Abstract
Epilepsy is a chronic neurological disorder. Antiepileptic drugs (AEDs) can cause vitamin B12 or D deficiency in children with intractable epilepsy. In this study, we measured salivary superoxide dismutase (SOD) and metalloproteinsases (MMP) levels in the patients with vitamin B12 and vitamin D treatment. Cytokines and chemokines were measured using ELISA. The mean salivary value of SOD activity in the control group was 1.75 ± 0.21 U/ml. In the treatment group, the value was 1.33 ± 0.18 U/ml. The salivary MMP 2, MMP 3, and MMP 9 levels of the patients with vitamin D and vitamin B12 treatment were lower than that in the patients without vitamin D and vitamin B12 treatment. Interleukin 1β (IL-1β), IL-6, IL-8, macrophage inflammatory protein 1β (MIP-1β), monocyte chemoattractant protein-1 (MCP-1) and IFN-inducible protein 10 (IP-10) were significantly decreased in the cortex of our patients with vitamin D and vitamin B12 treatment. In this study, a clear association between vitamin D and vitamin B12 treatment and epilepsy was identified. We now plan to investigate the genetic factors that underlie vitamin D and vitamin B12 treatment in patients treated with AEDs.
Keywords: Vitamin D, vitamin B12, antiepileptic drugs, MMP, cytokines
Introduction
Epilepsy is a chronic neurological disorder associated with spontaneous, recurrent and paroxysmal cerebral discharge which in turn results in neuronal damage and seizures [1]. Despite the high prevalence of epilepsy, 30% of patients do not have good control of their seizures [2]. Children with intractable epilepsy are exposed to many treatments and concomitant side effects [3,4]. Commonly used antiepileptic drugs (AEDs) can cause vitamin B12 or D deficiency, which is, in turn, associated with hyperhomocysteinemia [5,6]. Vitamin B12 is an essential water-soluble vitamin that is vitally important in hematopoiesis, nervous system functions, maintenance of the intact gastrointestinal mucosa and regulation of numerous other B12-dependent metabolic processes [7]. Vitamin D is a member of the steroid hormone family. Its insufficiency is associated with several diseases, including osteoporotic fractures [8], hypertension [9], and cancer [10]. There are now a number of papers recommending periodic vitamin B12 and D blood monitoring and vitamin B12 and D supplementation in children on long-term antiepileptic drugs [11,12].
However, to our knowledge, direct evidence for a role of vitamin B12 and D in epilepsy is limited. The aim of the present study was to investigate the influence of vitamin B12 and D supplementation on children with intractable epilepsy.
Methods and materials
Patients and study design
From January 2009 to December 2013, we recruited 42 patients at the Department of Neurology in First Hospital of China Medical University. The study protocol was approved by the local ethics committee and informed consent was collected from all participants. Twenty one patients were given 50,000 units of vitamin D2 weekly, as well as vitamin B12 200 mg/kg twice daily, as part of standard care.
Patients’s-B12, and s-D levels were measured
Blood samples were collected after an overnight fast, cooled on ice immediately, and centrifuged at 4°C. Serum was separated within 1 h and stored at -70°C. s-B12 level was measured by the immunoassay method using commercial kits (Siemens Healthcare Diagnostics Products Limited, Llanberis, Gwynedd, United Kingdom). Vitamin D status was assessed by measuring serum 25 (OH) D levels (DiaSorin, Stillwater, MN, USA).
Salivary superoxide dismutase (SOD) activity
Total activity of the SOD isoenzymes (Cu/Zn-SOD and Mn-SOD) was measured using the Xanthine Oxidase/XTT method described by Ukeda et al. [13]. This is an assay for SOD based on tetrazolium salt 3’-{1-[(phenylamino)-carbonyl]-3,4-tetrazolium}-bis(4-methoxy-6-nitro) benzenesulfonic acid hydrate reduction by xanthine-xanthine oxidase. One unit of the enzyme is defined as the amount of enzyme needed for 50% inhibition of absorption in the absence of the enzyme.
Salivary metalloproteinsases (MMPs) analysis
Immunoreactivity assays were employed for examination metalloproteinsases (MMPs). Salivary samples were centrifuged (800 × g, 10 min, 4°C), and the pellets were suspended in 150 μl of lysis buffer (45 mM HEPES, 0.4 M KCl, 1 mM EDTA, 10% glycerol, pH = 7.8). Following 30 min incubation at room temperature the samples were centrifuged (10,000 × g, 10 min, 4°C). Protein concentrations in the supernatants were determined. A volume containing 50 ng of protein was transferred to a 1.5 ml vial and all samples were brought to the same volume of 500 μl with the addition of PBS. The solutions were mixed well and 100 μl of each sample was added to ELISA-plate wells (Thermo Fisher Scientific, Waltham, MA, USA).
Imaging methods
Anatomic and functional MRI scanning was performed on a 1.5-T GE Signa Excite MRI system (GE Healthcare, Waukesha, WI, USA). A high-resolution whole brain T1-weighted anatomic MRI (3-D) spoiled gradient recall echo pulse sequence were: (120 slices, 1.5-mm slice thickness, field of view [FOV] 24 cm × 24 cm, 256 × 256 matrix, 0.75 mm × 0.75-mm in-plane resolution, repetition time [TR] = 20 msec, echo time [TE] = 5 msec). The T2*-weighted fMRI data were acquired from ten axial slices (matrix size 64 × 64, 5 mm thickness) covering visual cortex using echo planar imaging (EPI) sequence (TR/TE = 1000 ms/35 ms). The flip angles and band width of T1 weighted MR images were 7° and 200 Hz/Pixel, respectively, and those of T2* images were 40° and 1954 Hz/Pixel.
Measurement of cytokines and chemokines
Cortices from the fifteen patients without vitamin D and vitamin B12 treatment and the three patients with treatment were homogenized by using a total protein extraction kit (Chemicon, Temecula, CA, USA), then centrifuged at 4°C and stored at -80°C. Total protein concentration was calculated using the BCA assay kit (Pierce, Rockford, IL, USA). Levels of proinflammatory cytokines, including interferon (IFN)-γ, IL-1β, IL-2, IL-6, IL-8, TNF-α, granulocyte-macrophage colony stimulating factor (GM-CSF), anti-inflammatory cytokine IL-10, IFN-inducible protein 10 (IP-10), monocyte chemoattractant protein-1 (MCP-1), MCP-4, macrophage derived chemokine (MDC), and macrophage inflammatory protein 1β (MIP-1β) were measured using ELISA kits (Meso-Scale Discovery, Gaithersburg, MD, USA). Plates were analyzed using a microplate reader (Bio-Rad, Hercules, CA, USA).
Statistical analysis
Data are presented as the mean ± standard deviation (SD). Differences between groups were analyzed using Student’s t-test for continuous variables. Statistical analysis was performed using GraphPad 5.0 software (GraphPad Software, La Jolla, CA, USA) and significance was established at P < 0.05.
Results
Serum levels of vitamin D and vitamin B12 in the patients
A total of 42 epilepsy patients were identified in this study. Of these, 21 children were on regular vitamin D and vitamin B12 supplements. In the patients with vitamin D and vitamin B12 supplements, the serum 25-hydroxy vitamin D levels ranged between 18 and 42 ng/ml and vitamin B12 levels ranged between 204 and 758 pg/l, while the serum 25-hydroxy vitamin D and vitamin B12 levels in control group ranged between 3 and 22 ng/ml and between 100 and 453 pg/l, respectively (Figure 1, P < 0.05).
Figure 1.
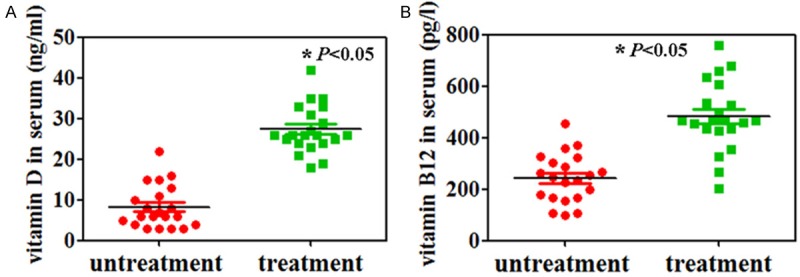
Serum levels of vitamin D (A) and vitamin B12 (B) were measured by using ELISA.
Salivary SOD activity and MMPs analysis
The mean salivary value of SOD activity in the control group was 1.75 ± 0.21 U/ml. In the treatment group, the value was 1.33 ± 0.18 U/ml (Figure 2A, P < 0.05). Furthermore, the salivary MMP 2, MMP 3, and MMP 9 levels of the patients with vitamin D and vitamin B12 treatment was lower than that in the patients without vitamin D and vitamin B12 treatment (Figure 2B, P < 0.05).
Figure 2.
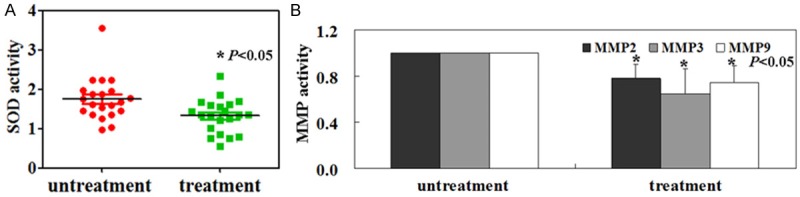
Salivary SOD (A) and metalloproteinases (MMP) activity (B) were measured as described in Methods.
Improvement in the brain of the patients with vitamin D and vitamin B12 treatment
Activation maps for the left temporal lobe epilepsy (LTLE) and right temporal lobe epilepsy (RTLE) were shown in Figure 3. Less activated sites were found in brain of the patients with vitamin D and vitamin B12 treatment (Figure 3). IL-1β, IL-6, IL-8, MIP-1β, MCP-1 and IP-10 were significantly decreased in the cortex of our patients with vitamin D and vitamin B12 treatment (Figure 4, P < 0.05).
Figure 3.
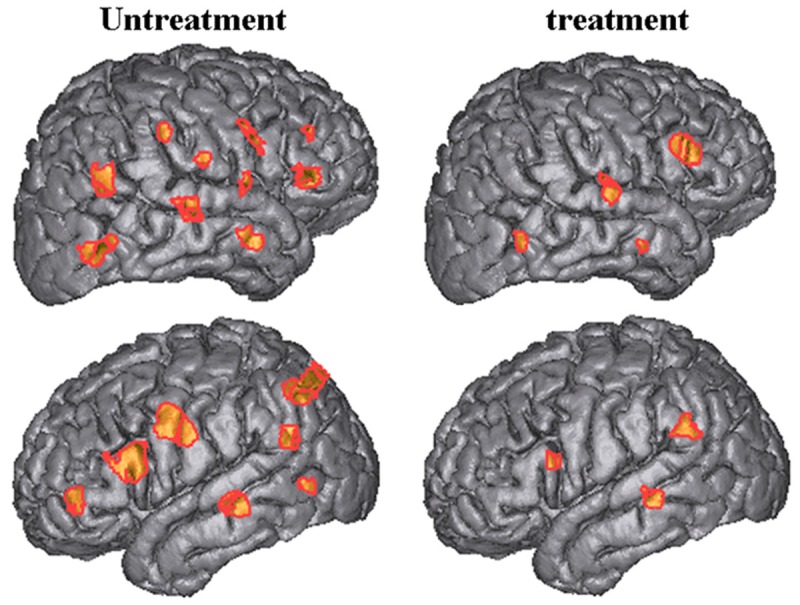
Activation maps for the left temporal lobe epilepsy (LTLE) and right temporal lobe epilepsy (RTLE) in the patients with or without vitamin D and vitamin B12 treatment.
Figure 4.
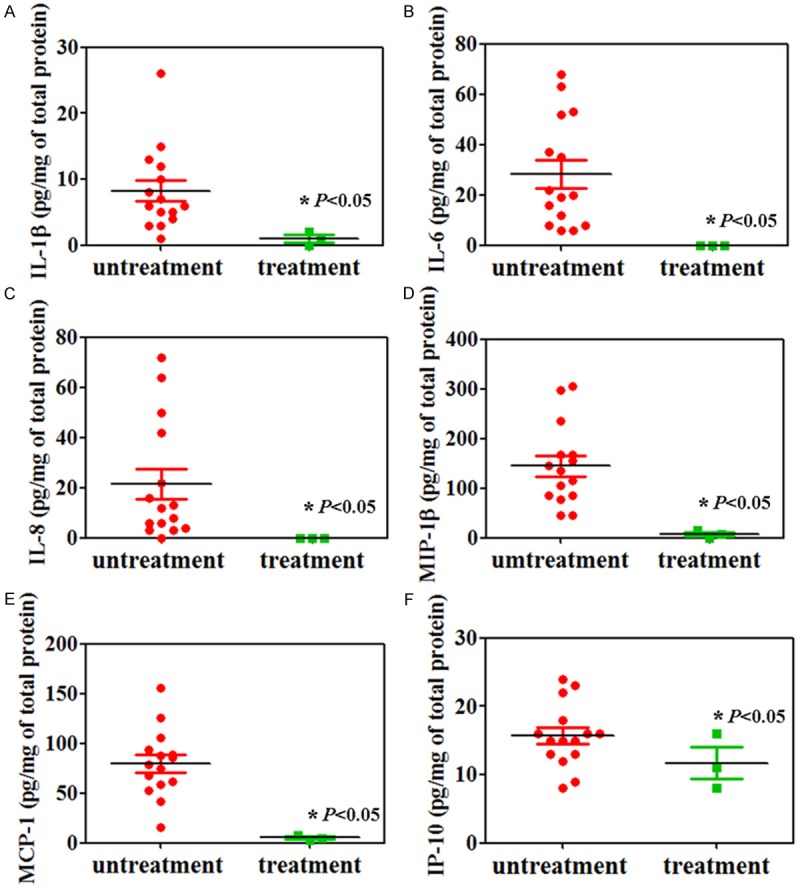
Cytokine analysis by ELISA. IL-1β (A), IL-6 (B), IL-8 (C), MIP-1β (D), MCP-1 (E) and IP-10 (F) were significantly decreased in brains of patients with epilepsy after vitamin D and vitamin B12 treatment.
Discussion
Chronic anti-epilepsy drugs may impair folate absorption and gastrointestinal transport by altering gastrointestinal pH [14]. The association between vitamin D, AEDs, and poor bone health in individuals with epilepsy was first recognized in 1979 [15]. Linnebank et al. [5] recommends that patients treated with AEDs should have their serum levels of folate and vitamin B12 closely monitored, or should receive prophylactic vitamin supplementation while receiving AED therapy. In this study, we found that vitamin D and vitamin B12 could be used as supplementations for epilepsy treatment.
Oxidative stress and MMPs may have a pivotal role in the pathogenesis of epilepsy [16]. Shahar et al. [16] found that salivary MMP 9 concentration was decreased by 23% in the patient with intractable epilepsy compared with healthy controls, while SOD activity in controls was decreased by 10% in the patient. In our study, we found that vitamin D and vitamin B12 could reverse the SOD and MMP 9 activities in the patients with intractable epilepsy.
Furthermore, marked glial activation and neuroinflammation in the surgically resected cortical tissue from patients with intractable epilepsy were observed. Cytokines could influence the activation of astrocytes and microglia. For example, Giulian et al. [17] found that injection of pro-inflammatory cytokines could induce astrogliosis in healthy animals. Cytokines contributed initially by inciting seizures in the developing brain after being induced by seizures or tissue injury, exacerbate tissue injury and promote further seizures [18]. In this study, we found that, after vitamin D and vitamin B12 treatment, IL-1β, IL-6, IL-8, MIP-1β, MCP-1 and IP-10 were significantly decreased in the cortex of the patients.
Although a clear association between vitamin D and vitamin B12 treatment and epilepsy, we now plan to investigate the genetic factors that underlie vitamin D and vitamin B12 treatment in patients treated with AEDs.
Acknowledgements
We would like to acknowledge the excellent technical support by Ji Li (First Hospital of China Medical University).
Disclosure of conflict of interest
None.
References
- 1.Folbergrová J, Kunz WS. Mitochondrial dysfunction in epilepsy. Mitochondrion. 2012;12:35–40. doi: 10.1016/j.mito.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Loscher W, Schmidt D. Modern antiepileptic drug development has failed to deliver: ways out of the current dilemma. Epilepsia. 2011;52:657–678. doi: 10.1111/j.1528-1167.2011.03024.x. [DOI] [PubMed] [Google Scholar]
- 3.Hanai T. Quality of life in children with epilepsy. Epilepsia. 1996;37:28–32. doi: 10.1111/j.1528-1157.1996.tb01816.x. [DOI] [PubMed] [Google Scholar]
- 4.McEwan MJ, Espie CA, Metcalfe J, Brodie MJ, Wilson MT. A systematic review of the contribution of qualitative research to the study of quality of life in children and adolescents with epilepsy. Seizure. 2004;13:3–14. doi: 10.1016/s1059-1311(03)00081-5. [DOI] [PubMed] [Google Scholar]
- 5.Linnebank M, Moskau S, Semmler A, Widman G, Stoffel-Wagner B, Weller M, Elger CE. Antiepileptic drugs interact with folate and vitamin B12 serum levels. Ann Neurol. 2011;69:352–359. doi: 10.1002/ana.22229. [DOI] [PubMed] [Google Scholar]
- 6.Fong CY, Riney CJ. Vitamin D deficiency among children with epilepsy in South Queensland. J Child Neurol. 2014;29:368–373. doi: 10.1177/0883073812472256. [DOI] [PubMed] [Google Scholar]
- 7.Gröber U, Kisters K, Schmidt J. Neuroenhancement with Vitamin B12-Underestimated Neurological Significance. Nutrients. 2013;5:5031–5045. doi: 10.3390/nu5125031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boonen S, Lips P, Bouillon R, Bischoff-Ferrari HA, Vanderschueren D, Haentjens P. Need for additional calcium to reduce the risk of hip fracture with vitamin D supplementation: evidence from a comparative meta analysis of randomized controlled trials. J Clin Endocrinol Metab. 2007;92:1415–1423. doi: 10.1210/jc.2006-1404. [DOI] [PubMed] [Google Scholar]
- 9.Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr. 2007;85:1586–1591. doi: 10.1093/ajcn/85.6.1586. [DOI] [PubMed] [Google Scholar]
- 10.Marins D, Wolf M, Pan D, Zadshir A, Tareen N, Thadhani R, Felsenfeld A, Levine B, Mehrotra R, Norris K. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: data from the third National Health and Nutrition Examination Survey. Arch Intern Med. 2007;167:1159–1165. doi: 10.1001/archinte.167.11.1159. [DOI] [PubMed] [Google Scholar]
- 11.Wirrell E. Vitamin D and bone health in children with epilepsy: fad or fact? Pediatr Neurol. 2010;42:394–395. doi: 10.1016/j.pediatrneurol.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Hochberg Z, Bereket A, Davenport M, Delemarre-Van de Waal HA, De Schepper J, Levine MA, Shaw N, Schoenau E, van Coeverden SC, Weisman Y, Zadik Z. European Society for Paediatric Endocrinology (ESPE) Bone Club. Consensus development for the supplementation of vitamin D in childhood and adolescence. Horm Res. 2002;58:39–51. doi: 10.1159/000063214. [DOI] [PubMed] [Google Scholar]
- 13.Ukeda H, Maeda S, Ishii T, Sawamura M. Spectrophotometric assay for superoxide dismutase based on tetrazolium salt 3’-1-(phenylamino)-carbonyl-3, 4-tetrazolium] -bis(4-methoxy-6-nitro)benzenesulfonic acid hydrate reduction by xanthine-xanthine oxidase. Anal Biochem. 1997;251:206–209. doi: 10.1006/abio.1997.2273. [DOI] [PubMed] [Google Scholar]
- 14.Belcastro V, Gaetano G, Italiano D, Oteri G, Caccamo D, Pisani LR, Striano P, Striano S, Ientile R, Pisani F. Antiepileptic drugs and MTHFR polymorphisms influence hyperhomocysteinemia recurrence in epileptic patients. Epilepsia. 2007;48:1990–1994. doi: 10.1111/j.1528-1167.2007.01164.x. [DOI] [PubMed] [Google Scholar]
- 15.Offermann G, Pinto V, Kruse R. Antiepileptic drugs and vitamin D supplementation. Epilepsia. 1979;20:3–15. doi: 10.1111/j.1528-1157.1979.tb04771.x. [DOI] [PubMed] [Google Scholar]
- 16.Shahar E, Attias U, Savulescu D, Genizin J, Gavish M, Nagler R. Oxidative stress, metalloproteinase and LDH in children with intractable and non-intractable epilepsy as reflected in salivary analysis. Epilepsy Res. 2014;108:117–124. doi: 10.1016/j.eplepsyres.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Giulian D, Li J, Li X, George J, Rutecki PA. The impact of microglia-derived cytokines upon gliosis in the CNS. Dev Neurosci. 1994;16:128–136. doi: 10.1159/000112099. [DOI] [PubMed] [Google Scholar]
- 18.Marchi N, Fan Q, Ghosh C, Fazio V, Bertolini F, Betto G, Batra A, Carlton E, Najm I, Granata T, Janigro D. Antagonism of peripheral inflammation reduces the severity of status epilepticus. Neurobiol Dis. 2009;33:171–181. doi: 10.1016/j.nbd.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


