Abstract
Atherosclerosis is a chronic inflammatory disease, starting with the accumulation of white blood cells and fatty materials in the arterial wall. ABCA1, a gene promotes phospholipid and cholesterol transfer from cells to poorly lapidated ApoA1, is considered to be related to the pathogenesis of coronary atherosclerosis. Meanwhile, disturbed miRNAs were reported to be related to coronary atherosclerosis. To understand the relationship between miRNA, ABCA1 and coronary atherosclerosis pathogenesis, we first screened the miRNAs that may directly target 3’UTR of ABCA1 and miR-33a was used as positive control. Through dual luciferase assay and western blot, we confirmed that miR-93 and miR-17 repress ABCA1 expression through directly targeting 3’UTR. The serum miR-33a, miR-93 and miR-17 levels in participants were detected by qRT-PCR and a significant reduction of miR-33a and miR-93 was found in the coronary patients. After statistical analysis we identified that a negative correlation was existed in the serum miR-93 and ABCA1 levels in coronary atherosclerosis patients. Meanwhile, our results indicate that the serum miR-93 positively correlates with the serum cholesterol level. This research may give insight into understanding of coronary atherosclerosis pathogenesis and create an opportunity to the diagnosis of coronary atherosclerosis.
Keywords: miRNA, coronary atherosclerosis, ABCA1
Introduction
Atherosclerosis is a chronic inflammatory disease, starting with the accumulation of white blood cells and fatty materials such as cholesterol in the arterial wall, is the principal cause of coronary artery disease coronary atherosclerosis). Plasma high-density lipoprotein (HDL) is thought to function as a sterol transporter which is a protective factor against coronary atherosclerosis. Meanwhile, the inverse correlation between plasma HDL-C and the incidence of coronary atherosclerosis is well established [1,2].
HDL formation occurs in the liver and intestine. The interaction between lipid poor apolipoprotein A1 (ApoA1) with the ATP binding cassette A1 (ABCA1) mediates this first step in HDL formation [3]. ABCA1 is a member of the ABC family of membrane transporters that promotes phospholipid and cholesterol transfer from cells to poorly lapidated ApoA1. Recently, genetic association study and functional study in mice have indicate an important role of ABCA1 during the pathogenesis of atherosclerosis [4-7].
MicroRNAs (miRNAs) are short non-coding RNAs which modulate gene expression by binding to complementary segments present in the 3’UTR of the mRNAs of protein coding genes. MiRNAs play very important roles in maintaining normal human body physiology conditions, and abnormal miRNA expressions have been found related to many human diseases spanning from psychiatric disorders to malignant cancers [8-10]. Increasing numbers of evidence have shown that the expression of a group of miRNAs was disturbed in atherosclerosis patients and some of them related to the pathogenesis of atherosclerosis [11].
In the present study, we screened 6 selected miRNAs that may target the 3’UTR of ABCA1 and found 2 of them repressed ABCA1 expression via targeting 3’UTR directly. Subsequently, the expression levels of selected miRNAs and ABCA1 were detected in the patients’ serum samples. A negative correlation was constructed between the serum ABCA1 and mir-93 levels.
Materials and methods
Clinical samples
This study includes 35 consecutive patients with coronary atherosclerosis and 35 age and sex paired healthy controls. All the participants were collected from Xuhui Central Hospital between September 2012 and November 2013. Clinical diagnosis of coronary atherosclerosis was evaluated by percutaneous coronary angiography, reviewed by two experienced cardiologists. Healthy control subjects, without coronary atherosclerosis, were selected in the same period. Written informed consent was obtained from all participants and this study was approved by the Ethics Committee of Xuhui Central Hospital.
10 ml peripheral venous blood was collected from each participant. Portion of blood sample was processed for serum separation which was stored at -20°C until used. The processing of these blood samples was started within 30 min after collection.
Cell culture
THP1 cells were cultured in RPMI 1640 supplemented with 10% (v/v) fetal bovine serum (Hyclone, Logan, UT, USA) at 37°C and 5% CO2. HEK293T cells were cultured in Dulbecco’s Modified Eagle Medium containing 10% fetal bovine serum (Hyclone, Logan, UT, USA), 100 IU/ml penicillin and 10 mg/mL streptomycin. All cells were maintained at 37°C under an atmosphere of 5% CO2.
Quantitative RT-PCR analysis of miRNA
Quantitative RT-PCR analysis was employed to determine the relative expression level of selected miRNAs in the serum. Total RNA was extracted from 100 μl serum samples, using Trizol LS Reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. The expression level of miRNAs was detected by TaqMan miRNA RT-Real Time PCR. Single-stranded cDNA was synthesized by using TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA) and then amplified by using TaqMan Universal PCR Master Mix (Applied Biosystems, Foster City, CA, USA) together with miRNA-specific TaqMan MGB probes (Applied Biosystems, Foster City, CA, USA). Each sample in each group was measured in triplicate and the experiment was repeated at least three times for the detection of miRNAs.
Dual luciferase assay
Full length of 3325 bp ABCA1 3’UTR were cloned into downstream of firefly luciferase coding region in pGLO3 basic plasmid (promega, Madison, WI, USA) to generate luciferase reporter vector. For luciferase reporter assays, HEK293T cells were seeded in 48-well plates. miRNAs mimics or antagonists and luciferase reporter vector were co-transfected by using lipofectamine 2000 (Invitrogen, Carlsbad, CA USA). pRL-TK plasmid containing renilla luciferase coding region was used as transfection control. Two days later, cells were harvested and assayed with the Dual-Luciferase Assay kit (Promega, Madison, WI USA). Each treatment was performed in triplicate in three independent experiments. The results were expressed as relative luciferase activity (Firefly LUC/Renilla LUC).
Western blotting
Protein extracts were boiled in SDS/β-mercaptoethanol sample buffer, and 20 μg samples were loaded into each lane of 8% polyacrylamide gels. The proteins were separated by electrophoresis, and the proteins in the gels were blotted onto PVDF membranes (Amersham Pharmacia Biotech, St. Albans, Herts, UK) by electrophoretic transfer. The membrane was incubated with mouse anti-ABCA1 monoclonal antibody (Abcam, Cambridge, MA, USA), mouse anti-β-actin monoclonal antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) for 1 h at 37°C. The specific protein antibody complex was detected by using horseradish peroxidase conjugated rabbit anti-mouse IgG. Detection by the chemiluminescence reaction was carried using the ECL kit (Pierce, Appleton, WI, USA). The β-actin signal was used as a loading control.
Enzyme linked immunosorbent assay (ELISA) for estimating ABCA1 protein
Serum ABCA1 level was estimated by using sandwich ELISA method and rabbit and mouse anti-ABCA1 antibodies (Abcam, Cambridge, MA, USA). The relative concentrations were compared using OD value directly
Blood biochemical indexes
Blood samples were drawn for measurement of serum levels of TC, HDL-C, LDL-C after a 12-hour overnight fast. Serum levels of TC (mmol/L), HDL-C (mmol/L), and LDL-C (mmol/L) were determined by colorimetric enzymatic assays with use of an Auto-Analyzer.
Statistical analysis
All the results were analyzed by using SPSS Statistical Package version 16. The data of two groups were analyzed by student’s t-test and the correlation analysis was processed by χ2-analysis. P < 0.05 was considered statistically significant.
Results
MiR-17 and -93 directly target ABCA1 3’UTR
The basic characteristics between the case and control groups were presented in Table 1. Coronary atherosclerosis is a chronic inflammatory disease, starting with the accumulation of white blood cells and fatty materials such as cholesterol in the arterial wall, and ABCA1, a gene promotes phospholipid and cholesterol transfer from cells to poorly lapidated ApoA1 is considered to be related to the pathogenesis of coronary atherosclerosis. Meanwhile, disturbed miRNAs were reported to be related to coronary atherosclerosis. To understand the relationship between miRNA, ABCA1 and coronary atherosclerosis pathogenesis, we first screened the miRNAs that may regulate ABCA1 expression directly. Depending on the results from online bioinformatics tool: TargetScan (http://www.targetscan.org/) we selected 6 miRNAs (miR-17, miR-19a, miR-93, miR-106a, miR-124 and miR-130a). MiR-33a, which has been confirmed to be reported was used as positive control. The full length of 3325 bp ABCA1 3’UTR were cloned into downstream of firefly luciferase coding region in pGLO3 basic plasmid to generate luciferase reporter vector (Figure 1A). Dual luciferase assay was employed to identify the direct interaction between miRNAs and ABCA1 3’UTR. HEK293T cells were transfected with ABCA1 3’UTR reporter vector and miRNA mimics, with pRL-TK as transfection control. As shown in Figure 1B, miR-93 and miR-17 repressed the luciferase activity for 56% and 49% compared with miR-control. Furthermore, the luciferase activity was up-regulated significantly by miR-93 and miR-17 antagonist (Figure 2A). These results indicate that miR-93 and miR-17 target the 3’UTR of ABCA1 leading to the change of luciferase activities.
Table 1.
Characteristics of cases and controls
| Characteristics | Cases (n = 35) | Controls (n = 35) | P value |
|---|---|---|---|
| Age | 65 | 65 | 1 |
| Sex (Male/female) | 19/16 | 19/16 | 1 |
| Diabetes (n, %) | 9 (25.7) | 5 (14.3) | 0.044 |
| Hypertension (n, %) | 29 (82.8) | 21 (60) | 0.0033 |
| Total cholesterol (mmol/L) | 4.74 (4.12-5.47) | 4.03 (3.48-5.20) | < 0.001 |
| HDL-C (mmol/L) | 1.18 (1.05-1.43) | 1.25 (1.03-1.64) | < 0.001 |
| LDL-C (mmol/L) | 3.09 (2.70-3.45) | 2.42 (2.04-3.01) | < 0.001 |
Figure 1.
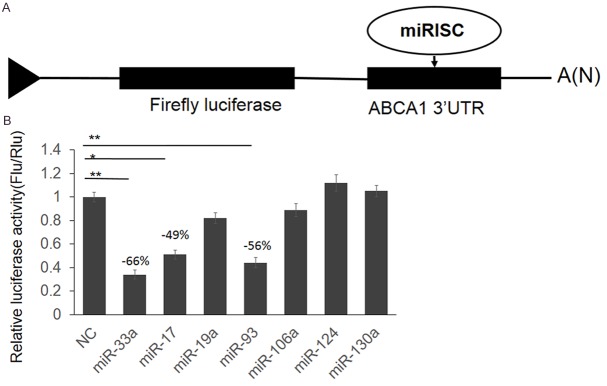
Screen miRNAs that target 3’UTR of ABCA1. A. The schematic diagram of ABCA1 3’UTR reporter vector construction. B. Dual luciferase assay. HEK293T cells were transfected with miRNA mimics with sequence scrambled short RNA as negative control. 48 hour post transfection, the cells were lysed and luciferase activities were detected. The results were analyzed by students’t-test and P < 0.05 was considered statistically significant. *P < 0.05, **P < 0.01.
Figure 2.
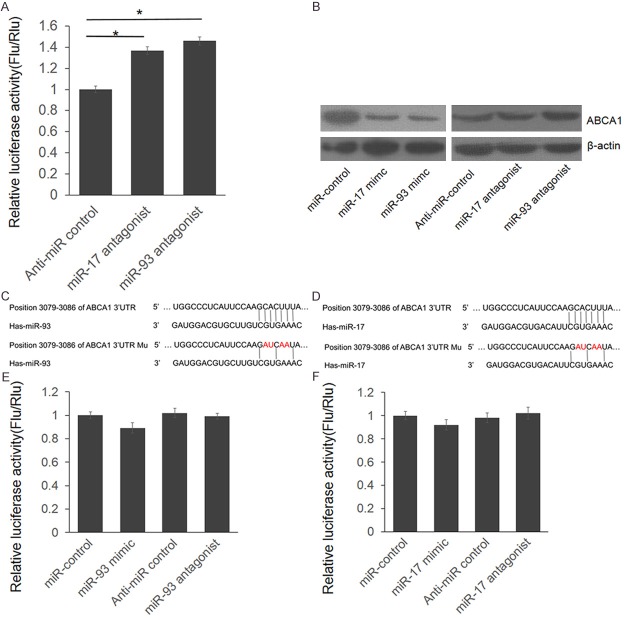
ABCA1 is a direct target gene of miR-93 and miR-17. A. HEK293T cells were transfected with ABCA1 3’UTR reporter vector and the antagonist of miR-93 or miR-17. 48 hours post transfection, the cells were lysed and luciferase activities were detected. The results were analyzed by students’t-test and p < 0.05 was considered statistically significant. *P < 0.05, **P < 0.01. B. THP1 cells were transfected with miRNA mimics or antagonists. 48 hours post transfection, the cells were lysed and the protein level of ABCA1 was detected by western blot. C, D. The schematic diagram of ABCA1 3’UTR mutant plasmid construction. E, F. HEK293T cells were transfected with ABCA1 3’UTR mutant reporter vector and miRNA mimics or antagonists. 8 hours post transfection, the cells were lysed and luciferase activities were detected. The results were analyzed by students’t-test and p < 0.05 was considered statistically significant. *P < 0.05, **P < 0.01.
MiR-17 and -93 repress endogenous ABCA1 expression
To further identify whether endogenous ABCA1 protein level was repressed by miR-93 and miR-17, we did the western blot assay. THP1 cells were transfected with miRNA mimics or antagonists. 48 hours after transfection, cells were lysed and the protein level of ABCA1 was detected. As shown in Figure 2B, the ABCA1 expression was significantly repressed by miR-93 and miR-17 mimics, meanwhile, it was significantly up-regulated by miR-93 and miR-17 antagonists.
Seed sequence mutant clone was constructed to identify the target site of miR-93 and miR-17. As shown in Figure 2C-F, when 4 nucleotides mutated, the luciferase activities were not influenced by the mimics or antagonists of miR-93 and miR-17 (P < 0.05). These results indicated that miR-93 and miR-17 repressed ABCA1 expression through targeting the 3’UTR of ABCA1 and ABCA1 is a direct target of miR-93 and miR-17.
Disturbed expression of miRNAs and ABCA1 exists in the serum samples of patients
To understand the expression of miRNAs and ABCA1 in vivo, we detected the serum miRNA levels via qRT-PCR and examined the serum ABCA1 level via ELISA. As shown in Figure 3A-C, the serum levels of miR-93 and miR-17 were reduced significantly in coronary atherosclerosis patients compared with healthy control (P < 0.01). Meanwhile, the patients’ serum ABCA1 level had a significant reduction (P < 0.05).
Figure 3.
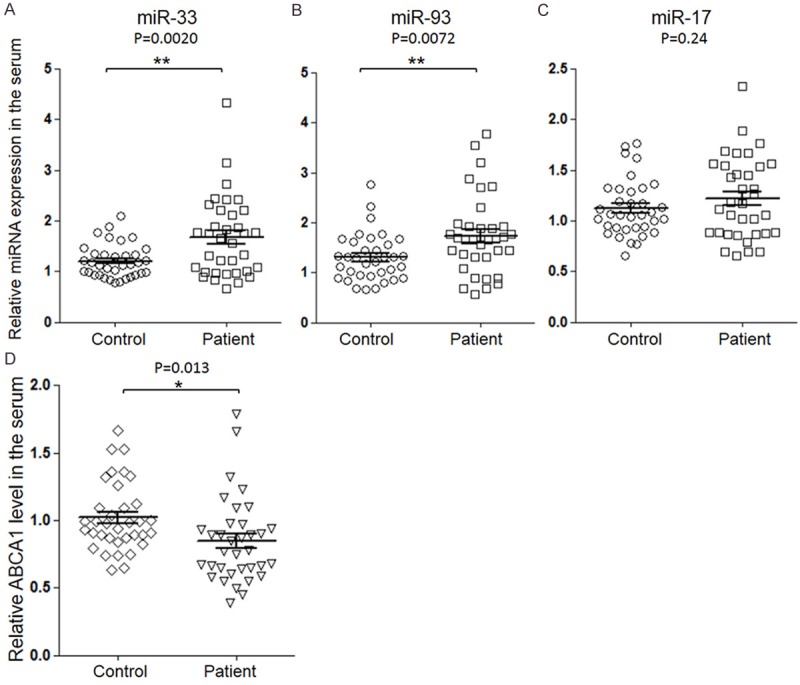
Determine the serum ABCA1 and miRNAs levels. QRT-PCR was employed to determine the serum level of miR-33a (A), miR-93 (B) and miR-17 (C). Serum ABCA1 level by ELISA (D). The results were analyzed by students’-test and P < 0.05 was considered statistically significant. *P < 0.05, **P < 0.01.
Examine the correlations between serum miRNAs, ABCA1 and cholesterol level
To further unveil the relationship between serum miRNA and ABCA1 levels, we did a correlation analysis. As shown in Figure 4B, there is a strong negative correlation between serum miR-93 and ABCA1 levels (r = -0.408, P = 0.015). However no relationship was found between serum miR-33a and ABCA1 levels (r = 0.011, P = 0.96). Subsequently, correlation analysis was also employed to examine the relationship between serum miRNAs and cholesterol levels in coronary atherosclerosis patients. As exhibited in Figure 4C and 4D, the serum cholesterol level presents postive correlations with the serum miR-33a (r = 0.49, P = 0.0027) and miR-93 (r = 0.41, P = 0.014).
Figure 4.
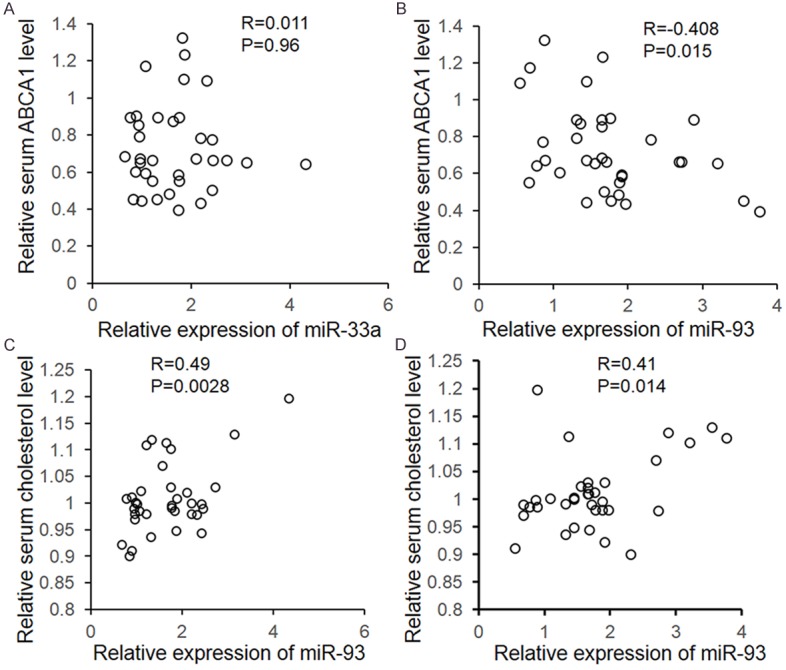
Correlation analysis. The correlation analysis was employed to determine the relationship between serum miR-93 and ABCA1 (A), miR-17 and ABCA1 (B), miR-93 and cholesterol (C), and miR-17 and cholesterol. The results were analyzed by χ2-analysis. P < 0.05 was considered statistically significant.
Discussion
Coronary atherosclerosis is a chronic inflammatory disease, starting with the accumulation of white blood cells and fatty materials in the arterial wall. ABCA1, a gene promotes phospholipid and cholesterol transfer from cells to poorly lapidated ApoA1, is considered to be related to the pathogenesis of coronary atherosclerosis. Meanwhile, disturbed miRNAs were reported to be related to coronary atherosclerosis. To understand whether there are some relations between miRNA, ABCA1 and coronary atherosclerosis pathogenesis, we first screened the miRNAs that may directly target 3’UTR of ABCA1 and miR-33a was used as positive control. Through dual luciferase assay and western blot, we identified that miR-93 and miR-17 repress ABCA1 expression through directly targeting 3’UTR. The serum miR-33a, miR-93 and miR-17 levels in participants were detected by qRT-PCR and a significant reduction of miR-33a and miR-93 was found in the coronary patients. After statistical analysis we identified that a negative correlation was existed in the serum miR-93 and ABCA1 levels in coronary atherosclerosis patients. Meanwhile, our results indicate that the serum miR-93 is positive correlate with the serum cholesterol level.
MiR-33a has been reported to be related to controlling lipoprotein metabolism [12-14]. There are also reports indicated that miR-33a regulates cholesterol accumulation by affecting HDL biogenesis (via ABCA1) pancreatic islets [15]. In this study, we failed to construct a negative correlation between serum miR-33a and ABCA1 level in coronary atherosclerosis patients. However, a positive correlation was found between serum miR-33a and cholesterol. These phenomenon may be results of the multifunction of miR-33a-miR-33a regulates serum cholesterol level through pathways except ABCA1.
In conclusion, we identified that ABCA1 is a direct target gene of miR-93 and miR-17. Meanwhile, up-regulated serum miR-93 is positive related to raise serum cholesterol level via targeting ABCA1 in coronary atherosclerosis patients. This research may give insight into understanding of coronary atherosclerosis pathogenesis and create an opportunity to approach the diagnosis of coronary atherosclerosis.
Disclosure of conflict of interest
None.
References
- 1.Miller GJ, Miller NE. Plasma-high-density-lipoprotein concentration and development of ischaemic heart-disease. Lancet. 1975;1:16–19. doi: 10.1016/s0140-6736(75)92376-4. [DOI] [PubMed] [Google Scholar]
- 2.Hausenloy DJ, Yellon DM. Targeting residual cardiovascular risk: Raising high-density lipoprotein cholesterol levels. Heart. 2008;94:706–714. doi: 10.1136/hrt.2007.125401. [DOI] [PubMed] [Google Scholar]
- 3.Duong PT, Collins HL, Nickel M, Lund-Katz S, Rothblat GH, Phillips MC. Characterization of nascent HDL particles and microparticles formed by ABCA1-mediated efflux of cellular lipids to apoA-I. J Lipid Res. 2006;47:832–843. doi: 10.1194/jlr.M500531-JLR200. [DOI] [PubMed] [Google Scholar]
- 4.Abd ET, Mohamed RH, Hagrass HA. Increased risk of premature coronary artery disease in Egyptians with ABCA1 (R219K), CETP (TaqIB), and LCAT (4886C/T) genes polymorphism. J Clin Lipidol. 2014;8:381–389. doi: 10.1016/j.jacl.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Koopal C, Visseren FL, Kastelein JJ, Westerink J. Premature atherosclerosis, extremely low HDL-cholesterol and concurrent defects in APOA1 and ABCA1 genes: A family case report. Int J Cardiol. 2014;177:e19–21. doi: 10.1016/j.ijcard.2014.07.172. [DOI] [PubMed] [Google Scholar]
- 6.Lv YC, Tang YY, Peng J, Zhao GJ, Yang J, Yao F, Ouyang XP, He PP, Xie W, Tan YL, Zhang M, Liu D, Tang DP, Cayabyab FS, Zheng XL, Zhang DW, Tian GP, Tang CK. MicroRNA-19b promotes macrophage cholesterol accumulation and aortic atherosclerosis by targeting ATP-binding cassette transporter A1. Atherosclerosis. 2014;236:215–226. doi: 10.1016/j.atherosclerosis.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Xu Y, Liu Q, Xu Y, Liu C, Wang X, He X, Zhu N, Liu J, Wu Y, Li Y, Li N, Feng T, Lai F, Zhang M, Hong B, Jiang JD, Si S. Rutaecarpine suppresses atherosclerosis in ApoE-/- mice through upregulating ABCA1 and SR-BI within RCT. J Lipid Res. 2014;55:1634–1647. doi: 10.1194/jlr.M044198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maes OC, Chertkow HM, Wang E, Schipper HM. MicroRNA: Implications for Alzheimer Disease and other Human CNS Disorders. Curr Genomics. 2009;10:154–168. doi: 10.2174/138920209788185252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu J, Li Y, Wang F, Wang X, Cheng B, Ye F, Xie X, Zhou C, Lu W. Suppressed miR-424 expression via upregulation of target gene Chk1 contributes to the progression of cervical cancer. Oncogene. 2013;32:976–987. doi: 10.1038/onc.2012.121. [DOI] [PubMed] [Google Scholar]
- 10.Farazi TA, Hoell JI, Morozov P, Tuschl T. MicroRNAs in human cancer. Adv Exp Med Biol. 2013;774:1–20. doi: 10.1007/978-94-007-5590-1_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rayner KJ, Fernandez-Hernando C, Moore KJ. MicroRNAs regulating lipid metabolism in atherogenesis. Thromb Haemost. 2012;107:642–647. doi: 10.1160/TH11-10-0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marquart TJ, Allen RM, Ory DS, Baldan A. MiR-33 links SREBP-2 induction to repression of sterol transporters. Proc Natl Acad Sci U S A. 2010;107:12228–12232. doi: 10.1073/pnas.1005191107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Najafi-Shoushtari SH, Kristo F, Li Y, Shioda T, Cohen DE, Gerszten RE, Naar AM. MicroRNA-33 and the SREBP host genes cooperate to control cholesterol homeostasis. Science. 2010;328:1566–1569. doi: 10.1126/science.1189123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rayner KJ, Suarez Y, Davalos A, Parathath S, Fitzgerald ML, Tamehiro N, Fisher EA, Moore KJ, Fernandez-Hernando C. MiR-33 contributes to the regulation of cholesterol homeostasis. Science. 2010;328:1570–1573. doi: 10.1126/science.1189862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wijesekara N, Zhang LH, Kang MH, Abraham T, Bhattacharjee A, Warnock GL, Verchere CB, Hayden MR. MiR-33a modulates ABCA1 expression, cholesterol accumulation, and insulin secretion in pancreatic islets. Diabetes. 2012;61:653–658. doi: 10.2337/db11-0944. [DOI] [PMC free article] [PubMed] [Google Scholar]