Abstract
More and more evidences suggestted that ApoE plays an important role in modulating the systemic and central nervous inflammatory responses. However, there is a lack of exacted mechanism of ApoE. In this study, we aimed to investigate whether apolipoprotein E (ApoE) induced inflammatory responses and apoptosis in neonatal mice brain from ApoE deficient (ApoE-/-) and wildtype (WT). Compared to control group, the microglia cell from ApoE-/- mice showed more severe inflammation and cell death such as iNOS and IL-1β. Furthermore, anti-inflammatory such as TGF-β, IL-10 from microglia and astrocytes in ApoE-/- mice were decreased. On the other way, TGF-β from astrocytes can inhibit inflammation factors secretion from microglia. Our findings suggested that the anti- inflammation factor such as IL-10 mainly from microglia and TGF-β mainly from astrocyte is significant decreased after Loss of ApoE function in ApoE-/- mice which induced severe inflammation. Furthrtmore, anti- inflammation factor such as IL-10 and TGF-β Therefore, we conclude that apolipoprotein E knockout induced inflammatory responses related to microglia in neonatal mice brain via astrocytes.
Keywords: Apolipoprotein E, mice, cerebral palsy, inflammatory responses
Introduction
Apolipoprotein E (ApoE) is a lipid transport protein abundantly expressed in brain cells [1]. In central neural system, ApoE is packaged with cholesterol and phospholipid to form lipid-protein complexes which are then released into the extracellular space. The complexes bind to ApoE receptors on the surfaces of nerve cells, allowing them to be internalized and providing a mechanism for the maintenance and repair of cell membranes, neurotransmissions, and brain response to hazards [1,2]. ApoE has emerged as a key contributing factor as inflammation in a number of neurodegenerative diseases, including Alzheimer’s disease (AD), Parkinson’s disease (PD), amyotrophic lateral sclerosis (ALS), traumatic brain injury, and HIV-encephalitis cerebral palsy (CP) [3,4]. However, there is need more mechanism study.
Microglia are pivotal in immune surveillance and also facilitate the coordinated responses between the immune system and the brain [5,6]. For example, microglia interprets and propagates inflammatory signals that are initiated in the periphery. However, Recent evidence suggests that astrocytes may play a key role in regulating demyelinating CNS diseases [3,7-9]. Furthermore, more and more evidences also suggests that ApoE plays an important role in modulating the systemic and central nervous inflammatory responses which dependent the interaction between microglia and astrocyte [10,11]. However, the exactly mechanism need to study via ApoE deficient (ApoE-/-) mouse model. In our research, we evaluated the microglia features in apolipoprotein E deficient (ApoE-/-) mice. Compared to control group, the microglia cell from ApoE-/- mice showed more severe inflammation and cell death such as iNOS and IL-1β. Furthermore, anti-inflammatory such as TGF-β, IL-10 from microglia and astrocytes in ApoE-/- mice were decreased. On the other way, TGF-β from astrocytes can inhibit inflammation factors secretion from microglia. However, the above findings related the ApoE pathway. Therefore, we conclude that apolipoprotein E knockout induced inflammatory responses related to microglia in neonatal mice brain via astrocytes.
Materials and methods
Animals
Twenty C57BL/6 ApoE deficient mice (ApoE-/-) and Twenty C57BL/6 wild-type (WT) mice were bought from Vital River company, Beijing. Mice were maintained under specific pathogen-free conditions and housed with a 12-h light-dark cycle. Free access to a standard laboratory chow diet and drinking water was provided.
Microglia-astrocyte transwell co-cultures
Primary cultures of normal and ApoE-/- mice hippocampi astrocytes were prepared according to the previous method [12,13]. And then they were plated at 50,000-75,000 cells/well in a 24-well transwell plate (Corning Life Sciences). After incubation for 3 h, astrocytes were added to a removable 0.4 lm polycarbonate membrane at an equal number as microglia from normal and ApoE-/- mice Hippocampi. In other way, the exogenous TGF-β with 100 pg/ml was used to treat with microglia from normal and ApoE-/- mice Hippocampi to make sure whether anti-inflammatory role of astrocytes on microglia. After 24 h, the supernatants.
Determination of IL-1β, TGF-β, IL-10 concentration
After cell culture, the supernatants of microglia or astrocyte in the hippocampi and co-culture of microglia-astrocyte were collected and stored at -80°C till assay. Then, the content of IL-1β, TGF-β, IL-10 of microglia or astrocyte in the hippocampi and co-culture of microglia-astrocyte were assayed using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions.
The detection of iNOS from microglia
INOS activity was measured by using an assay kit (Jiancheng Bioengineering Institute, Nanjing, China). The assay for iNOS activity depends on the ability of the synthase to catalyze arginine (Arg) to form NO, which can further react with nucleophilic substances to produce chromophoric compound, which has a peak absorbance at 530 nm. iNOS activity can be determined based on fact that nNOS and eNOS were Ca2+-dependent, while iNOS was Ca2+-independent. One unit of iNOS activity was defined as the amount that formed 1 nmol NO in 1 min per milliliter medium. In brief, the microglia from different groups were ultrasonic broken cells and homogenized in 250 μl chilled 50 mM NOx (NO and NOz) was measured using a Sievers 280 Nitric Oxide Chemiluminescent Analyzer (Sievers Instruments, Boulder, CO). Acid and reducing agents (vanadium III chloride in 1 M hydrochloric acid) at 95°C were added to brain samples, converting NO3- and NO2- to NO. An inert gas was then used to purge NO from the solution, which was detected by chemiluminescence, with a sensitivity limit of detection of 20 nM. Standard curves were constructed, and the amount of NO3- and NO2- in brain was determined.
Western blotting
Primary cultures of normal and ApoE-/- mice hippocampi astrocytes or microglia from WT and ApoE-/- group (5/group) were prepared in 50 mM Tris-HCl, 150 mM NaCl, 0.1% Triton X-100, 0.25% sodium deoxycholate, 0.1% SDS, 1% protease inhibitor cocktail and centrifuged 30 min at 13 000 g at 4°C. Total protein amount of supernatant was measured by the Bradford assay, using bovine serum albumin as a standard. Samples containing equal amount of protein were boiled in SDS-mercaptoethanol sample buffer, separated by 10-12% SDS-PAGE gel and electrically transferred to PVDF (Millipore Corporation; Billerica, MA, USA) membranes. Nonspecific binding was blocked by pre-incubation in skim milk. The PVDF membranes were then incubated overnight at 4°C with primary antibodies against p-STAT-3 (Santa Cruz, CA, USA), lab1 (Cell Signaling Technology, Danvers, MA, USA), cleaved caspase-3 (Cell Signaling Technology, Danvers, MA, USA), β-tubulin (Cell Signaling Technology, Danvers, MA, USA). Bound primary antibody was detected with HRP-conjugated secondary antibody (1:10000; Cell Signaling Technology, Danvers, MA, USA).
Statistical analysis
Results are expressed as mean ± SD. Data were analyzed by one-way ANOVA followed by Tukey’s post hoc test for multiple comparisons using Prism software A P-value<0.05 was regarded as significant.
Results
Loss of apolipoprotein E function induced microglia cell apoptosis
To test the role of ApoE in microglia from WT and ApoE-/- mice, the protein level of active and total caspase-3 of microglia of hippocampi were analyzed in WT and ApoE-/- mice with WB. As shown in Figure 1, the protein from microglia of ApoE-/- mice expression was significantly higher than WT controls (P<0.05).
Figure 1.
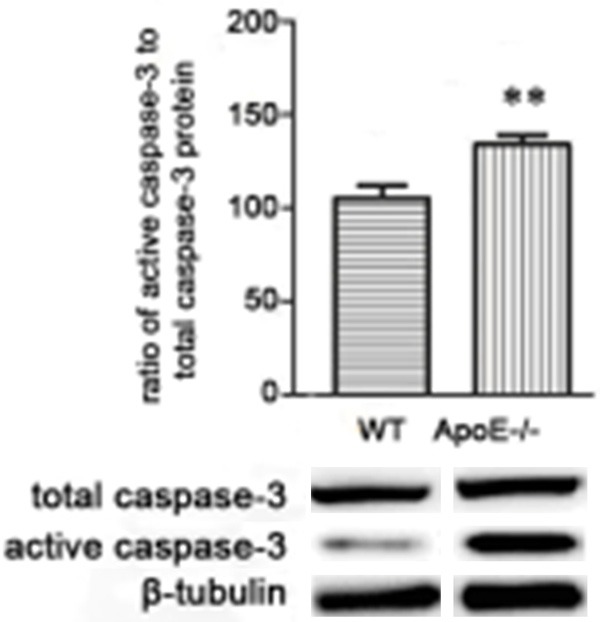
Loss of Apolipoprotein E function induced cell apoptosis. N = 20, *P<0.5, **P<0. 1, ***P<0.01.
Related inflammation factors were high production in microglia of ApoE-/- mice hippocampi
As shown in Figure 2A, the activity of iNOS protein of microglia in ApoE-/- group was significantly higher than WT controls (P<0.01). However, the activity of iNOS from normal and ApoE-/- mice microglia were decreased after treated with exogenous TGF-βas anti- inflammation factors with 100 pg/ml. Furthermore, the expression of iNOS protein in ApoE-/- group was significantly higher than WT controls and also decreased after exogenous TGF-β treatment (Figure 2B, P<0.01). These data suggested that loss of Apolipoprotein E function induced inflammation.
Figure 2.
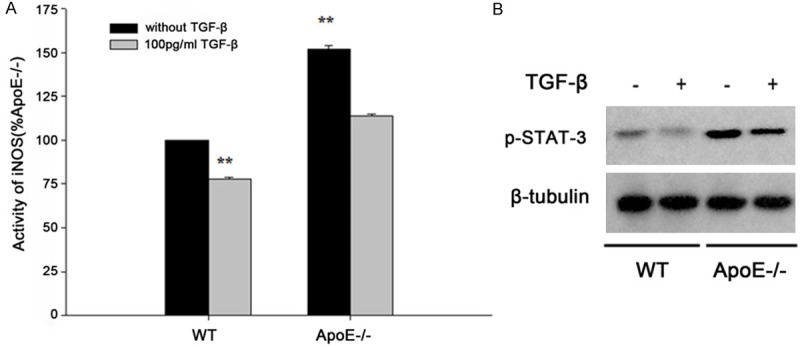
A. The iNOS activity of wild type (WT) and ApoE deficient (ApoE-/-) mice. B. The protein level of iNOS was detected by western blot. P<0.05 was regarded as statistically significant.
Related inflammation of microglia dependent astrocytes in ApoE-/- mice hippocampi
Then, we detected the related inflammation of IL-10, IL-1β, TGF-β concentration in the microglia or astrocyte in the hippocampi and co-culture of microglia-astrocyte from WT and ApoE-/- mice. As shown in Figure 3A-C, the concentration of IL-10 in WT microglia is significant higher than in ApoE-/- mice (P<0.01). The concentration of IL-1β in ApoE-/- microglia is significant higher than in WT mice (P<0.001). However, the concentration of TGF-β in WT astrocyte is significant higher than in ApoE-/- mice (P<0.01). Furthermore, to make sure the exactly relation between microglia and astrocyte from WT and ApoE-/- mice, as shown in Figure 3D-F, the concentration of IL-10 in ApoE-/- A+ApoE-/- M group is significant decreased than in WT A+WT M group (P<0.01). The concentration of IL-1β in ApoE-/- A+ApoE-/- M group is significant higher than in WT A+WT M group (P<0.001). However, the concentration of TGF-β in ApoE-/- A+ApoE-/- M group is significant decreased than in WT A+WT M group (P<0.01). Therefore, our findings suggested that the anti- inflammation factor such as IL-10 mainly from microglia and TGF-β mainly from astrocyte is significant decreased after loss of Apolipoprotein E function in ApoE-/- mice which induced severe inflammation.
Figure 3.
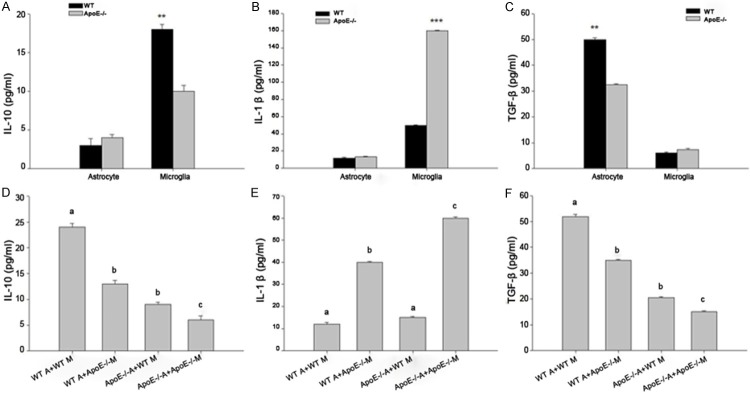
The related inflammation of microglia dependent astrocytes in ApoE-/- mice hippocampi. A. The concentration of IL-10 in microglia or astrocyte in WT and ApoE-/- mice. B. The concentration of IL-1β in microglia or astrocyte from WT and ApoE-/- mice. C. The concentration of TGF-βin microglia or astrocyte from WT and ApoE-/- mice. D. The concentration of IL-10 in microglia-astrocyte mixed from WT and ApoE-/- mice. E. The concentration of IL-1β in microglia-astrocyte mixed from WT and ApoE-/- mice. F. The concentration of TGF-β in microglia-astrocyte mixed from WT and ApoE-/- mice. n = 20, *P<0.5, **P<0.1, ***P<0.01.
ApoE deficient related to IL-10 and TGF-β expresstion
As shown at Figure 4, IL-10 and TGF-β as anti- inflammatory factor in WT microglia and astrocyte are significant higher than in ApoE-/- mice (P<0.01). Therefore, we detected the key protein relented to IL-10 and TGF-β expresstion by WB. As shown in Figure 4, the p-STAT-3 from ApoE-/- microglia and Lab1 from ApoE-/- astrocyte are significant decreased than in WT group.
Figure 4.
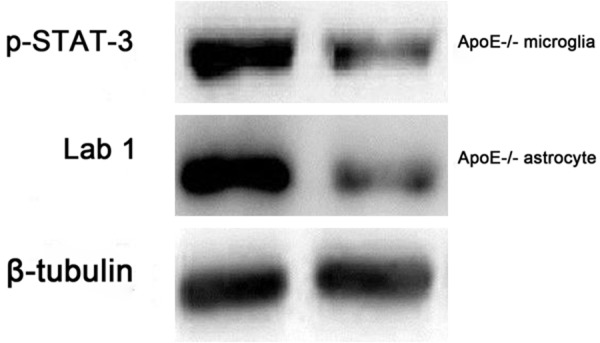
ApoE deficient related to IL-10 and TGF-β expression.
Discussion
Apolipoprotein E plays a significant role in lipid metabolism in mammals and has been implicated in growth and injured neurons repair [14,15]. ApoE has emerged as a key contributing factor in a number of neurodegenerative diseases, including Alzheimer’s disease (AD), Parkinson’s disease (PD), amyotrophic lateral sclerosis (ALS), traumatic brain injury, and HIV-encephalitis cerebral palsy (CP) [3,4]. However, the exactly mechanism need to study via Apo E deficient (ApoE-/-) mouse model. Here in this study, using apolipoprotein E deficient mouse model, our findings suggested that the anti- inflammation factor such as IL-10 mainly from microglia and TGF-β mainly from astrocyte is significant decreased after loss of ApoE function in ApoE-/- mice which induced severe inflammation in ApoE-/- mice. Furthrtmore, anti- inflammation factor such as IL-10 and TGF-β Therefore, we conclude that apolipoprotein E knockout induced inflammatory responses related to microglia in neonatal mice brain via astrocytes
In our study, we first detected the inflammation factor such as iNOS and IL-1β. The activity of iNOS protein in ApoE-/- group was significantly higher than WT controls (P<0.01). Furthermore, the expression of iNOS protein in ApoE-/- group was significantly higher than WT controls (P<0.01). Then, we detected the anti- inflammation factors such as IL-10 and TGF-β concentration in the microglia or astrocyte in the hippocampi and co-culture of microglia-astrocyte from WT and ApoE-/- mice. As shown in Figure 3A-C, the concentration of IL-10 in WT microglia is significant higher than in ApoE-/- mice (P<0.01). However, the concentration of IL-1β in ApoE-/- microglia is significant higher than in WT mice (P<0.01). Then, the concentration of TGF-β in WT astrocyte is significant higher than in ApoE-/- mice (P<0.01). After mixed, the concentration of IL-10 in ApoE-/- A+ApoE-/- M group is significant decreased than in WT A+WT M group (P<0.01). The concentration of IL-1β in ApoE-/- A+ApoE-/- M group is significant higher than in WT A+WT M group (P<0.01). However, the concentration of TGF-β in ApoE-/- A+ApoE-/- M group is significant decreased than in WT A+WT M group (P<0.01). As we known, Furthermore, inhibition of TGF-β signaling in the brain resulted in prolonged sickness behavior and amplified pro-inflammatory cytokine expression in mice challenged with lipopolysaccharide. Taken together, IL-10 stimulated the production of TGF-β by astrocytes, which in turn, attenuated microglial activation [13,16]. Therefore, our findings suggested that anti-inflammatory such as TGF-β, IL-10 from microglia and astrocytes in ApoE-/- mice were decreased. On the other hand, the inflammatory responses of Apolipoprotein E knockout mice in hippocampi microglia related to TGF-β of astrocytes.
In stefin B-deficient BMDMs, down-regulation of the IL-10 synthesis is due to the decreased activation STAT-3. ecreased phosphorylation of STAT-3 at Tyrosine 705 was detected in stefin B-deficient than in WT BMDMs, in agreement with the decreased IL-10 expression [17]. Furthermore, the latency associated protein (LAP) is involved in the folding and synthesis of TGF-β via monomers form a dimer by joining another LAP molecule by LTBP directs TGF-β to the extracellular matrix [18,19]. Therefore, we detected the key protein relented to IL-10 and TGF-β expresstion by WB. As shown in Figure 4, the p-STAT-3 from ApoE-/- microglia and Lab1 from ApoE-/- astrocyte are significant decreased than in WT group. However, the mechanism of ApoE in regulating p-STAT-3 and Lab1 need father study.
Acknowledgements
This study was supported by the Wuxi Guidance of Science and Technology Project in 2012, no. CSZ00N1240.
Disclosure of conflict of interest
None.
References
- 1.Nakashima Y, Plump AS, Raines EW, Breslow JL, Ross R. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler Thromb. 1994;14:133–140. doi: 10.1161/01.atv.14.1.133. [DOI] [PubMed] [Google Scholar]
- 2.Braga LW, Borigato EV, Speck-Martins CE, Imamura EU, Gorges AM, Izumi AP, Dantas RC, Nunes LG. Apolipoprotein E genotype and cerebral palsy. Dev Med Child Neurol. 2010;52:666–671. doi: 10.1111/j.1469-8749.2009.03465.x. [DOI] [PubMed] [Google Scholar]
- 3.Verghese PB, Castellano JM, Holtzman DM. Apolipoprotein E in Alzheimer’s disease and other neurological disorders. Lancet Neurol. 2011;10:241–252. doi: 10.1016/S1474-4422(10)70325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lien E, Andersen GL, Bao Y, Gordish-Dressman H, Skranes JS, Vik T, Blackman JA. Apolipoprotein E polymorphisms and severity of cerebral palsy: a cross-sectional study in 255 children in Norway. Dev Med Child Neurol. 2013;55:372–377. doi: 10.1111/dmcn.12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 6.Schwarzmaier SM, Plesnila N. Contributions of the immune system to the pathophysiology of traumatic brain injury-evidence by intravital microscopy. Front Cell Neurosci. 2014;8:358. doi: 10.3389/fncel.2014.00358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mishra MK, Wang J, Keough MB, Fan Y, Silva C, Sloka S, Hayardeny L, Brück W, Yong VW. Laquinimod reduces neuroaxonal injury through inhibiting microglial activation. Ann Clin Transl Neurol. 2014;1:409–22. doi: 10.1002/acn3.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mucke L, Eddleston M. Astrocytes in infectious and immune-mediated diseases of the central nervous system. FASEB J. 1993;7:1226–1232. doi: 10.1096/fasebj.7.13.8405808. [DOI] [PubMed] [Google Scholar]
- 9.De Keyser J, Mostert JP, Koch MW. Dysfunctional astrocytes as key players in the pathogenesis of central nervous system disorders. J Neurol Sci. 2008;267:3–16. doi: 10.1016/j.jns.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 10.Laskowitz DT, Song P, Wang H, Mace B, Sullivan PM, Vitek MP, Dawson HN. Traumatic brain injury exacerbates neurodegenerative pathology: improvement with an apolipoprotein E-based therapeutic. J Neurotrauma. 2010;27:1983–1995. doi: 10.1089/neu.2010.1396. [DOI] [PubMed] [Google Scholar]
- 11.Maezawa I, Maeda N, Montine TJ, Montine KS. Apolipoprotein E-specific innate immune response in astrocytes from targeted replacement mice. J Neuroinflammation. 2006;3:10. doi: 10.1186/1742-2094-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fenn AM, Henry CJ, Huang Y, Dugan A, Godbout JP. Lipopolysaccharide-induced interleukin (IL)-4 receptor-α expression and corresponding sensitivity to the M2 promoting effects of IL-4 are impaired in microglia of aged mice. Brain Behav Immun. 2012;26:766–777. doi: 10.1016/j.bbi.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Norden DM, Fenn AM, Dugan A, Godbout JP. TGFβ produced by IL-10 redirected astrocytes attenuates microglial activation. Glia. 2014;62:881–895. doi: 10.1002/glia.22647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuroda MM, Weck ME, Sarwark JF, Hamidullah A, Wainwright MS. Association of apolipoprotein E genotype and cerebral palsy in children. Pediatrics. 2007;119:306–313. doi: 10.1542/peds.2006-1083. [DOI] [PubMed] [Google Scholar]
- 15.McMichael GL, Gibson CS, Goldwater PN, Haan EA, Priest K, Dekker GA, MacLennan AH. Association between apolipoprotein E genotype and cerebral palsy is not confirmed in a Caucasian population. Hum Genet. 2008;124:411–416. doi: 10.1007/s00439-008-0564-y. [DOI] [PubMed] [Google Scholar]
- 16.Liu W, Tang Y, Feng J. Cross talk between activation of microglia and astrocytes in pathological conditions in the central nervous system. Life Sci. 2011;89:141–146. doi: 10.1016/j.lfs.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 17.Maher K, Završnik J, Jerič-Kokelj B, Vasiljeva O, Turk B, Kopitar-Jerala N. Decreased IL-10 expression in stefin B-deficient macrophages is regulated by the MAP kinase and STAT-3 signaling pathways. FEBS Lett. 2014;588:720–726. doi: 10.1016/j.febslet.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 18.Arjaans M, Oude Munnink TH, Timmer-Bosscha H, Reiss M, Walenkamp AM, Lub-de Hooge MN, de Vries EG, Schröder CP. Transforming growth factor (TGF)-β expression and activation mechanisms as potential targets for anti-tumor therapy and tumor imaging. Pharmacol Ther. 2012;135:123–132. doi: 10.1016/j.pharmthera.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Giannelli G, Mazzocca A, Fransvea E, Lahn M, Antonaci S. Inhibiting TGF-β signaling in hepatocellular carcinoma. Biochim Biophys Acta. 2011;1815:214–223. doi: 10.1016/j.bbcan.2010.11.004. [DOI] [PubMed] [Google Scholar]


