Abstract
A retrospective study was conducted to compare the performance of Child-Pugh and Model for End-Stage Liver Diseases (MELD) scores for predicting the in-hospital mortality of acute upper gastrointestinal bleeding (UGIB) in patients with liver cirrhosis. A total of 145 patients with a diagnosis of liver cirrhosis and acute UGIB between July 2013 and June 2014 were retrospectively analyzed (male/female: 94/51; mean age: 56.77±11.33 years; Child-Pugh class A/B/C: 46/64/35; mean Child-Pugh score: 7.88±2.17; mean MELD score: 7.86±7.22). The in-hospital mortality was 8% (11/145). Areas under receiving-operator characteristics curve (AUROC) for predicting the in-hospital mortality were compared between MELD and Child-Pugh scores. AUROCs for predicting the in-hospital mortality for Child-Pugh and MELD scores were 0.796 (95% confidence interval [CI]: 0.721-0.858) and 0.810 (95% CI: 0.736-0.870), respectively. The discriminative ability was not significant different between the two scoring systems (P=0.7241). In conclusion, Child-Pugh and MELD scores were similar for predicting the in-hospital mortality of acute UGIB in cirrhotic patients.
Keywords: Liver cirrhosis, gastrointestinal bleeding, Child-Pugh, MELD, prognosis
Introduction
Child-Pugh classification, including total bilirubin, albumin, international normalized ratio (INR) or prothrombin time, hepatic encephalopathy, and ascites, is the most commonly used scoring system for evaluating the prognosis of liver cirrhosis [1]. Two of the five variables are subjective, and the remaining three variables are acquired from laboratory tests. In 2000, Model for End-Stage Liver Disease (MELD) score, a mathematical formula which is composed of serum creatinine, total bilirubin, and INR, is firstly introduced by the investigators from Mayo Clinic to predict the mortality of patients undergoing transjugular intrahepatic portosystemic shunt (TIPS) insertions [2]. Notably, all of the variables in MELD scoring system were objective [3]. Salerno et al. confirmed the superiority of MELD score over Child-Pugh score in predicting the 3-month survival in such patients [4]. However, Schepke et al. showed that MELD score was only slightly superior to Child-Pugh class for the predicting the long-term survival after TIPS [5]. At present, MELD score is also widely used to prioritize the organ allocation in candidates for liver transplantation [3]. However, there is a debate about whether MELD can replace Child-Pugh score for predicting the survival in non-transplanted patients with chronic liver disease [6,7].
Acute upper gastrointestinal bleeding (UGIB) is a lethal complication of liver cirrhosis [8,9]. The major predictors for early mortality of acute UGIB in liver cirrhosis include hepatic encephalopathy, Child-Pugh score or class, MELD score, shock, renal failure, infection, hepatocellular carcinoma, active bleeding, portal vein thrombosis, and hepatic venous pressure gradient [9,10]. However, it remains unclear about whether Child-Pugh or MELD score is better for predicting the in-hospital mortality of acute UGIB in cirrhotic patients. Herein, we conducted a retrospective observational study to explore this issue.
Methods
All patients with a diagnosis of liver cirrhosis who were admitted to the General Hospital of Shenyang Military Region between July 2013 and June 2014 were retrospectively included in the present study. Inclusion criteria were as follows. 1) Patients were diagnosed with liver cirrhosis based on the history of liver disease, clinical manifestations, laboratory tests, imaging tests, and liver biopsy, if necessary. 2) Patents with hepatocellular carcinoma and other malignancies were excluded by the disease history and imaging examinations. 3) Patients presented with acute UGIB. The time fame for the acute bleeding episodes should be 120 hours (5 days) according to the Baveno V criteria [11]. 4) Source of acute UGIB was not restricted. This was primarily because not all patients underwent endoscopic examinations at their emergent admissions. 5) Patients with absence of complete laboratory tests were excluded. The study protocol was approved by the ethic committee of our hospital.
Clinical records were reviewed by two investigators (YP and JD), and checked by another investigator (XQ). The primary data collected at admission were: the demographic data, causes of liver diseases, severity of bleeding, vital signs of hospitalized patients, laboratory data, Child-Pugh score/class, and MELD score. Additionally, we also collected endoscopic findings (i.e., location and grade of varices and red color sign), treatment options (endoscopic ligation or sclerotherapy, vasoactive drug, and/or surgery, etc.), in-hospital death, and causes of death.
Child-Pugh score was calculated based on the severity of hepatic encephalopathy, ascites, total bilirubin, albumin, and INR (1).
MELD score=9.57 × ln (creatinine [µmol/L] × 0.01) + 3.78 × ln (bilirubin [µmol/L] × 0.05) + 11.2 × ln (INR) + 0.643 (3).
Statistical analysis
Categorical variables were reported as frequency (percentage) and continuous variables were reported as mean ± standard deviations. Receiving-operator characteristics (ROC) curve analysis was performed to identify the discriminative capacity of Child-Pugh and MELD scores in predicting the risk of in-hospital death. A cut off value of Child-Pugh score or MELD score was chosen as both sensitivity and specificity were optimal. Areas under the ROC curves (AUROC) with 95% confidence intervals (CIs) for these two scoring systems were also reported. We compared the performance of the two scoring systems by using the DeLong tests. P < 0.05 was considered statistically significant. All statistical analyses were performed by using the MedCalc software version 11.4.2.0.
Results
Overall, 849 patients with liver cirrhosis were admitted to our hospital during the enrollment period. Among them, 179 cirrhotic patients without malignancy presented with acute UGIB. Thirty-four patients were further excluded, because some laboratory data for liver and renal function were missing. Finally, 145 patients were included in the present study (Figure 1).
Figure 1.

Patient selection.
Baseline characteristics at admission were shown in Table 1. A majority of patients had a history of viral hepatitis and alcohol abuse. Endoscopic examinations were performed in 80% of patients. Child-Pugh and MELD scores at admission were 7.88±2.17 and 7.86±7.22, respectively.
Table 1.
Baseline characteristics of 145 patients
| Variables | Values |
|---|---|
| Sex (male/female) | 94/51 |
| Age (years) | 56.77±11.33 |
| Causes of liver diseases, n (%) | |
| Hepatitis B virus | 46 (31.7) |
| Hepatitis C virus | 11 (7.6) |
| Alcohol | 35 (24.1) |
| Hepatitis B virus + Alcohol | 3 (2.1) |
| Hepatitis B virus + Hepatitis C virus | 1 (0.7) |
| Unknown | 35 (24.1) |
| Others | 14 (9.7) |
| Vital signs | |
| Systolic blood pressure (mmHg) | 118.83±20.12 |
| Diastolic blood pressure (mmHg) | 67.34±11.26 |
| Heart rate (b.p.m.) | 84.74±15.37 |
| Interval between diagnosis of liver cirrhosis and admission (months) | 55.33±71.60 |
| Interval between bleeding and admission (hours) | 32.54±31.88 |
| Manifestation, n (%) | |
| Haematemesis | 37 (25.5) |
| Melena | 54 (37.2) |
| Haematemesis and melena | 54 (37.2) |
| Diabetes (yes/no) | 29/116 (20%) |
| Laboratory tests | |
| RBC (10*12/L) | 2.65±0.70 |
| Hb (g/L) | 74.91±22.19 |
| WBC (10*12/L) | 5.66±4.36 |
| PLT (10*9/L) | 66.00±62.58 |
| TBIL (umol/L) | 28.18±25.58 |
| DBIL (umol/L) | 14.27±18.40 |
| IBIL (umol/L) | 13.87±11.08 |
| ALB (g/L) | 30.35±6.79 |
| ALT (U/L) | 31.99±32.06 |
| AST (U/L) | 53.76±134.89 |
| ALP (U/L) | 84.03±61.08 |
| GGT (U/L) | 65.21±93.64 |
| BUN (mmol/L) | 8.84±6.03 |
| CR (umol/L) | 67.00±42.48 |
| K (mmol/L) | 4.07±0.52 |
| Na (mmol/L) | 138.37±4.56 |
| Ca (mmol/L) | 2.02±0.24 |
| Blood ammonia (umol/L) | 54.76±58.75 |
| PT (second) | 18.08±6.24 |
| APTT (second) | 41.58±8.38 |
| INR | 1.54±0.79 |
| Ascites, n (%) | |
| No | 67 (46.2) |
| Mild | 22 (15.2) |
| Moderate and severe | 56 (38.6) |
| Hepatic encephalopathy, n (%) | |
| No | 132 (91.0) |
| Grade I-II | 7 (4.8) |
| Grade III-IV | 6 (4.1) |
| Endoscopy (yes/no) | 116/29 (80%) |
| Varices, n (%) | |
| Mild-Moderate | 13 (9.0) |
| Severe | 103 (71.0) |
| NA | 29 (20.0) |
| Location of varices, n (%) | |
| No | 1 (0.7) |
| Esophageal varices | 63 (43.4) |
| Gastric varices | 16 (11.0) |
| Esophageal and gastric varices | 35 (24.1) |
| Unknown | 1 (0.7) |
| NA | 29 (20.0) |
| Portal hypertensive gastropathy, n (%) | |
| Yes | 2 (1.4) |
| No | 114 (78.6) |
| NA | 29 (20) |
| Erosive gastritis, n (%) | 1 (0.7) |
| Child-Pugh class, n (%) | |
| A | 46 (31.7) |
| B | 64 (44.1) |
| C | 35 (24.1) |
| Child-Pugh score | 7.88±2.17 |
| MELD score | 7.86±7.22 |
Abbreviations: RBC, red blood cell; Hb, hemoglobin; WBC, white blood cell; PLT, platelet; TBIL, total bilirubin; DBIL, direct bilirubin; IBIL, indirect; ALB, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; GGT, gamma-glutamyltranspeptidase; BUN, blood urea nitrogen; CR, creatinine; NA, not available; MELD, model for end stage liver disease; PT, prothrombin time; APTT, activated partial thromboplastin time; INR, international nomalized ratio.
Treatment options after admission were shown in Table 2. Blood transfusion was given in 91 patients. Somatostatin or its analogs were prescribed in nearly all patients received. Endoscopic therapy was performed in 104 patients. Splenectomy with devascularization was performed in 3 patients. Neither Sengstaken-blakemore tube nor TIPS was performed in any patients. The in-hospital mortality was 8% (11/145). Cause of death was uncontrolled UGIB in all of the 11 patients.
Table 2.
Treatment in 145 patients
| Treatment | Values |
|---|---|
| Transfusion (yes/no), n (%) | 91/54 (62.8/37.2) |
| Transfusion of RBC unit | 4.40±4.04 |
| Drugs, n (%) | |
| Somatostatin (yes/no) | 144/1 (99.3/0.7) |
| Proton pump inhibitor (yes/no) | 145/0 (100/0) |
| Endosopic therapy, n (%) | |
| None | 12 (8.3) |
| Ligation | 54 (37.2) |
| Sclerotherapy | 3 (2.1) |
| Tissue adhesive | 22 (15.2) |
| Ligation + sclerotherapy | 1 (0.7) |
| Ligation + tissue adhesive | 23 (15.9) |
| Sclerotherapy + tissue adhesive | 1 (0.7) |
| NA | 29 (20) |
| Surgery, n (%) | |
| None | 142 (97.9) |
| Shunt | 0 (0) |
| Splenectomy + devascularization | 3 (2.1) |
| Sengstaken-blakemore tube, n (%) | 0 |
Abbreviations: RBC, red blood cell; NA, not available.
In the ROC analysis, Child-Pugh score had a cut-off value of 9 with a specificity of 63.6% and a sensitivity of 79.1% (Figure 2). AUROC was 0.796 (95% CI: 0.721-0.858). In the ROC analysis, MELD score had a cut-off value of 12 with a specificity of 83.6% and a sensitivity of 72.7% (Figure 3). AUROC was 0.810 (95% CI: 0.736-0.870). The discriminative ability was not significant different between the two scoring systems (Figure 4) (P=0.7241).
Figure 2.

ROC analysis of Child-Pugh scores for predicting the in-hospital mortality of acute UGIB in liver cirrhosis.
Figure 3.

ROC analysis of MELD scores for predicting the in-hospital mortality of acute UGIB in liver cirrhosis.
Figure 4.
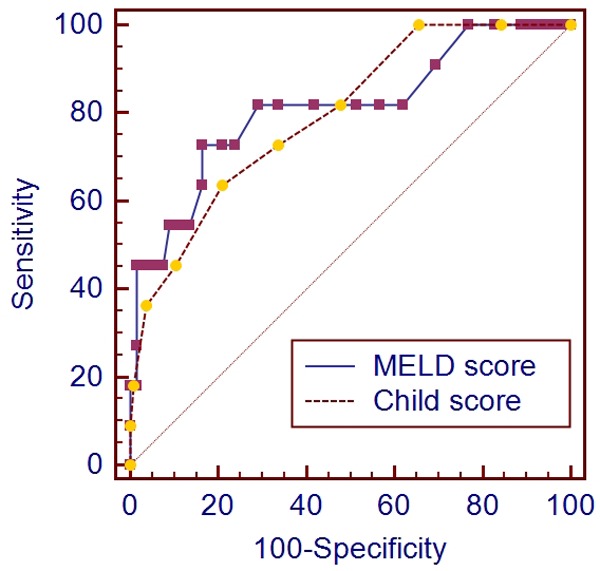
Comparison of the performance of Child-Pugh and MELD scores for predicting the in-hospital mortality of acute UGIB in liver cirrhosis.
Discussion
Numerous studies have compared the performance of Child-Pugh score with that of MELD score for the prognostic prediction in patients with liver diseases. As for the cirrhotic patients with acute variceal bleeding, their superiority remained controversial among studies. Chalasani et al. collected 239 cirrhotic patients with acute variceal bleeding from 4 large academic hospitals, and compared the performance of the two scoring systems in predicting the in-hospital and 1-year mortality rates [12]. The MELD score was highly predictive of both in-hospital (AUROC=0.82) and 1-year (AUROC=0.75) mortality rates. But its advantages over Child-Pugh score were not significant. Amitrano et al. retrospectively analyzed the 6-week and 3-month mortality of 172 cirrhotic patients with the first episode of oesophageal variceal bleeding after drug and endoscopic therapy [13]. AUROC for the MELD and Child-Pugh scores for predicting the 6-week mortality was 0.80 and 0.76, respectively. AUROC for the MELD and Child-Pugh scores for predicting the 3-month mortality was 0.79 and 0.76, respectively. Cerqueira et al. included 102 cirrhotic patients consecutively admitted with oesophageal variceal bleeding [14]. AUROC for the MELD and Child-Pugh score for predicting the in-hospital mortality was 0.760 (95% CI: 0.644-0.876) and 0.719 (95% CI: 0.585-0.853), respectively. More recently, Reverter et al. analyzed 178 patients with cirrhosis and acute esophageal variceal bleeding [15]. AUROC for the MELD and Child-Pugh scores for predicting the 6-week mortality was 0.79 and 0.74, respectively (P=0.2179). These studies by Amitrano, Cerqueira, and Reverter suggested the superiority of MELD score over Child-Pugh score [13-15]. However, it should be noted that the difference was not statistically significant. Orloff et al. enrolled 211 consecutive patients with liver cirrhosis and esophageal variceal bleeding after endoscopic sclerotherapy or emergency portacaval shunt [16]. The investigators found that Child-Pugh score was similar to MELD score in predicting the survival, recurrent encephalopathy, and rebleeding. Additionally, Child-Pugh score was superior to MELD score in predicting the hospital readmissions and readmission days.
As for the cirrhotic patients with unstable UGIB (heart rate > 100 beats/minute or systolic blood pressure < 100 mmHg), Hsu et al. retrospectively analyzed the performance of Glasgow-Blatchford, Rockall, and MELD scores. MELD scores had a significant discriminative ability for predicting the mortality (AUROC=0.736, 95% CI: 0.629-0.842, P=0.001). By comparison, Glasgow-Blatchford and Rockall scores did not have any significant discriminative ability for predicting the mortality (AUROC=0.527, 95% CI: 0.393-0.661, P= 0.709; AUROC=0.591, 95% CI: 0.465-0.717, P=0.208) [17].
Our target population has the following features. 1) All patients had a diagnosis of liver cirrhosis. 2) All patients presented with acute UGIB. Indeed, at the emergency admission for UGIB, especially massive haematemesis, not all patients had the opportunity to undergo the endoscopic examinations to identify the sources of bleeding. 3) Child-Pugh and MELD scores, two most important scoring systems for the prognosis of liver cirrhosis, were compared in our cohort. 4) The in-hospital mortality of acute UGIB was the only endpoint of our study. We found that both scoring systems had good discriminative abilities for the in-hospital mortality of acute UGIB in liver cirrhosis, and that the AUROC for MELD score might be slightly superior to that for Child-Pugh score, but the difference was not statistically significant between them.
The potential limitations of our study should be clarified. First, the comparisons of long-term follow-up outcome between the two scoring systems were lacking. Second, 20% of included patients did not undergo the endoscopic examination. Thus, we did not strictly limit the source of UGIB (variceal or non-variceal). Third, none of patients underwent TIPS for acute UGIB. Indeed, a recent randomized controlled trial suggested that early TIPS should be more effective for improving the survival of acute variceal bleeding in high-risk cirrhotic patients [18]. This consideration is also supported by a meta-analysis [19]. Thus, the mortality would be lower in our patients, if TIPS was employed.
In conclusion, the discriminative ability for predicting the in-hospital mortality of acute UGIB in liver cirrhosis was similar between Child-Pugh and MELD scores.
Disclosure of conflict of interest
None.
References
- 1.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 2.Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864–871. doi: 10.1053/he.2000.5852. [DOI] [PubMed] [Google Scholar]
- 3.Kamath PS, Kim WR. The model for end-stage liver disease (MELD) Hepatology. 2007;45:797–805. doi: 10.1002/hep.21563. [DOI] [PubMed] [Google Scholar]
- 4.Salerno F, Merli M, Cazzaniga M, Valeriano V, Rossi P, Lovaria A, Meregaglia D, Nicolini A, Lubatti L, Riggio O. MELD score is better than Child-Pugh score in predicting 3-month survival of patients undergoing transjugular intrahepatic portosystemic shunt. J Hepatol. 2002;36:494–500. doi: 10.1016/s0168-8278(01)00309-9. [DOI] [PubMed] [Google Scholar]
- 5.Schepke M, Roth F, Fimmers R, Brensing KA, Sudhop T, Schild HH, Sauerbruch T. Comparison of MELD, Child-Pugh, and Emory model for the prediction of survival in patients undergoing transjugular intrahepatic portosystemic shunting. Am J Gastroenterol. 2003;98:1167–1174. doi: 10.1111/j.1572-0241.2003.07515.x. [DOI] [PubMed] [Google Scholar]
- 6.Cholongitas E, Papatheodoridis GV, Vangeli M, Terreni N, Patch D, Burroughs AK. Systematic review: The model for end-stage liver disease--should it replace Child-Pugh’s classification for assessing prognosis in cirrhosis? Aliment Pharmacol Ther. 2005;22:1079–1089. doi: 10.1111/j.1365-2036.2005.02691.x. [DOI] [PubMed] [Google Scholar]
- 7.Durand F, Valla D. Assessment of the prognosis of cirrhosis: Child-Pugh versus MELD. J Hepatol. 2005;(Suppl 42):S100–107. doi: 10.1016/j.jhep.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 8.Cremers I, Ribeiro S. Management of variceal and nonvariceal upper gastrointestinal bleeding in patients with cirrhosis. Therap Adv Gastroenterol. 2014;7:206–216. doi: 10.1177/1756283X14538688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D’Amico G, de Franchis R. Upper digestive bleeding in cirrhosis. Post-therapeutic outcome and prognostic indicators. Hepatology. 2003;38:599–612. doi: 10.1053/jhep.2003.50385. [DOI] [PubMed] [Google Scholar]
- 10.Augustin S, Muntaner L, Altamirano JT, Gonzalez A, Saperas E, Dot J, Abu-Suboh M, Armengol JR, Malagelada JR, Esteban R, Guardia J, Genescà J. Predicting early mortality after acute variceal hemorrhage based on classification and regression tree analysis. Clin Gastroenterol Hepatol. 2009;7:1347–1354. doi: 10.1016/j.cgh.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 11.de Franchis R. Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2010;53:762–768. doi: 10.1016/j.jhep.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Chalasani N, Kahi C, Francois F, Pinto A, Marathe A, Bini EJ, Pandya P, Sitaraman S, Shen J. Model for end-stage liver disease (MELD) for predicting mortality in patients with acute variceal bleeding. Hepatology. 2002;35:1282–1284. doi: 10.1053/jhep.2002.32532. [DOI] [PubMed] [Google Scholar]
- 13.Amitrano L, Guardascione MA, Bennato R, Manguso F, Balzano A. MELD score and hepatocellular carcinoma identify patients at different risk of short-term mortality among cirrhotics bleeding from esophageal varices. J Hepatol. 2005;42:820–825. doi: 10.1016/j.jhep.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 14.Cerqueira RM, Andrade L, Correia MR, Fernandes CD, Manso MC. Risk factors for in-hospital mortality in cirrhotic patients with oesophageal variceal bleeding. Eur J Gastroenterol Hepatol. 2012;24:551–557. doi: 10.1097/MEG.0b013e3283510448. [DOI] [PubMed] [Google Scholar]
- 15.Reverter E, Tandon P, Augustin S, Turon F, Casu S, Bastiampillai R, Keough A, Llop E, González A, Seijo S, Berzigotti A, Ma M, Genescà J, Bosch J, García-Pagán JC, Abraldes JG. A MELD-based model to determine risk of mortality among patients with acute variceal bleeding. Gastroenterology. 2014;146:412–419. e413. doi: 10.1053/j.gastro.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 16.Orloff MJ, Vaida F, Isenberg JI, Wheeler HO, Haynes KS, Jinich-Brook H, Rapier R, Hye RJ, Orloff SL. Child-Turcotte score versus MELD for prognosis in a randomized controlled trial of emergency treatment of bleeding esophageal varices in cirrhosis. J Surg Res. 2012;178:139–146. doi: 10.1016/j.jss.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Hsu SC, Chen CY, Weng YM, Chen SY, Lin CC, Chen JC. Comparison of 3 scoring systems to predict mortality from unstable upper gastrointestinal bleeding in cirrhotic patients. Am J Emerg Med. 2014;32:417–420. doi: 10.1016/j.ajem.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Pagan JC, Caca K, Bureau C, Laleman W, Appenrodt B, Luca A, Abraldes JG, Nevens F, Vinel JP, Mössner J, Bosch J Early TIPS (Transjugular Intrahepatic Portosystemic Shunt) Cooperative Study Group. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med. 2010;362:2370–2379. doi: 10.1056/NEJMoa0910102. [DOI] [PubMed] [Google Scholar]
- 19.Qi X, Jia J, Bai M, Guo X, Su C, Garcia-Pagan JC, Han G, Fan D. Transjugular Intrahepatic Portosystemic Shunt for Acute Variceal Bleeding: A Meta-analysis. J Clin Gastroenterol. 2014 doi: 10.1097/MCG.0000000000000205. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]


