Abstract
Introduction: Ghrelin is a novel brain-gut peptide hormone consisted of 28 amino-acid. In the plasma, it exists in two major molecular forms, acylated and des-acyled ghrelin, filtered in glomeruli or secreted by nephrons. Primary biological effects of hormones are regulating appetite, foods intake and energy metabolism. We investigated the changing and relationships between serum and urine ghrelin levels in acute stroke patients to provide more information whether diagnostic parameter. Methods: Thirty acute stroke patients and thirty consecutive volunteers included in study prospectively. To analyze serum and urine ghrelin levels, at the time of diagnose, all of participant blood and fresh urine (1 ml serum, 2 ml urine respectively) samples were obtained. Serum ghrelin levels analyzed ELISA technique, and urine ghrelin levels studied by validation technique. To compare quantitative data student’s t test, and for qualitative data chi-square and Fisher’s Exact Chi-square test was used. P<0.05 was considered statistically significant. Results: Urine acyl ghrelin levels found statistically significant between patient and control groups (P=0.001), but there were no statistically significant differences between both groups (P>0.05) in serum acyl gherelin, des-acyl ghrelin and urine des-acyle ghrelin levels. Conclusions: The results indicate that urine acyl ghrelin levels may be considered as a diagnostic parameter in acute ischemic stroke patients. Further studies delineating the mechanism of these observed results are warranted.
Keywords: Stroke, acute, ghrelin, serum, urine
Introduction
Ischemic stroke is the second most common cause of death and major cause of disability worldwide. The stroke is generally defined as any disease process that interrupts blood flow to the brain and causes focal neurologic sendroms [1,2]. Injury is related to the loss of oxygen and glucose substrates necessary for high-energy phosphate production and the presence of mediators of secondary ischemic cellular injury. When a cell becomes sufficiently depleted of oxygen and nutrients, pro-apoptotic genes involved in cell death are activated. There are three principal types of cell death induced by cerebral ischemia and hypoxic injury; apoptosis, necrosis, and autophagy [2]. The more delayed cell death that occurs in the ischemic penumbra (3-24 hours and beyond) via principally apoptotic and autophagic mechanisms, against which ghrelin may be useful [3]. Relationship between stroke and endocrine system has been investigated for many years. It is known that stroke may causes transient or permanent changes of hormones secreted by circadian rhythm [4,5].
Ghrelin is a novel brain-gut peptide hormone consisted of 28 amino-acid was discovered by Japan scientists Kojima et al. in 1999 [6,7]. In the plasma, it exist in two major molecular forms, acyl and des-acyl ghrelin, the latter of which is the result of post-translational octanoylation of pro-ghrelin by the enzyme ghrelin-O-acyltransferase (GOAT) [8,9]. Circulating ghrelin consists of more than 90% of des-acyl ghrelin and less than 10% acyl ghrelin. They are secreted in a pulsated manner as its level increases before the onset of meal, during fasting, and decreases after feeding [10]. Both ghrelin forms have been reported to prevent cell death by increasing mitochondrial function in cultured neurons exposed to oxygen and glucose deprivation (an in vitro model of ischemia) [2,11,12]. Ghrelin also protects against inflammation that plays a significant role which contrubutes to brain injury in hours to day after ischemic events [13]. Mean circulating half life of total ghrelin (acyl + desacyl ghrelin) was in the range of 35 min [10]. Ghrelin circulates into the bloodstream bound to lipoproteins [14], and filtered in the glomeruli or secreted by nephrons [15].
Circulating ghrelin is predominantly produced by specific cells in the stomach’s oxyntic glands X/A-like cells of the fundus mucosa representing about 20% of gastric mucosal cells in humans [16], where 65-90% of the circulating ghrelin is synthesized [17-19]. The small intestine is the second major source of ghrelin synthesis and secretion but there are other sources of ghrelin, for example in the hypothalamus, pituitary, arcuate nucleus and various parts of the central nervous system (CNS), salivary and thyroid glands, breast, small intestine, kidney and heart that should be taken into consideration [20]. The major biological functions of ghrelin include the secretion of growth hormone, the stimulation of appetite and food intake, the modulation of gastric acid secretion and motility, and the modulation of the endocrine and exocrine pancreatic secretions. The hormone exerts biological effects via the activation of growth hormone secretagogue receptor-1a (GHSR-1a), the presence of which was confirmed in different parts of the gastrointestinal (GI) tract and midbrain structures [9,10,21]. Only acyl form of ghrelin stimulates growth hormone (GH) release. In contrast, des-acyl ghrelin is the most abundant form in plasma and does not bind GHS-R1a. It has no GH stimulating activity or any effect on other anterior pituitary function. A desacyl ghrelin not only a reservoir but also both types of ghrelin exhibit similar biological activities [22]. Some of variety biological effects of hormones are regulating appetite, foods intake and energy metabolism via mediating endogenous GH [23,24]. Ghrelin also exerts numerous peripheral effects such as direct effect on endocrine and exocrine pancreatic functions, the cardiovascular functions, gastric secretion, gastrointestinal (GI) motility, glucose release, cell proliferation, reproductive functions, and sleep [9,20].
It is also now evident that ghrelin can be a powerful neuroprotective agent in experimental models of cerebral ischemia. Exogenous ghrelin also improves neurological deficit, infarct size, and survival of cortical neurons in rodents after transient focal ischemia-reperfusion (middle cerebral artery occlusion; MCAO) [25,26]. Local expression of ghrelin receptor has also been shown in the cerebral cortex, and ghrelin has been shown to inhibit apoptosis, stabilizing the mitochondrial membrane potential, and improving mitochondrial function by regulating reactive oxygen species (ROS), respiration, enzyme activity and mitochondrial gene expression in transient focal cerebral ischemia [12,25,27]. Middle cerebral artery occlusion (MCAO) rat models had significantly showed lower gastric kinetic and elevated serum ghrelin levels in the early stage of cerebral ischemia. This can be explained as a compensatory reaction in the early ischemic stage. The up-regulation of serum ghrelin may prevent brain damage and impose decreased intestinal motility so as to decrease injury to the mucosal barrier [28].
We conducted this study to investigate prospectively serum and urine ghrelin levels in patients with acute stroke.
Materials and methods
Study design
This prospective study was conducted between June 22 and December 5, 2007 in the ED of a tertiary care hospital. The study was approved by the local ethics committee. Informed consent approval was obtained from each patient before study enrollment. The informed consent was composed of four parts: patient name, aim and expected benefits of the study, and the rights of patients during the study.
Study setting and population
Consecutive 30 patients with acute ischemic stroke presenting to ED during the study period and 30 healthy volunteers for control group who were at the same age group with patients was included study. Mean admission time to the ED of patients with acute stroke is three hours (range 0-36 h). Because ghrelin values may be affected from one or more of the following conditions, this patients were excluded from the study: the presence of active infection or gastric ulcer; recent antineoplastic therapy such as chemotherapy, radiation therapy or surgery; other primary cachectic states such as thyroid disease or severe liver disease, and chronic renal impairment, hemorrhagic stroke, subarachnoid hemorrhage (SAH), intracranial mass, ischemic stroke beyond 48 h, chronic obstructive pulmonary disease, congestive heart failure, cardiac or cancer related cachexia, hyperthyroidism, hypogonadism, cushing’s syndrome, polycystic ovarian syndrome, anorexia or bulimia nervosa , fasting and chronic food restriction, obesity, type 1 DM, type 2 DM and the patients who ingesting some hormones, drug and nutrients (Table 1). The eligibility of patients for the study was determined by an attending emergency physician between 8:00 a.m. and midnight and by a senior emergency medicine (EM) resident from midnight to 8:00 a.m.
Table 1.
Shows study exclusion criteria
| Some disease and conditions | Some hormons, drug and nutrients |
|---|---|
| Gastric ulcer | Acetylcholine agonists |
| Hyperthyroidism | Glucose load |
| Severe liver disease | Arginine |
| Chronic renal impairment | GH, GHRH, or GHRH plus |
| Hemorrhagic stroke | Oxyntomodulin |
| Subarachnoid hemorrhage | Leptin |
| Ischemic stroke beyond 48 hours | Testosterone |
| Chronic obstructive pulmonel disease | Insulin |
| Congestive heart failure | Urocortin |
| Hypogonadism | Somatostatin |
| Cushing’s syndrome | Oestrogen |
| Polycystic ovarian syndrome | Thyroid hormones |
| Anorexia or Bulimia nervosa | Glucocorticoids |
| Fasting and chronic food restriction | Cortistatin |
| Obesity | Sex steroids |
| Type 1 DM, Type 2 DM | Oral protein load |
| Cardiac or cancer related cachexia | |
| Presence of active infection | |
| Recent antineoplastic therapy (chemotherapy, radiation therapy or surgery) |
Abbreviations: DM, Diabetes mellitus; GH, Growth hormone; GHRH, Growth hormone releasing hormone.
Study protocol
Data of study were collected by EM residents. The diagnose was confirmed with patient history, physical examination, cranial computerized tomography, carotid doppler ultrasound, magnetic resonance imaging (MRI) and diffusion imaging in all patients. MRI was used as the gold standard test for diagnosed with acute ischemic stroke. All patients were treated in the neurologic care unit of the university hospital.
Laboratory measurements
Blood and fresh urine (1 ml serum, 2 ml urine respectively) samples of all participants were obtained for analyze serum and urine ghrelin levels at the time of diagnose simultaneously. To prevent peptide hormones from cellular protease for each 1 ml of blood 20-30 µl aprotinin was added to each samples tube. After centrifugations, 1/10 volume ratio 1N HCL was added to derived samples, and stored at -80°C until they were assayed [10,19,29]. Ghrelin plasma levels were measured using an enzyme-linked immunosorbent assay (ELISA) technique with Biotec L 800 device and urine ghrelin levels were studied by validation technique.
Data analysis
The study data were analyzed by descriptive statistical methods (mean ± standard deviation). To compare quantitative data student’s t test, and for comparison of qualitative data, chi-square and Fisher’s Exact Chi-square test was used. P value <0.05 was considered significant.
Results
A total of 30 patients with acute ischemic stroke were admitted to the ED during the study period. The patients included 13 females (43.3%) and 17 men (56.7%). The control group included 14 females (46.7%) and 16 men (53.3%). The study subjects had a mean age of 69.77±1.76 (50-86) for patient and 66.23±2 (35-88) for control group, there were no significant statistical age and gender differences between both groups (P>0.05) (Table 2).
Table 2.
Shows demographic data
| Patient (n=30) | Control (n=30) | P | ||
|---|---|---|---|---|
| Age (years) | 69.77±1.76 | 66.23±2.35 | P>0.05 | |
| Gender | Female | 13 (43.3%) | 14 (46.7%) | P>0.05 |
| Male | 17 (56.7%) | 16 (53.3%) | P>0.05 | |
The serum acyl ghrelin levels were 19.88±1.44 pg/ml in patients and 26.63±6.26 pg/ml in control group. There were no statistically significant differences between both groups (P>0.05) (Table 3; Figure 1). Urine acyl ghrelin levels were 18.20±1.96 pg/ml in patients, and 9.80±1.19 pg/ml in control group. Urine acyl ghrelin levels were determined statistically significant between patient and control group (P=0.001) (Table 3; Figure 2).
Table 3.
Serum and Urine acyl ghrelin level
| Patient (n=30) | Control (n=30) | P | |
|---|---|---|---|
| Serum (pg/ml) | 19.88±1.44 | 26.63±6.26 | P>0.05 |
| Urine (pg/ml) | 18.20±1.96 | 9.80±1.19 | P=0.01 |
Figure 1.
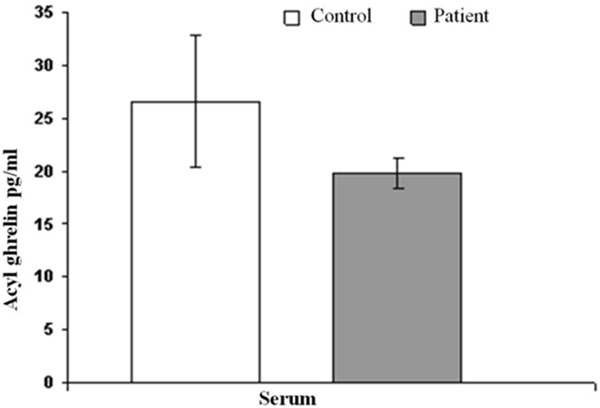
Shows variation between patient and control groups serum acyl ghrelin levels.
Figure 2.
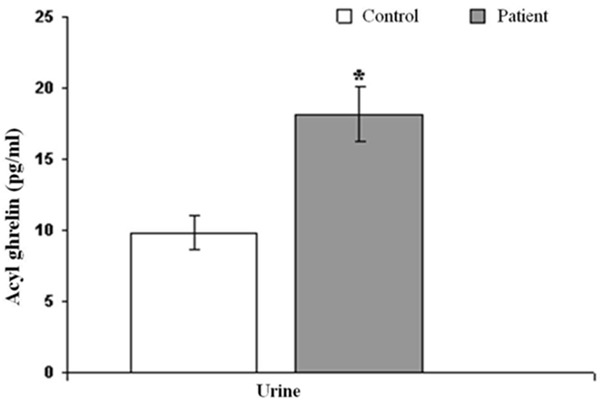
Shows variation between patient and control group’s urine acyl ghrelin levels.
The serum des- acyl ghrelin levels were 937.80±144.14 pg/ml in patients and 705.30±102.19 pg/ml in control group. There were no statistically significant differences between both groups (P>0.05) (Table 4; Figure 3). Urine des-acyl ghrelin levels were 299.25±183.74 pg/ml in patient group, and 127.67±31.57 pg/ml in control group. There was no statistically significant difference between both groups (P>0.05) (Table 4; Figure 4).
Table 4.
Serum and Urine des-acyl ghrelin levels
| Patient (n=30) | Control (n=30) | P | |
|---|---|---|---|
| Serum (pg/ml) | 937.80±144.14 | 705.30±102.19 | P>0.05 |
| Urine (pg/ml) | 299.25±183.74 | 127.67±31.57 | P>0.05 |
Figure 3.
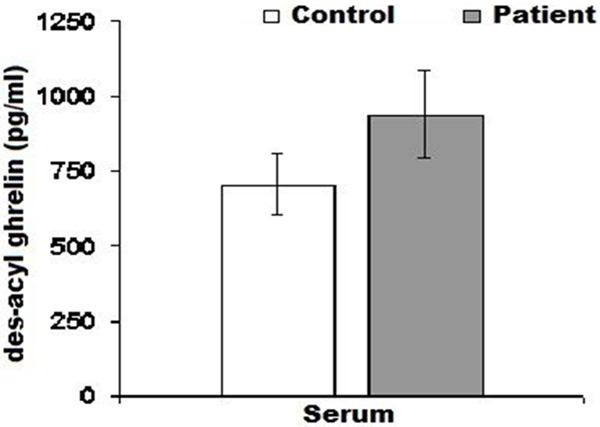
Shows variation between patient and control groups serum des-acyl ghrelin levels.
Figure 4.
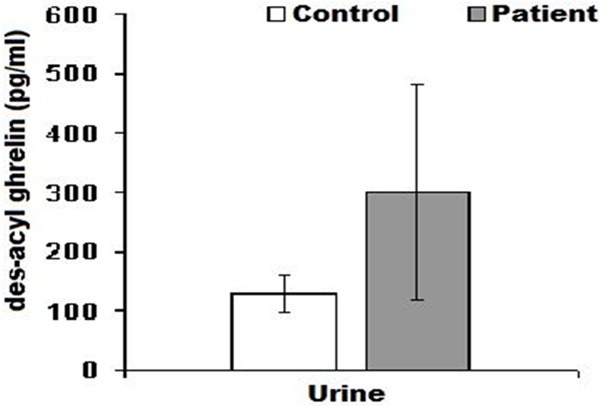
Shows variation between patient and control groups urine des-acyl ghrelin levels.
Discussion
In present study, we observed that urine acyl ghrelin levels were determined statistically significant between patient and healthy control subjects, whereas there were no statistically significances between urine des-acyl ghrelin levels and serum acyled and des-acyled ghrelin values in both groups. To the best of our knowledge, because this is first study in literature analyzing of urine acyl and des-acyl grelin levels in patients with acute ischemic stroke, these results may be important.
Takeda et al. found that administration of ghrelin subcutaneously to acute renal ischemic rats showed that the ghrelin protective effects realized through increasing serum IGF-1 levels [30]. El Eter at al. investigated acute gastric ischemia and reperfusion injury generated by clamping rat celiac artery and reported that ghrelin protects gastric mucosa against ischemic injury of the stomach through its antioxidant properties [31]. Miao et al. found that the expression of ghrelin’s receptor GHSR-1a in cerebral cortex of rat were obviously decreased by ischemia/reperfusion injury and increased by ghrelin (i.v.). They also showed that ghrelin (i.v.) prevented cortical neurons from injury induced by in vivo ischemia/reperfusion [25]. Massadi et al. reported that, pre-administration of a GHSR1a antagonist completely reversed the neuroprotective effect of acylated ghrelin but not des-acyl ghrelin, suggesting that des-acylated ghrelin has neuroprotective effects independent of GHSR1a [18].
Krazewski et al. conducted a study concerning with ischemia and reperfusion injury on animals, and found that ghrelin significantly reduced in cortical neuronal apoptosis [32]. Hwang et al. examined the role of prostate apopitosisrespons-4 (Par-4), a proapoprotic gene the expression of which is increased after ischemic injury, in ghrelin-mediated neuroprotection during middle cerebral artery occlusion (MCAO). Finally they demonstrate that peripheral administration of acylated and des-acylated ghrelin protected cortical neurons from ischemic injury caused by MCAO. The protective effects of ghrelin and des-acyl ghrelin appear to be related with an inhibition of the expression of Par-4. Another probable mechanism of ghrelin and deacyl ghrelin is anti-apoptotic effects by targeting Bcl-2 protein family, inhibiting cytochrom c release and caspase-3 activity. Their data suggest that ghrelin, independent of its acylation, may have therapeutic potential for the treatment of stroke and can function as a neoroprotective agent [9]. William et al. conducted a rat study and showed that ghrelin can cross the blood-brain barrier (BBB) and plays neuroprotective role in ischemic area of the brain [33].
Cheyuo et al. studied the effect of ghrelin in permanent focal cerebral ischemia and the role of the vagus nerve in its action in rat models underwent right-sided permanent middle cerebral artery occlusion (MCAO) with or without prior bilateral truncal vagotomy and they said that human ghrelin is a neuroprotective agent that inhibits inflammation, nNOS activity, and apoptosis in focal cerebral ischemia through a vagal pathway [25]. Ersahin et al. investigate to elucidate the putative neuroprotective effects of ghrelin in subarachnoid hemorrhage (SAH)-induced brain injury, and declared that ghrelin alleviates SAH-induced oxidative brain damage, and exerts neuroprotection by maintaining a balance in oxidant-antioxidant status, by inhibiting proinflammatory mediators, and preventing the depletion of endogenous antioxidants evoked by SAH [34].
The circulating concentration ghrelin has been found to be related to a meal pattern learning independent of the nutrient status. Total plasma ghrelin levels increase pre-prandialy and decrease post-prandialy [16,22]. The mechanisms responsible for preprandial surges in ghrelin are not known completely, but they are probably triggered by the sympathetic nervous system. In rats and humans, ghrelin secretion varies markedly throughout the day, with peaks preceding food intake. The nocturnal increase in plasma ghrelin concentration is blunted in obese subjects and by sleep deprivation. It is suggested that the depth and duration of prandial suppression of ghrelin concentration is related to the number of calories ingested, and that ingested carbohydrates proteins and lipids suppress ghrelin levels which latter less effectively than others [35]. Increases in circulating ghrelin expression have also been correlated with the disease severity and sequelae. Additionally ghrelin levels correlated with the expression of proinflammatory cytokines, more specifically, TNF-α, IL-6 and IL-1β as classic markers of inflammation. Elevated levels of S-100 in plasma after head trauma, subarachnoid hemorrhage, and stroke have been correlated with the extent of brain damage and indicate blood–brain barrier dysfunction [16].
Various hormones, drugs and condition also affect serum and plasma ghrelin levels. Insulin, either oral or intravenous glucose, somatostatin and its natural analogue cortistatin, and GH suppress systemic concentrations of ghrelin. It has been recently demonstrated that plasma ghrelin is decreased in obesity and negatively correlated with BMI. However, little was known about circulating ghrelin levels in patients with stroke [29,35].
Some studies suggested that ghrelin levels are reduced in clinical stroke populations. Miao et al. outlined that the expression of ghrelin’s receptor (GHSR-1a) in cerebral cortex of rat were obviously decreased by ischemia/reperfusion injury and increased by ghrelin infusion (i.v.). In particular, ghrelin levels have been reported to be lower in male patients after cardio embolic stroke compared with the healthy controls [36,37]. Age may be an important indicator and parallel to differences in the circulating levels of total and acylated ghrelin. Two recent studies in elderly subjects have revealed diminished levels of ghrelin in people 70 years or older and that these lower levels correlated with a decline in nutritional status [22].
Circulating ghrelin is lower in patients who have experienced stroke than in the general population when controlling for factors such as age and obesity [2]. In this study, the time of acute stroke period we found that the patients’ serum and plasma ghrelin level did not changed secondary to against ischemia when compared with control subjects. Given the apparent neuroprotective properties of ghrelin, restoring circulating ghrelin levels after stroke therefore may produce significant beneficial outcomes on neurological and cognitive functions [38,39]. The mechanisms of these effects are unclear; however, a combination of the anti-apoptotic and inflammatory modulatory effects of ghrelin may play a role.
Conclusion
Recently, the studies about ghrelin being popular marker have increasingly continued. We suggest that ghrelin having neuroprotective properties should be infused to the patients, especially aged peoples, who experienced acute stroke episode to improve prognosis. Also, our results demonstrate that urine acyl ghrelin level elevated in patients with acute stroke. But we do not know whether these results may be used as diagnostic or prognostic scale, comprehensive studies are needed to support these results.
Disclosure of conflict of interest
None.
References
- 1.Korkmaz T, Ersoy G, Kutluk K. An Evaluation of Pre-Admission Factors Affecting the Admission Time of Patients with Stroke Symptoms. Turk J Emerg Med. 2011;11:28–31. [Google Scholar]
- 2.Spencer SJ, Miller AA, Andrews ZB. The Role of Ghrelin in Neuroprotection after Ischemic Brain Injury. Brain Sci. 2013;3:344–59. doi: 10.3390/brainsci3010344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broughton BR, Reutens DC, Sobey CG. Apoptotic mechanisms after cerebral ischemia. Stroke. 2009;40:e331–9. doi: 10.1161/STROKEAHA.108.531632. [DOI] [PubMed] [Google Scholar]
- 4.Schwarz S, Schwab S, Klinga K, Maser-Gluth C, Bettendorf M. Neuroendocrine changes in patients with acute space occupying ischaemic stroke. J Neurol Neurosurg Psychiatry. 2003;74:725–7. doi: 10.1136/jnnp.74.6.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skvortsova VI, Platonova IA, Ostroutsev IV, Zhuravleva EIu, Chigraĭ ZA, Efremova NM, Ogareva NV. The influence of hormones of stres-promoting system on the course of acut ischemik stroke. Zh Nevrol Psikhiatr Im S S Korsakova. 2000;100:22–7. [PubMed] [Google Scholar]
- 6.Kojima M, Hosada H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–659. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 7.Ukkola O. Endocrinological activities of ghrelin: new insights. Eur J Intern Med. 2003;14:351–356. doi: 10.1016/s0953-6205(03)90000-8. [DOI] [PubMed] [Google Scholar]
- 8.Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell. 2008;132:387–96. doi: 10.1016/j.cell.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 9.Hwang S, Moon M, Kim S, Hwang L, Ahn KJ, Park S. Neuroprotective effect of ghrelin is associated with decreased expression of prostate apoptosis response-4. Endocr J. 2009;56:609–17. doi: 10.1507/endocrj.k09e-072. [DOI] [PubMed] [Google Scholar]
- 10.Delporte C. Structure and physiological actions of ghrelin. Scientifica (Cairo) 2013;2013:518909. doi: 10.1155/2013/518909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chung H, Seo S, Moon M Park S. Phosphati-dylinositol-3-kinase/Akt/glycogen synthase kinase-3 beta and ERK1/2 pathways mediate protective effects of acylated and unacylated ghrelin against oxygen-glucose deprivation-induced apoptosis in primary rat cortical neuronal cells. J Endocrinol. 2008;198:511–21. doi: 10.1677/JOE-08-0160. [DOI] [PubMed] [Google Scholar]
- 12.Andrews ZB. The extra-hypothalamic actions of ghrelin on neuronal function. Trends Neurosci. 2011;34:31–40. doi: 10.1016/j.tins.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J Neuroimmunol. 2007;184:53–68. doi: 10.1016/j.jneuroim.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vriese CD, Perret J, Delporte C. Focus on the short-and long-term effects of ghrelin on energy homeostasis. Nutrition. 2010;26:579–584. doi: 10.1016/j.nut.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 15.Yoshimito A, Mori K, Sugawara A, Mukoyama M, Yahata K, Suganami T, Takaya K, Hosoda H, Kojima M, Kangawa K, Nakao K. Plasma ghrelin and desacyl ghrelin concentrations in renal failure. J Am Soc Nephrol. 2002;13:2748–2752. doi: 10.1097/01.asn.0000032420.12455.74. [DOI] [PubMed] [Google Scholar]
- 16.Castañeda TR, Tong J, Datta R, Culler M, Tschöp MH. Ghrelin in the regulation of body weight and metabolism. Front Neuroendocrinol. 2010;31:44–60. doi: 10.1016/j.yfrne.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 17.Lorenzi T, Meli R, Marzioni D, Morroni M, Baragli A, Castellucci M, Gualillo O, Muccioli G. Ghrelin: a metabolic signal affecting the reproductive system. Cytokine Growth Factor Rev. 2009;20:137–152. doi: 10.1016/j.cytogfr.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Al Massadi O, Tschöp MH, Tong J. Ghrelin acylation and metabolic control. Peptides. 2011;32:2301–8. doi: 10.1016/j.peptides.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 19.Aydın S. Discovery of Ghrelin Hormone: Research and Clinical Applications. Turk J Biochem. 2007;32:82–95. [Google Scholar]
- 20.Dong XY, Xu J, Tang SQ, Li HY, Jiang QY, Zou XT. Ghrelin and its biological effects on pigs. Peptides. 2009;30:1203–11. doi: 10.1016/j.peptides.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Pawlık MW, Obuchowıcz R, Bıernat J, Szczepanski W, Pajdo R, Kwiecień S, Brzozowski T, Konturek SJ, Pawlik WW. Effects of peripherally and centrally applied ghrelin in the pathogenesis of ischemia-reperfusion induced injury of the small intestine. J Physiol Pharmacol. 2011;62:429–439. [PubMed] [Google Scholar]
- 22.Baatar D, Patel K, Taub DD. The effects of ghrelin on inflammation and the immune system. Mol Cell Endocrinol. 2011;340:44–58. doi: 10.1016/j.mce.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 23.Kojima M, Kangawa K. Ghrelin: structure and function. Physiol Rev. 2005;85:495–522. doi: 10.1152/physrev.00012.2004. [DOI] [PubMed] [Google Scholar]
- 24.Kierson JA, Dimmetteo DM, Loche RG, Mackley AB, Spear ML. Ghrelin is cholecsytokinin in term and preterm human breast milk. Acta Paediatr. 2006;95:991–995. doi: 10.1080/08035250600669769. [DOI] [PubMed] [Google Scholar]
- 25.Cheyuo C, Wu R, Zhou M, Coppa G, Wang P. Ghrelin suppresses inflammation and neuronal nitric oxide synthase in focal cerebral ischemia via the vagus nerve. Shock. 2011;35:258–265. doi: 10.1097/SHK.0b013e3181f48a37. [DOI] [PubMed] [Google Scholar]
- 26.Miao Y, Xia Q, Hou Z, Pan H, Zhu S. Ghrelin protects cortical neuron against focal ischemia/reperfusion in rats. Biochem Biophys Res Commun. 2007;359:795–800. doi: 10.1016/j.bbrc.2007.05.192. [DOI] [PubMed] [Google Scholar]
- 27.Shimada T, Furuta H, Doi A, Ariyasu H, Kawashima H, Wakasaki H, Nishi M, Sasaki H, Akamizu T. Des-acyl ghrelin protects microvascular endothelial cells from oxidative stress-induced apoptosis through sirtuin 1 signaling pathway. Metabolism. 2014;63:469–74. doi: 10.1016/j.metabol.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 28.Xu X, Zhu Y, Chuai J. Changes in serum ghrelin and small intestinal motility in rats with ischemic stroke. Anat Rec (Hoboken) 2012;295:307–312. doi: 10.1002/ar.21490. [DOI] [PubMed] [Google Scholar]
- 29.Chen Y, Ji XW, Zhang NA, Lv JC, Zhang JG, Zhao CH. Prognostic Value of Plasma Ghrelin in Predicting the Outcome of Patients with Chronic Heart Failure. Arch Med Res. 2014:263–269. doi: 10.1016/j.arcmed.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 30.Takeda R, Nishimatsu H, Suzuki E, Satonaka H, Nagata D, Oba S, Sata M, Takahashi M, Yamamoto Y, Terauchi Y, Kadowaki T, Kangawa K, Kitamura T, Nagai R, Hirata Y. Ghrelin improves renal function in mice with ischemic acute renal failure. J Am Soc Nephrol. 2006;17:113–121. doi: 10.1681/ASN.2004080626. [DOI] [PubMed] [Google Scholar]
- 31.El Eter E, Al Tuwaijiri A, Hagar H, Arafa M. In vivo and in vitro antioxidant activity of ghrelin: Attenuation of gastric ischemic injury in the rat. J Gastroenterol Hepatol. 2007;22:1791–1799. doi: 10.1111/j.1440-1746.2006.04696.x. [DOI] [PubMed] [Google Scholar]
- 32.Krajewski S, Krajewska M, Ellebry LM. Release of caspase-9 from mitochondria during neuronal apoptosis and cerebral ischemia. Proc Natl Acad Sci U S A. 1999;96:5752–5757. doi: 10.1073/pnas.96.10.5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Willam AB, Tschöp M, Sandra A, Heiman ML. Extent and direction of transport across the blood-brain barrier is determined by its unique primary structure. J Pharmacol Exp Ther. 2002;302:822–827. doi: 10.1124/jpet.102.034827. [DOI] [PubMed] [Google Scholar]
- 34.Ersahin M, Toklu HZ, Erzik C, Cetinel S, Akakin D, Velioğlu-Oğünç A, Tetik S, Ozdemir ZN, Sener G, Yeğen BC. The anti-inflammatory and neuroprotective effects of ghrelin in subarachnoid hemorrhage induced oxidative brain damage in rats. J Neurotrauma. 2010;27:1143–1155. doi: 10.1089/neu.2009.1210. [DOI] [PubMed] [Google Scholar]
- 35.Leite-Moreira AF, Soares JB. Physiological, pathological and potential therapeutic roles of ghrelin. Drug Discov Today. 2007;12:276–88. doi: 10.1016/j.drudis.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 36.Miao Y, Xia Q, Hou Z, Zheng Y, Pan H, Zhu S. Ghrelin protects cortical neuron against focal ischemia/reperfusion in rats. Biochem Biophys Res Commun. 2007;359:795–800. doi: 10.1016/j.bbrc.2007.05.192. [DOI] [PubMed] [Google Scholar]
- 37.Kantorova E, Chomova M, Kurca E, ivak S, Zelenak K, Kučera P, Galajda P. Leptin, adiponectin and ghrelin, new potential mediators of ischemic stroke. Neuro Endocrinol Lett. 2011;32:716–721. [PubMed] [Google Scholar]
- 38.Kenny R, Cai G, Bayliss JA, Clarke M, Choo YL, Miller AA, Andrews ZB, Spencer SJ. Endogenous ghrelin’s role in hippocampal neuroprotection after global cerebral ischemia: does endogenous ghrelin protect against global stroke? Am J Physiol Regul Integr Comp Physiol. 2013;304:R980–90. doi: 10.1152/ajpregu.00594.2012. [DOI] [PubMed] [Google Scholar]
- 39.Lopez NE, Lindsay G, Karina LR, Mary HA, Putnam J, Eliceiri B, Coimbra R, Bansal V. Ghrelin decreases motor deficits after traumatic brain injury. J Surg Res. 2014;187:230–6. doi: 10.1016/j.jss.2013.09.030. [DOI] [PubMed] [Google Scholar]


