Abstract
Objective: Tenofovir disoproxil fumarate (TDF) and entecavir (ETV) have been accepted as the standard treatment drugs for hepatitis B virus (HBV) reactivation. We aim to compare the efficacy and safety of TDF and ETV initial treatment of chronic hepatitis B (CHB) patients. Methods: We retrospectively analyzed the efficacy and safety of TDF treatment on 33 CHB patients and of ETV treatment on 65 CHB patients by comparing the HBV DNA levels, HBV DNA undetectable rate, HBV DNA negative conversion multi-factor analysis, alanine amino transferase (ALT) normalization rate, and the adverse event incidence at weeks 4, 12, 24, 36, 48, 72 before and after treatment in each group. Results: The HBV DNA levels in the ETV group were significantly lower than that in the TDF group at week 4 (95.05 ± 39.49 versus 103.3 ± 80.25 U/L, P = 0.005). The differences in HBV DNA levels at the other times between these two treatment groups were not statistically significant. No significant differences were observed with HBV DNA undetectable rate and ALT normalization rate between the two groups (P = 0.114, 0.656, respectively). HBV DNA negativity multi-factor analysis showed that the differences in TDF and ETV treatment were not statistically significant (P = 0.116). Therefore, the proportion of Creatine Kinase (CK) levels that were 2 times over the upper limit of normal (2ULN) showed no significant differences in any time points between the two groups (P > 0.05). Conclusion: TDF and ETV treatment both exhibited rapid inhibiting effects on HBV DNA replication in the early phase of naïve CHB patients in Mainland China.
Keywords: TDF, ETV, chronic hepatitis B, treatment
Introduction
Chronic Hepatitis B (CHB) patients infected by Hepatitis B virus (HBV) resulted in a public health problem worldwide and the mortality rate of HBV infection is about 1 million worldwide [1]. It had been proven that more viral load of HBV DNA increases the risk of hepatitis B related liver cirrhosis and liver cancer [2]. At present, the primary focus of treatment for CHB is maximizing the inhibition of HBV replication [3]. Tenofovir disoproxil fumarate (TDF) and entecavir (ETV) have recently been proposed as first-line potent antiviral drugs for the treatment of CHB by the American Association for the Study of Liver Diseases (AASLD) and European Association for the Study of the Liver (EASL) [3]. The clinical comparison research between TDF and ETV treatment has been performed in the United States and European countries on TDF over the years [4-6]. However, the treatment effect comparison between these two drugs in Chinese HBV patients has not been characterized.
TDF has not been public used in Mainland China yet while some patients in our hospital has purchased from Hong Kong and used them in other ways. To investigate the effects of TDF initial treatment of CHB patients in China, our team has retrospectively analyzed and compared the effects of TDF and ETV treatment in naïve CHB patients in this study.
Materials and methods
Patient selection
All the patients were selected in our follow-up study group from the Third Affiliated Hospital, Sun Yat-sen Universty.
Follow-up time
From June, 2012 to June, 2014, 98 nucleotide analogs (NAs) treatment patients participated in this follow-up research study, including 33 in the TDF initial treatment group and 65 in the ETV initial treatment group. All the patients were followed up once at least every 3 months in order to collect the serum for relative testing. All the patients corresponded to the guideline of prevention and treatment for chronic hepatitis B, which was implemented by the Chinese Society of Hepatology and the Chinese Society of Infectious Diseases, which are branches of Chinese Medical Association 7. The demographics of the patients are shown in Table 1.
Table 1.
The Demographics of TDF and ETV treated CHB patients
| TDF group | ETV group | Statistics | P | |
|---|---|---|---|---|
|
|
||||
| (n = 33) | (n = 65) | |||
| Age (years) | 35 (26-61) | 39 (20-67) | t = 1.849 | 0.067 |
| Sex (male, %) | 69.7 (23/33) | 81.5 (53/65) | χ2 = 1.763 | 0.184 |
| BMI | 22.63 ± 2.73 | 22.85 ± 2.86 | t = 0.355 | 0.723 |
| Follow-up time (months) | 13.4 (6.2-28.0) | 16 (6.0-27.0) | t = 0.656 | 0.513 |
| The proportion of Alcohol history (%) | 18.2 (6/33) | 24.6 (16/65) | χ2 = 0.520 | 0.471 |
| The proportion of Smoking history (%) | 42.4 (14/33) | 35.4 (23/65) | χ2 = 0.462 | 0.497 |
| Family history of Hepatitis B (%) | 33.3 (11/33) | 30.8 (20/65) | χ2 = 0.067 | 0.796 |
| ALT baseline (U/L) | 194.1 ± 128.5 | 157.6 ± 216.8 | t = 1.043 | 0.300 |
| HBV DNA baseline (Log10 IU/ml) | 6.50 ± 0.69 | 6.15 ± 1.36 | t = 1.701 | 0.092 |
| Rate of Hepatitis B E antigen positive (%) | 60.6 (20/33) | 55.4 (36/65) | χ2 = 0.244 | 0.622 |
Patients inclusion and exclusion criteria
Inclusion criteria
Patients must be (1) diagnosed with CHB and never have taken any nucleoside or NAs before this study, and they (2) must correspond to the antiviral indication in the guideline of prevention and treatment for chronic hepatitis B drawn up by the Chinese Society of Hepatology and the Chinese Society of Infectious Diseases, Chinese Medical Association.
Exclusion criteria
Patients were excluded from this study if they were (1) co-infected with other hepatitis virus or suffered from co-morbidities with alcoholic, drug-induced, or autoimmune liver diseases. In addition, they were excluded if they were (2) pregnant or lactating women. In the present work, we retrospectively analyzed 321 TDF and ETV initial treatment patients from the Third Affiliated Hospital, Sun Yat-sen Universty. Among them, 98 initial treatment patients were selected since their medical records were complete and met the inclusion and exclusion criteria and follow-up time requirements.
Therapeutic method
All the patients purchased and used TDF (Viread, GSK Co.) by their self. The patients in the TDF monotherapy group took TDF 300 mg/day while the patients in the ETV treatment group took ETV 0.5 mg/day (Baraclude, Squibb Co.). They participated in our follow-up study group under their own consent.
Detection methods
Liver and kidney functions were tested using Hitachi 7180 (Hitachi, Ltd., Tokyo, Japan) and Olympus 64 (Olympus Co., Tokyo, Japan). Normal range of ALT is 5-35 U/L. Serum HBV DNA (measured by Daan Gene Co., Guangzhou, China) lower detection limit is 2log10 IU/mL.
Observing index
The biochemical index and virology indicators of the patients in each group were measured before and after treatment at weeks 4, 12, 24, 36, 48, 72.
Regulation and ethics statement
All of the patients’ information (including the research and informed consent forms) corresponded to the requirements of the ethics committee in the Third Affiliated Hospital, Sun Yat-sen Universty. All the patients signed the written informed consent form.
Statistical methods
Clinical data analysis was performed using SPSS13.0. Count data was compared using χ2 test and other data were compared using Student’s Test. P < 0.05 means statistically significant difference. HBV DNA cumulative undetectable rate map was analyzed via survival Kaplan-Meimer analysis.
Results
Overall result
In the TDF and ETV group, the median ages of the patients were 35 (26-61) and 39 (20-67), respectively. The median follow-up times were 13.4 (6.2-28.0) months and 16.0 (6.0-27.0) months, respectively, and the rate of Hepatitis B E antigen positive HBeAg (+) patients were 60.6% (20/33) and 55.4% (36/65), respectively. HBV DNA baselines were 6.50 ± 0.69 (Log10 IU/ml) for the TDF group and 6.15 ± 1.36 (Log10 IU/ml) for the ETV group. The HBeAg seroconversion rates at the end of follow-up were 6.1% (2/33) and 9.1% (5/65), respectively. The rates of virologic breakthrough in both groups were 0. Differences between the two groups in all the parameters above as well as ALT baselines, family history of hepatitis B, alcohol history, and smoking history were not statistically significant (Table 1).
The decrease pattern of HBV DNA levels along with the antiviral time (Figure 1).
Figure 1.
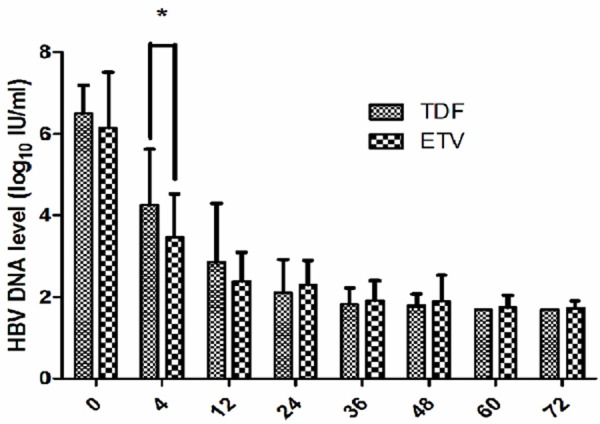
Comparisons of HBV DNA levels between TDF and ETV group. *HBV DNA level in ETV significantly lower than that in TDF at week 4. (t = 2.913, P = 0.005).
The HBV DNA levels of the patients in both groups were decreased after treatment. The HBV DNA levels showed a statistically significant difference between the two groups at week 4 of treatment (t = 2.913, P = 0.005), while having no significant differences at weeks 12, 24, 36, 48. 72 (t = 1.759, 1.400, 0.438, 0.820, 0.772; P = 0.086, 0.166, 0.780, 0.414, 0.443, 0.423, respectively). However, pairwise comparison in each group showed that in the TDF group the difference between week 0 and week 4 was statistically significant (6.50 ± 0.69 versus 4.26 ± 1.37 log10 IU/ml, t = 8.451, P < 0.001). Furthermore, the differences between week 0 and weeks 12, 36, 48, 72 were all statistically significant (P < 0.001) as were the differences from week 4 to week 12 and from week 12 to week 24 (3.52 ± 1.40 V.S. 2.86 ± 1.44 log10 IU/ml, t = 4.057, P < 0.001; 2.86 ± 1.44 V.S. 2.11 ± 0.81 log10 IU/ml; t = 2.615, P = 0.012, respectively). Performing pairwise comparison between week 24 and weeks 36, 48 and 72 showed no statistically significant differences (P > 0.05). In the ETV group, the difference between week 0 and week 4 was statistically significant (6.15 ± 1.36 V.S. 3.47 ± 1.06 log10 IU/ml, t = 12.548, P < 0.001), and the differences between week 0 and weeks 12, 24, 36, 48, 72 were all statistically significant (P < 0.001) as was the difference between week 4 and week 12 (3.47 ± 1.06 V.S. 2.39 ± 0.71 log10 IU/ml, t = 6.795, P < 0.001). Pairwise comparison between week 12 and weeks 24, 36, 48 and 72 showed no statistically significant differences (P > 0.05).
HBV DNA undetectable rates (Figure 2)
Figure 2.
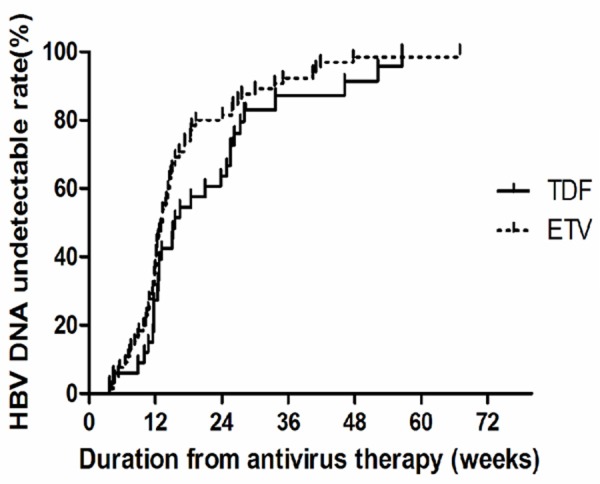
Comparisons of HBV DNA undetectable rates between TDF and ETV group.
The HBV DNA undetectable rates increased in every observed time point with the extension of antiviral drugs treatment in both TDF and ETV group. Kaplan-Meier survival analysis was performed to compare the HBV DNA undetectable rates in the two groups, and the results showed no statistically significant difference (Log Rank χ2 = 2.501, P = 0.114). Pairwise comparison in the TDF group showed that the difference between week 4 and week 12 was not statistically significant (χ2 = 1.728, P = 0.289). In contrast, the differences between week 4 and week 24 as well as week 12 and week 24 were statistically significant (χ2 = 11.000, P = 0.001; χ2 = 6.346, P = 0.012, respectively). Pairwise comparison between week 24 and weeks 36, 48 and 72 revealed no statistically significant differences (P > 0.05). Pairwise comparison in ETV group showed that the difference between week 4 and week 12 was statistically significant (χ2 = 69.617, P < 0.001). Comparing week 12 to weeks 24, 36, 48 and 72 revealed no statistically significant differences (P > 0.05). In addition, the median time of the negative conversion of HBV DNA (under the lower detection limit of HBV DNA levels) was calculated via survival analysis. The results showed that the medium time were 15.57 (3.71-56.57) weeks and 13.29 (4.00-67.00) weeks in TDF and ETV groups, respectively.
The decrease pattern of ALT levels along with the antiviral time
The ALT levels progressively decreased with the extension of antiviral drugs treatment in both groups. The difference in the ALT levels between the two groups at week 12 was statistically significant (TDF: 60.18 ± 44.57 U/L V.S. ETV: 38.26 ± 30.11 U/L, t = 2.545, P = 0.014), while the differences at other time points were not (P > 0.05). Pairwise comparison in TDF group showed that the difference between week 0 and week 4 was statistically significant (194.12 ± 128.53 U/L V.S. 103.33 ± 80.25 U/L, t = 3.442, P = 0.001). Comparing week 0 to weeks 12, 36, 48, 60 and 72 showed statistical significance (P < 0.001). In addition, the difference between week 4 and week 12 as well as week 12 and week 24 showed statistical significance (103.33 ± 80.25 U/L V.S. 60.18 ± 44.57 U/L, t = 2.700, P = 0.009; 60.18 ± 44.57 U/L V.S. 32.52 ± 11.66 U/L, t = 3.450, P = 0.001, respectively). Pairwise comparison between week 24 and weeks 36, 48, 60 and 72 showed no statistical significance (P > 0.05).
Similarly, pairwise comparison before and after treatment in ETV group showed that the difference between week 0 and week 4 was statistically significant (157.63 ± 216.8 V.S. 95.04 ± 39.49 U/L, t = 2.289, P = 0.025). Comparing week 0 to weeks 12, 24, 36, 48, 60 and 72 showed statistical significance as well (P < 0.001). The difference between week 4 and week 12 as well as week 12 to week 24 showed statistical significance (95.04 ± 39.49 V.S. 38.26 ± 30.11 U/L, t = 9.217, P < 0.001; 38.26 ± 30.11 V.S. 28.25 ± 17.37 U/L, t = 2.324, P = 0.022, respectively). Pairwise comparison between week 24 and weeks 36, 48, 60 and 72 showed no statistical significance (P > 0.05).
ALT normalization rates
The ALT normalization rates have gradually increased with the antiviral drugs treatment (Figure 3). The Kaplan-Meier survival analysis (Figure 4) was performed to compare the ALT normalization rates in the two groups, and the results showed no statistically significant difference (Log Rank χ2 = 0.200, P = 0.656). Pairwise comparison in the TDF group showed that the difference between week 4 and week 12 was statistically significant (12.1% V.S. 36.4%, χ2 = 5.280, P = 0.022). However, the difference between week 12 and week 24 was not significant (36.4% V.S. 57.6%, χ2 = 2.981, P = 0.084). Pairwise comparison at other time points all showed no statistical significance (P > 0.05). Pairwise comparison in ETV group showed that the differences between week 4 and week 12 as well as week 12 and week 24 were statistically significant (10.8% V.S. 55.4%, χ2 = 29.225, P < 0.001 and 55.4% V.S. 76.9%, χ2 = 6.734, P = 0.009, respectively). Pairwise comparison at other time points showed no statistic significance (P > 0.05).
Figure 3.
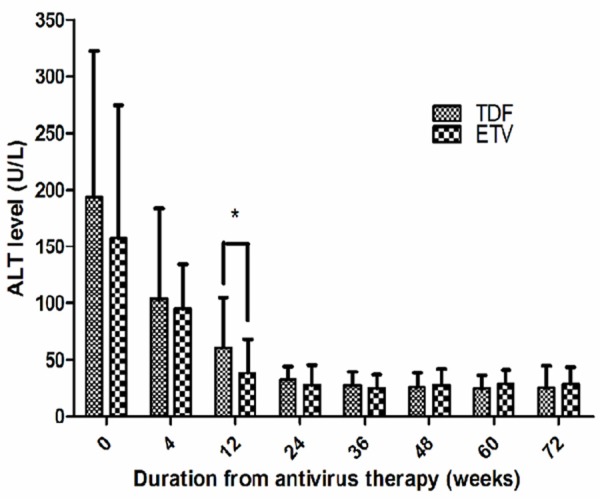
Comparisons of ALT levels between TDF and ETV group.*ALT level in ETV significantly lower than in TDF at week 12. (t = 2.545, P = 0.014).
Figure 4.
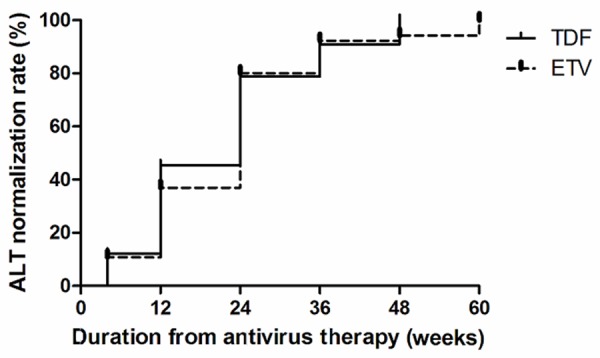
Comparisons of ALT normalization rates between TDF and ETV groups.
Multi-factor analysis of HBV DNA negative conversion rate
The results of multi-factor survival analysis (Cox regression analysis-Stepwise regression method) of HBV DNA negative conversion in all the patients are showed in Tables 2, 3. As shown in the tables, comparing the influence factors of HBV DNA negative conversion in TDF and ETV groups showed a statistically significant difference (χ2 = 2.469, P = 0.016). The HBV DNA negative conversion was positively influenced by age (P = 0.010) and negatively correlated with alcohol history (P = 0.002). Other indicators such as sex, smoking history, family history of hepatitis B, HBV DNA baseline, ALT baseline, and HBeAg condition all showed no statistically significant correlation to the HBV DNA negative conversion (P > 0.05).
Table 2.
Multi-factor analysis of HBV DNA negative conversion (all factors)
| Score | df | Sig. | |
|---|---|---|---|
| Age | 6.624 | 1 | 0.010 |
| Sex | 0.280 | 1 | 0.597 |
| BMI | 1.372 | 1 | 0.242 |
| Smoking history | 3.100 | 1 | 0.078 |
| Family history of hepatitis B | 6.429 | 1 | 0.011 |
| Alcohol history | 9.645 | 1 | 0.002 |
| Antivirus (TDF or ETV) | 2.469 | 1 | 0.116 |
| HBV DNA baseline (Log10 IU/ml) | 5.019 | 1 | 0.025 |
| ALT baseline (U/L) | 4.999 | 1 | 0.025 |
| Hepatitis B E antigen positive | 0.414 | 1 | 0.520 |
a. Residual Chi Square = 32.470 with 10 df, P < 0.001.
Table 3.
Multi-factor analysis of HBV DNA negative conversion (age, alcohol history)
| B | SE | Wald | df | Sig. | Exp (B) | 95.0% CI for Exp (B) | |||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| Lower | Upper | ||||||||
| Step 1 | Alcohol history | -0.809 | 0.266 | 9.265 | 1 | 0.002 | 0.445 | 0.264 | 0.750 |
| Step 2 | Age | 0.025 | 0.010 | 6.579 | 1 | 0.010 | 1.025 | 1.006 | 1.045 |
| Alcohol history | -0.813 | 0.267 | 9.277 | 1 | 0.002 | 0.443 | 0.263 | 0.748 | |
Adverse event incidence
The patients in the two groups showed good tolerance to drugs and no severe illnesses or deaths using the antiviral drugs treatment during the follow-up study. CK levels 2 times over the upper limit of normal (2ULN) occurred in only one case in the TDF group. The maximum value was 308 U/L, which occurred at week 36 and returned to normal at week 48. CK elevation exceeding 2ULN did not occur in any other patients. In the ETV group, the 2.3% of the patients had CK elevation over 2ULN. The average at weeks 0, 4, 12, 24, 36, 48, 60 and 72 were 3.1% (2/65), 1.5% (1/65), 1.5% (1/65), 1.5% (1/65), 3.4% (2/59), 1.7% (1/58) and 4.2% (2/48), respectively. The differences between all time points showed no statistical significance (χ2 = 1.827, P = 0.969). The highest CK elevation occurred at week 12 with a maximum value of 474 U/L. The CK elevation returned to normal levels in every patient after 2-4 weeks. No CK related adverse event including myolysis, lactic acidosis, etc. occurred, and no serum creatinine (Cr) elevation over ULN was detected in any patients in both groups.
Discussion
Currently, TDF and ETV are ranked as the primary anti-HBV NAs in many countries and areas in the world, including Hong Kong, China. The clinical research of TDF and ETV has been performed abroad on TDF over the years [4-6]. Results from this past research have indicated not only the positive treatment effects of NAs but also the potent inhibitory effects on HBV DNA replication and the capacity to ameliorate liver fibrosis and cirrhosis. However, the research endeavors indicated above were mainly focused on the HBV infection in the USA and Europe; no related clinical studies have yet been performed in China. Currently, the treatment of most of the CHB patients was changed from TDF to TDF monotherapy or a combination treatment. There are very few naïve CHB patients treated with TDF. Furthermore, no comparison between TDF and ETV monotherapy has been performed in China. In our present work, we retrospectively analyzed the clinical features of 33 CHB patients initially treated with TDF and 65 patients initially treated with ETV. The results suggested that both options showed potent and rapid inhibitory effects of HBV DNA replication on the patients.
As shown in this study, TDF and ETV both exhibited potent antiviral effects on the patients. The difference of HBV DNA levels between the two groups showed no statistical significance through week 12 even though the levels were much lowthese two treatment schemes showed a two-phase decline pattern, a rapid decrease before week 12 but slowing after week 24. The HBV DNA levels in most er in ETV group compared to the TDF group at week 4. The HBV DNA levels in patients have decreased to 2 log10 IU/mL (lower detection limit) until 24 weeks. Thus, the TDF and ETV treatment both showed potent antiviral effects and consistently inhibited virus to below the lower detection limit. The Marcellin P group have reported that the HBV DNA levels after treatment with TDF for 48 weeks in the HBeAg (+) naïve CHB patients was 2.46 log10 IU/ml and in the HBeAg (-) patients was 2.31 log10 IU/ml (the detection limit was 2.6 log10 IU/ml) [8]. However, the TDF achieved similar affects after treatment for only 24 weeks in our study.
The HBV DNA undetectable rate is another important indicator commonly used to reflect the ability of inhibiting a virus. The results in this study showed that the HBV DNA undetectable rates were increased with prolonged antiviral drug treatment in both group. However, there was no significant difference in terms of the HBV DNA undetectable rates between the TDF and ETV treatment groups. The results were similar to a previous study [9], which showed similar inhibition rates of HBV DNA with TDF and ETV initial treatment for 48 weeks. Our results also showed an increase of HBV DNA inhibition rates at week 24 compared to other time points in each group, bur no significant differences were found after 24 weeks. These results suggest that TDF and ETV treatment both had potent inhibition ability of HBV DNA at an early phase. In addition, the multi-factor analysis of HBV DNA negative conversion also showed no significant difference of inhibition ability of HBV DNA between TDF and ETV treatment.
The high ALT normalization rate was observed in CHB patients treated with TDF and EVT and the ALT normalization rate of both treatment groups increased with time. The difference of ALT normalization rate between the two groups was not statistically significant. The ALT normalization rate in the TDF group progressively increased from week 4 and had no significant difference after week 24 while in the ETV group it progressively increased until week 36 and had no significant difference after week 36. Thus, in the TDF group, the ALT normalization rate peaked at week 24 and peaked at week 36 in the ETV group. The Lampertico P group has reported that the ALT normalization rate peaked at week 24-36 in TDF initial treatment patients, which was similar to the peak time of ALT normalization rate of the TDF group in our study but slightly earlier than the peak time of ALT normalization rate of the ETV group [10].
The TDF and ETV group both showed good tolerance and safety with low incidence of adverse events during treatment. The patients with CK elevation mostly showed mild or moderate increase over normal levels and had no significant difference compared to the baseline of CK elevation. The CK never happened to exceed ULN of the patients in our study, which is similar to a previous study by Heathcote EJ [11]. In addition, TDF maintained a favorable safety for up to 3 years’ treatment.
In conclusion, our study showed that TDF and ETV treatment both exhibited rapid inhibiting effects on HBV DNA replication in the early phase of naïve CHB patients in Mainland China. With high ALT normalization rate, the treatments could prove to be suitable antiviral treatment schemes.
Acknowledgements
This project was supported by the National Science and Technology Major Project (2012ZX10002004, 2012ZX10004-902).
Disclosure of conflict of interest
None.
References
- 1.Lee WM. Hepatitis B virus infection. N Engl J Med. 1997;337:1733–45. doi: 10.1056/NEJM199712113372406. [DOI] [PubMed] [Google Scholar]
- 2.Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, Huang GT, Iloeje UH REVEAL-HBV Study Group. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]
- 3.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167–85. doi: 10.1016/j.jhep.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Gerada J, Borg E, Formosa D, Magro R, Pocock J. Tenofovir as rescue therapy following clinical failure to Lamivudine in severe acute hepatitis B. Mediterr J Hematol Infect Dis. 2013;5:e2013035. doi: 10.4084/MJHID.2013.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keskin O, Ormeci AC, Baran B, Kabaçam G, Tüzün A, Karatayli E, Akyüz F, Karatayli S, Bozdayi AM, Onel D, Badur S, Idilman R, Kaymakoglu S, Yurdaydin C. Efficacy of tenofovir in adefovir-experienced patients compared to treatment-naive patients with chronic hepatitis B. Antivir Ther. 2014;19:543–50. doi: 10.3851/IMP2732. [DOI] [PubMed] [Google Scholar]
- 6.Fung S, Kwan P, Fabri M, Horban A, Pelemis M, Hann HW, Gurel S, Caruntu FA, Flaherty JF, Massetto B, Dinh P, Corsa A, Subramanian GM, McHutchison JG, Husa P, Gane E. Randomized Comparison of Tenofovir Disoproxil Fumarate vs Emtricitabine and Tenofovir Disoproxil Fumarate in Patients With Lamivudine-Resistant Chronic Hepatitis B. Gastroenterology. 2014;146:980–988. doi: 10.1053/j.gastro.2013.12.028. [DOI] [PubMed] [Google Scholar]
- 7.Chinese Society of Hepatology and Chinese Society of Infectious Diseases, Chinese Medical Association. The guideline of prevention and treatment for chronic hepatitis B (2010 version) Chin J Hepatol. 2011;19:13–24. doi: 10.3760/cma.j.issn.1007-3418.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Marcellin P, Heathcote EJ, Buti M, Gane E, de Man RA, Krastev Z, Germanidis G, Lee SS, Flisiak R, Kaita K, Manns M, Kotzev I, Tchernev K, Buggisch P, Weilert F, Kurdas OO, Shiffman ML, Trinh H, Washington MK, Sorbel J, Anderson J, Snow-Lampart A, Mondou E, Quinn J, Rousseau F. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med. 2008;359:2442–55. doi: 10.1056/NEJMoa0802878. [DOI] [PubMed] [Google Scholar]
- 9.Doğan ÜB, Kara B, Gümürdülü Y. Comparison of the efficacy of tenofovir and entecavir for the treatment of nucleos(t)ide-naive patients with chronic hepatitis B. Turk J Gastroenterol. 2012;23:247–52. doi: 10.4318/tjg.2012.0380. [DOI] [PubMed] [Google Scholar]
- 10.Lampertico P, Soffredini R, Viganò M, Yurdaydin C, Idilman R, Papatheodoris G, Margheriti K, Buti M, Esteban R, Zaltron S, Vavassori A, Carosi G, Minola E, Vinci M, Pinzello G, Giorgini A, Zuin M, Salmi A, Del Poggio P, De Filippi F, Bruno S, Pasulo L, Fagiuoli S, Andreoletti M, Colli A, Fumagalli Maldini F, Milanese M, Colombo AE, Bellati GA, Angeli E, Angeli E, Gubertini G, Rizzardini G, Fasano M, Santantonio T, Terreni N, Spinzi G, Facchetti F, Invernizzi F, Colombo M. 2 year effectiveness and safety of tenofovir in 302 NUC-naïve patients with chronic hepatitis B: a multicenter European study in clinical practice. Hepatology. 2011;54(Suppl 1) Abstract 1433. [Google Scholar]
- 11.Heathcote EJ, Marcellin P, Buti M, Gane E, De Man RA, Krastev Z, Germanidis G, Lee SS, Flisiak R, Kaita K, Manns M, Kotzev I, Tchernev K, Buggisch P, Weilert F, Kurdas OO, Shiffman ML, Trinh H, Gurel S, Snow-Lampart A, Borroto-Esoda K, Mondou E, Anderson J, Sorbel J, Rousseau F. Three-year efficacy and safety of tenofovir disoproxil fumarate treatment for chronic hepatitis B. Gastroenterology. 2011;140:132–143. doi: 10.1053/j.gastro.2010.10.011. [DOI] [PubMed] [Google Scholar]


