherosclerosis. 2006;186:132–139. doi: 10.1016/j.atherosclerosis.2005.06.041. [DOI] [PubMed] [Google Scholar]
- 18.Apostolakis S, Baritaki S, Kochiadakis GE, Igoumenidis NE, Panutsopulos D, Spandidos DA. Effects of polymorphisms in chemokine ligands and receptors on susceptibility to coronary artery disease. Thromb Res. 2007;119:63–71. doi: 10.1016/j.thromres.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 19.Singh N, Sinha N, Kumar S, Pandey CM, Agrawal S. Polymorphism in chemokine receptor genes and risk of acute myocardial infarction in North Indian population. Mol Biol Rep. 2012;39:2753–2759. doi: 10.1007/s11033-011-1031-8. [DOI] [PubMed] [Google Scholar]
- 20.Sharda S, Gilmour A, Harris V, Singh VP, Sinha N, Tewari S, Ramesh V, Agrawal S, Mastana S. Chemokine receptor 5 (CCR5) deletion polymorphism in North Indian patients with coronary artery disease. Int J Cardiol. 2008;124:254–258. doi: 10.1016/j.ijcard.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 21.Karaali ZE, Sozen S, Yurdum M, Cacina C, Toptas B, Gok O, Agachan B. Effect of genetic variants of chemokine receptors on the development of myocardial infarction in Turkish population. Mol Biol Rep. 2010;37:3615–3619. doi: 10.1007/s11033-010-0011-8. [DOI] [PubMed] [Google Scholar]
- 22.Ye H, Zhao Q, Huang Y, Wang L, Liu H, Wang C, Dai D, Xu L, Ye M, Duan S. Meta-analysis of low density lipoprotein receptor (LDLR) rs2228671 polymorphism and coronary heart disease. Biomed Res Int. 2014;2014:564940. doi: 10.1155/2014/564940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hao PP, Chen YG, Wang JL, Wang XL, Zhang Y. Meta-analysis of aldehyde dehydrogenase 2 gene polymorphism and Alzheimer’s disease in East Asians. Can J Neurol Sci. 2011;38:500–506. doi: 10.1017/s0317167100011938. [DOI] [PubMed] [Google Scholar]
- 24.Cullen P, Funke H, Schulte H, Assmann G. Lipoproteins and cardiovascular risk-from genetics to CHD prevention. J Atheroscler Thromb. 1997;4:51–58. doi: 10.5551/jat1994.4.51. [DOI] [PubMed] [Google Scholar]
- 25.Kotake H. Association of cholesteryl ester transfer protein-TaqIB polymorphyisms with HDL cholesterol levels and the risk of coronary artery disease. J Atheroscler Thromb. 2007;14:152–153. doi: 10.5551/jat.14.152. [DOI] [PubMed] [Google Scholar]
- 26.Nohara A, Kobayashi J, Mabuchi H. Retinoid X receptor heterodimer variants and cardiovascular risk factors. J Atheroscler Thromb. 2009;16:303–318. doi: 10.5551/jat.no786. [DOI] [PubMed] [Google Scholar]
- 27.Zhang H, Zhu S, Chen J, Tang Y, Hu H, Mohan V, Venkatesan R, Wang J, Chen H. Peroxisome proliferator-activated receptor gamma polymorphism Pro12Ala Is associated with nephropathy in type 2 diabetes: evidence from meta-analysis of 18 studies. Diabetes Care. 2012;35:1388–1393. doi: 10.2337/dc11-2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kodama S, Saito K, Tanaka S, Horikawa C, Fujiwara K, Hirasawa R, Yachi Y, Sone Y, Tada Iida K, Shimano H, Ohashi Y, Yamada N, Sone H. Fasting and post-challenge glucose as quantitative cardiovascular risk factors: a meta-analysis. J Atheroscler Thromb. 2012;19:385–396. doi: 10.5551/jat.10975. [DOI] [PubMed] [Google Scholar]
- 29.Arababadi MK, Naghavi N, Hassanshahi G, Mahmoodi M. Is CCR5-Delta32 mutation associated with diabetic nephropathy in type 2 diabetes? Ann Saudi Med. 2009;29:413. doi: 10.4103/0256-4947.55177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song GG, Lee YH. A Meta-analysis of the relation between chemokine receptor 5 delta32 polymorphism and multiple sclerosis susceptibility. Immunol Invest. 2014;43:299–311. doi: 10.3109/08820139.2013.845204. [DOI] [PubMed] [Google Scholar]
- 31.Berce V, Repnik K, Potocnik U. Association of CCR5-delta32 mutation with reduced risk of nonatopic asthma in Slovenian children. J Asthma. 2008;45:780–784. doi: 10.1080/02770900802386024. [DOI] [PubMed] [Google Scholar]
- 32.Shanthanna H, Singh B, Guyatt G. A systematic review and meta-analysis of caudal block as compared to noncaudal regional techniques for inguinal surgeries in children. Biomed Res Int. 2014;2014:890626. doi: 10.1155/2014/890626. [DOI] [PMC free article] [PubMed] [Google Scholar]


