Abstract
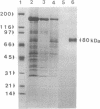
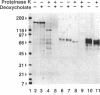
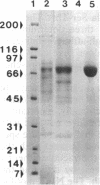
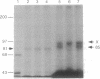
Clathrin-coated vesicles are known to be involved in the transport of proteins from the Golgi to the vacuole in plant cells. The mechanisms by which proteins are directed into this pathway are not known. Here we identify an integral membrane protein of approximately 80 kDa, extracted from clathrin-coated vesicles of developing pea (Pisum sativum L.) cotyledons, that bound at neutral pH to an affinity column prepared with the N-terminal targeting determinant of the vacuolar thiol protease, proaleurain, and eluted when the pH was lowered to 4. The protein was not retained on a control column prepared with the N-terminal sequence of a homologous, secreted thiol protease, endopeptidase B. The 80-kDa protein also accumulated in a membrane fraction that is less dense than clathrin-coated vesicles. In vitro studies demonstrated a binding constant of 37 nM between the approximately 80 kDa protein and the proaleurain targeting determinant. A peptide with a vacuolar targeting determinant from prosporamin weakly competed for binding to the approximately-80 kDa protein, while a peptide carrying a single amino acid substitution known to abolish prosporamin vacuolar targeting had no measurable binding affinity for the protein. The binding protein is a glycoprotein with a transmembrane orientation in which the C terminus is exposed to the cytoplasm. The binding domain is located in the N-terminal luminal portion of the protein. These properties of the binding protein are consistent with the function of a receptor that would select proteins in the trans-Golgi for sorting to clathrin-coated vesicles and delivery to the vacuole.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Bednarek S. Y., Raikhel N. V. Intracellular trafficking of secretory proteins. Plant Mol Biol. 1992 Oct;20(1):133–150. doi: 10.1007/BF00029156. [DOI] [PubMed] [Google Scholar]
- Bednarek S. Y., Raikhel N. V. The barley lectin carboxyl-terminal propeptide is a vacuolar protein sorting determinant in plants. Plant Cell. 1991 Nov;3(11):1195–1206. doi: 10.1105/tpc.3.11.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner M., Chrispeels M. J. Involvement of the Golgi Apparatus in the Synthesis and Secretion of Hydroxyproline-rich Cell Wall Glycoproteins. Plant Physiol. 1975 Mar;55(3):536–541. doi: 10.1104/pp.55.3.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley S. M., Beevers L. Coated Vesicles Are Involved in the Transport of Storage Proteins during Seed Development in Pisum sativum L. Plant Physiol. 1989 Oct;91(2):674–678. doi: 10.1104/pp.91.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley S. M., Beevers L. Isozymes of beta-N-Acetylhexosaminidase from Pea Seeds (Pisum sativum L.). Plant Physiol. 1987 Dec;85(4):1118–1122. doi: 10.1104/pp.85.4.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holwerda B. C., Padgett H. S., Rogers J. C. Proaleurain vacuolar targeting is mediated by short contiguous peptide interactions. Plant Cell. 1992 Mar;4(3):307–318. doi: 10.1105/tpc.4.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch T., Beevers L. Uncoating of clathrin-coated vesicles by uncoating ATPase from developing peas. Plant Physiol. 1993 Sep;103(1):205–212. doi: 10.1104/pp.103.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld S., Mellman I. The biogenesis of lysosomes. Annu Rev Cell Biol. 1989;5:483–525. doi: 10.1146/annurev.cb.05.110189.002411. [DOI] [PubMed] [Google Scholar]
- Kornfeld S. Structure and function of the mannose 6-phosphate/insulinlike growth factor II receptors. Annu Rev Biochem. 1992;61:307–330. doi: 10.1146/annurev.bi.61.070192.001515. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matsuoka K., Nakamura K. Propeptide of a precursor to a plant vacuolar protein required for vacuolar targeting. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):834–838. doi: 10.1073/pnas.88.3.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos M., Jr, Nguyen N. Y., Liu T. Y. Reproducible high yield sequencing of proteins electrophoretically separated and transferred to an inert support. J Biol Chem. 1988 May 5;263(13):6005–6008. [PubMed] [Google Scholar]
- Nakamura K., Matsuoka K. Protein targeting to the vacuole in plant cells. Plant Physiol. 1993 Jan;101(1):1–5. doi: 10.1104/pp.101.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus J. M., Sticher L., Meins F., Jr, Boller T. A short C-terminal sequence is necessary and sufficient for the targeting of chitinases to the plant vacuole. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10362–10366. doi: 10.1073/pnas.88.22.10362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saalbach G., Jung R., Kunze G., Saalbach I., Adler K., Müntz K. Different legumin protein domains act as vacuolar targeting signals. Plant Cell. 1991 Jul;3(7):695–708. doi: 10.1105/tpc.3.7.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahagian G. G., Steer C. J. Transmembrane orientation of the mannose 6-phosphate receptor in isolated clathrin-coated vesicles. J Biol Chem. 1985 Aug 15;260(17):9838–9842. [PubMed] [Google Scholar]
- Schroeder M. R., Borkhsenious O. N., Matsuoka K., Nakamura K., Raikhel N. V. Colocalization of barley lectin and sporamin in vacuoles of transgenic tobacco plants. Plant Physiol. 1993 Feb;101(2):451–458. doi: 10.1104/pp.101.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong P. Y., Tollefsen S. E., Kornfeld S. The cation-independent mannose 6-phosphate receptor binds insulin-like growth factor II. J Biol Chem. 1988 Feb 25;263(6):2585–2588. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]