Abstract
Background: The efficacy and safety of new intravenous P2Y12 inhibitor (cangrelor) for patients with coronary artery disease (CAD) remain unclear. Methods and Results: Trials were identified in PubMed, Web of Science, Embase, and Cochrane Database searches. We included four randomized, placebo-controlled reports in the meta-analysis. The database consisted of 36, 081 patients on cangrelor compared with clopidogrel or placebo. Major adverse cardiac events (MACE) were defined as the primary efficacy endpoint and major or severe bleeding at 48 hours was defined as the primary safety endpoint. Cangrelor significantly decreased risk of MACE (OR: 0.87, P = 0.002) and stent thrombosis (OR: 0.53, P < 0.001). However, at the same time, an increase in TIMI minor bleeding (OR: 1.49, P = 0.04) and in GUSTO moderate bleeding (OR: 1.43, P = 0.04) were observed by cangrelor. Conclusions: Intravenous administration of cangrelor is benefit to reduce risk of MACE and stent thrombosis in patients with CAD excepting for increased minor bleeding events.
Keywords: Cangrelor, coronary artery disease, meta-analysis
Introduction
Dual antiplatelet therapy with clopidogrel and aspirin has been the standard antiplatelet therapy for acute coronary syndromes (ACSs) since 2001 [1], given its clear superiority in reducing myocardial infarction (MI),composite risk for death and stent thrombosis in comparison to aspirin alone [2,3]. Clopidogrel, an irreversible antagonist of the P2Y12 adenosine-diphosphate (ADP) platelet receptor, has a highly variable effect on platelet inhibition [4]. Though increased loading doses is an alternative choice [5], recent papers have indicated that doubling dose of clopidogrel has no evident benefit on mortality in patients with percutaneous coronary intervention [6,7].
Emerging P2Y12 inhibitors, such as prasugrel, ticagrelor, cangrelor and elinogrel, have faster onset of action and are more potent than clopidogrel, showing better antiplatelet effects for coronary artery disease (CAD) patients. According to individual properties, new P2Y12 inhibitors can be classified as intravenous drugs (cangrelor and elinogrel) and oral (ticagrelor and prasugrel). Some studies have indicated the superior antiplatelet effects of new oral P2Y12 inhibitors compared with clopidogrel [8,9]. However, the benefit of the new intravenous P2Y12 inhibitor (cangrelor) still remains unclear. So the goal of this study is to synthesize the available prospective data to help evaluate the impact of cangrelor on risk of ischemic and bleeding events in patients with CAD.
Methods
Search strategy
We conducted PubMed, Web of Science, Embase, and Cochrane Database searches (until March 2014) using medical subject heading and keyword terms included the following terms: (cangrelor) AND (acute coronary syndromes OR myocardial infarction OR angina OR coronary artery disease OR percutaneous coronary intervention OR PCI). No language restrictions were applied. Randomized controlled trials (RCT), cohort studies, case series and case control studies were included. Review articles, meeting abstracts, individual case reports and editorials were excluded. Literatures were reviewed by two researchers (Tang and Chen) independently of each other.
Study selection and data extraction
Studies were extracted if they met the following criteria: 1) RCT enrolling patients with CAD or ACS; 2) studies compare cangrelor with clopidogrel; 3) the study supplied data on ischemic and bleeding events. Two researchers (Tang and Chen) extracted data independently. In case of disagreements, a third investigator (Zhang) made a decision by discussion. Data extraction included: study name, publication year, population, the length of follow-up, characteristics of participants, efficacy and safety outcomes.
Endpoints and definitions
The primary efficacy end point was major adverse cardiac events (MACE). We also examined all-cause death, myocardial infarction (MI) and stent thrombosis. MI was defined according to American College of Cardiology/American Heart Association definitions [10,11] or the universal definition of MI [12]. Definitions of stent thrombosis were refereed to Academic Research Consortium definitions [13]. The primary safety end point for this meta-analysis was the rate of major bleeding defined by TIMI or GUSTO criteria [14]. All endpoints were checked at the points of 48 hours after PCI procedure in every study.
Quality assessment
The quality assessment of enrolled studies was performed by risk of bias in line with the Cochrane Collaboration methods [15]. Specifically, sources of sequence generation, incomplete outcome data, selective outcome reporting, allocation concealment, masking of outcome assessors, and other bias were assessed in detail. Two independent reviewers (Tang, Chen) carried out the quality assessment, and any disagreements were settled by consensus or adjudicated by a third reviewer (Zhang).
Statistical analysis
Review Manager 5.2 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, Denmark) was used for analysis. The measure of treatment effect for each study was the odds ratio (OR) with 95% confidence interval (CI). Heterogeneity was quantified by using the I2 statistic: low, moderate and high represented I2 values of 25, 50 and 75%, respectively. In case of high heterogeneity, sensitivity analyses were conducted by removing each study individually to explore possible reasons and to compare the influence of various exclusion criteria on overall risk estimate. In addition, subgroup analysis was conducted if significant heterogeneity was identified. A two-tailed P value < 0.05 was considered statistically significant for each test.
Results
The search strategy revealed 374 potentially eligible study reports. Totally, 216 irrelevant citations and 152 duplicates were excluded by evaluating title and abstract. When abstracts for inclusion and exclusion criteria were reviewed, there were 6 studies requiring further review. Among these, 2 studies were missed for not getting necessary data by communicating with authors. Finally, 4 RCTs were selected in the meta-analysis referring to the review process in Figure 1 [10,11,16,17]. The database consisted of 36,081 patients on cangrelor compared with clopidogrel or placebo. Clopidogrel loading doses ranged from 300 mg to 600 mg. The endpoints were observed at 48 hours after randomization. Characteristics of the trials included in the analysis are shown in Table 1.
Figure 1.
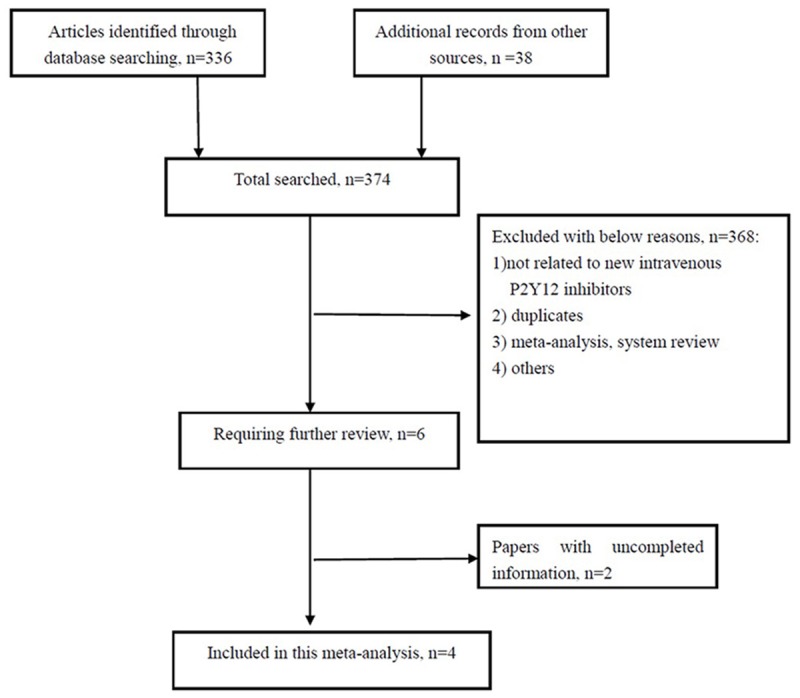
Flow diagram for the meta-analysis.
Table 1.
Main features of included studies
| Study year | n | Population | PCI | Follow-up | Intervention | Reference LD/MD | MACEs definitions | Major bleeding |
|---|---|---|---|---|---|---|---|---|
| Bhatt 2009 | 5362 | NSTEMI: 60% UA: 35% SCAD: 5% | 99% | 30 days | Cangrelor IV 30 ug/kg bolus, 4 ug/kg/min 2-4 h, then clopidogrel then clopidogrel 600 mg | Placebo + clopidogrel 600 mg at the end of PCI | Death/MI/IDR | TIMI |
| Harrington 2009 | 8877 | STEMI: 11% NSTEMI: 49% UA: 25% SCAD: 15% | 100% | 30 days | Cangrelor IV 30 mg/kg bolus and 4 mg/kg/min 2 h | Placebo + clopidogrel 600 mg 30 min before PCI | Death/MI/IDR | TIMI |
| Leonardi 2012 | 10900 | STEMI: 0% NSTEMI: 57% UA: 31% SCAD: 12% | 100% | 48 h | Cangrelor IV 30 mg/kg bolus and 4 mg/kg/min 2-4 h | Placebo + clopidogrel 600 mg at the end of PCI | Death/MI/IDR | TIMI |
| Bhatt 2103 | 10942 | STEMI: 18% NSTEMI: 26% SCAD: 56% | 100% | 48 h | Cangrelor IV 30 mg/kg bolus and 4 mg/kg/min 2-4 h | Clopidogrel 600 or 300 mg LD | Death/MI/IDR/ST | TIMI |
PCI, percutaneous coronary intervention; NSTEMI, none ST elevation myocardial infarction; STEMI, ST elevation myocardial infarction; UA, unstable angina; SCAD, stable coronary artery disease; LD, loading dose; MD, maintenance dose; MACCE, major adverse cardiac and cerebrovascular events; IV: intravenous; MI: myocardical infarction; IDR, ischemia-driven revascularization; ST, stent thrombosis; TIMI, thrombolysis in myocardial infarction criteria.
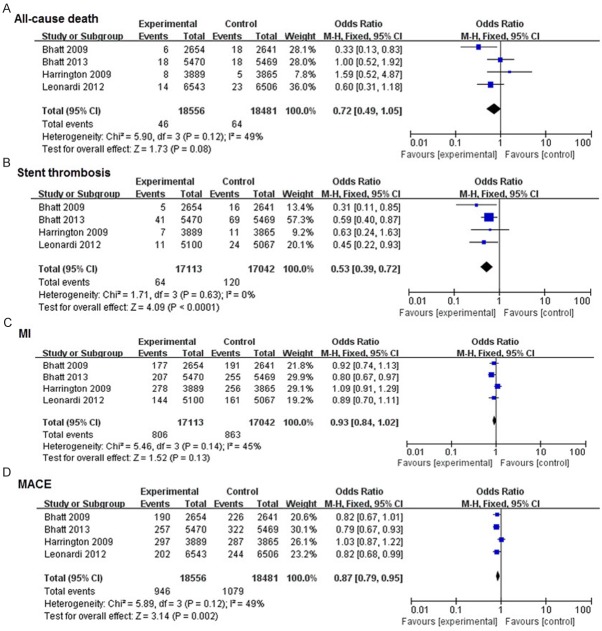
In this meta-analysis, all the included studies were double-blind, and almost all of the candidates received PCI procedure. Results were reported in Figures 2, 3. There was no significant heterogeneity for the analyses of MACE, all-cause death, stent thrombosis, and major or minor bleeding endpoints (P > 0.1, and I2 < 50%). No significant differences were observed in all-cause death (P = 0.08), MI (P = 0.13), TIMI major bleeding (P = 0.99) and GUSTO severe bleeding (P = 0.49) between cangrelor group and clopidogrel group. There was a 13% decrease in MACE (OR: 0.87, 95% CI: 0.79-0.95, P = 0.002), and 47% decrease in stent thrombosis (OR: 0.53, 95% CI: 0.39-0.72, P < 0.01), along with a significant increase in TIMI minor bleeding (OR: 1.49, 95% CI: 1.02-2.17, P = 0.004) and GUSTO moderate bleeding (OR: 1.43, 95% CI: 1.02-2.00, P = 0.004).
Figure 2.
Effects of new intravenous P2Y12 inhibitor compared with clopidogrel on efficacy events in patients with CAD.
Figure 3.
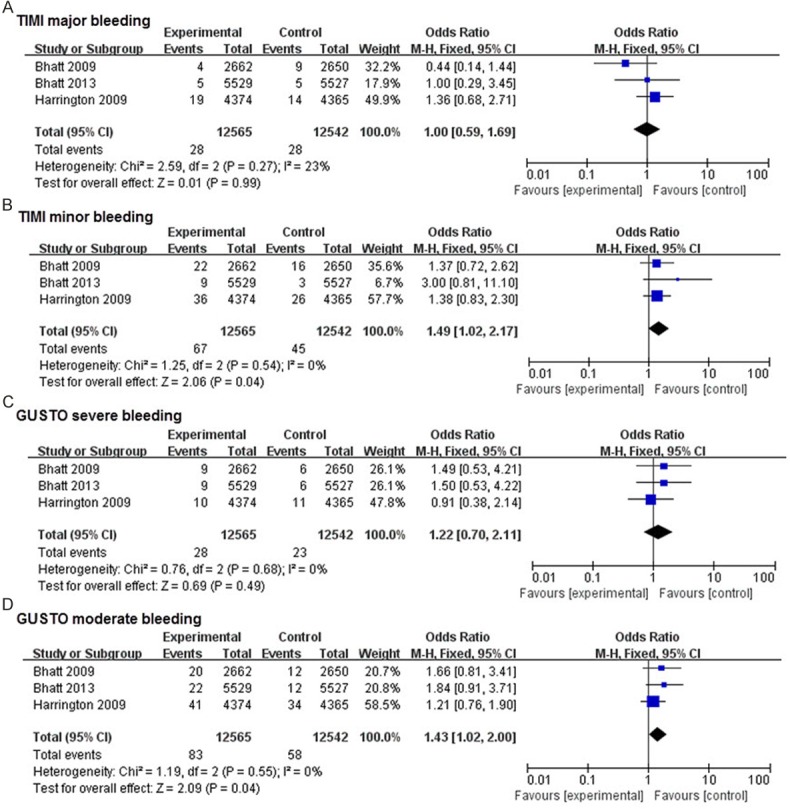
Effects of new intravenous P2Y12 inhibitor compared with clopidogrel on safety events in patients with CAD.
Discussion
This meta-analysis systematically addresses the question that whether new intravenous P2Y12 inhibitor (cangrelor) is associated with decreased efficacy or safety end points. The main findings could be summarized as follows: 1) Comparing with clopidogrel, administration of cangrelor leads to a significant reduction in the incidence of major ischemic events (MACE, stent thrombosis and Q-wave MI) in patients with CAD. 2) Cangrelor significantly increases risk of minor bleeding in comparison with clopidogrel.
Although dual antiplatelet therapy is the cornerstone of treating CAD patients, responsiveness to clopidogrel varies obviously among individuals [18,19]. Recent articles cannot reach an agreement about whether high-dose clopidogrel is benefit to reduce the risk of ischemic events in patients, without increasing rate of bleeding complications [5-7]. Some articles supported that high-dose clopidogrel inhibits platelet function effectively along with other effects, such as improving endothelial nitric oxide bioavailability and diminishing biomarkers of oxidant stress, and retarding the progression of established lesions and promotion of cell apoptosis [20,21]. However, recent papers pointed that double dose of clopidogrel has no obvious benefit on mortality with standard dose of clopidogrel in patients [6,7], and it did not reduce the incidence of major ischemic events, and it has no benefit effect on survival [22]. So, more rapid and stronger inhibition of platelet aggregation is necessary for emerging antiplatelet agents, with the expectation of further improving outcomes for patients with CAD.
The new P2Y12 inhibitor drugs with both intravenous (cangrelor and elinogrel) and oral (prasugrel and ticagrelor) formulations, have faster onset of action and greater potency than clopidogrel. Prasugrel is a kind of thienopyridine oral pro-drug, which can be changed into an irreversible P2Y12 receptor inhibitor, while ticagrelor is a kind of oral, direct acting, and reversible P2Y12 inhibitor. Previous researches revealed that both drugs showed superior antiplatelet effects compared with standard or higher doses of clopidogrel, with features of inhibiting platelet aggregation more rapidly and consistently [9,23,24]. However, to our knowledge, few meta-analysis systematically evaluates the impact of new intravenous P2Y12 inhibitor (cangrelor) on risk of ischemic and bleeding events in patients with CAD. Cangrelor is an intravenous, direct-acting and reversible P2Y12 inhibitor. And elinogrel, which can be administration by either intravenously or orally, is a direct-acting, competitive and reversible inhibitor of the P2Y12 receptor. These features may offer specific theoretical advantages on safety and efficacy. In our meta-analysis, 13% significantly decrease in MACE and 47% significantly decrease in stent thrombosis were observed with cangrelor compared with clopidogrel.
New intravenous P2Y12 inhibitor could decrease the primary end point including MACE and stent thrombosis, and there was no difference in major bleeding event between new intravenous P2Y12 inhibitors and clopidogrel. One previous meta-analysis about comparing cangrelor with clopidogrel or placebo for prevention of thrombotic complications during and after PCI, suggested that cangrelor reduced PCI periprocedural thrombotic complications at the expense of increased bleeding [25]. Dissimilarly, our pooled analysis consisted of 36,803 CAD patients, and we found that new intravenous P2Y12 inhibitor was only related to increase minor bleeding in patients with CAD.
It is well known that new oral P2Y12 antagonists (prasugrel and ticagrelor) provide more rapid and consistent platelet inhibition than clopidogrel, especially for patients with STEMI. However, these oral agents still require hours to reach effective function of platelet inhibition, and moreover they are limited by the uncontrollable bleeding profile [8,26,27]. While the plasma half-life of cangrelor is approximately several minutes, so platelet function can be restored within 1 hour after stop of the infusion [28]. Based on these points, our meta-analysis supported that cangrelor is superior for clinical implications, in spite of further investigation are still required.
There are several limitations in our systemic review. Firstly, as with any meta-analysis, it is limited by the follow-up period of the enrolled trials and the sample sizes, along with other substrate-modifying strategies. For example, in this meta-analysis, the method of agents’ administration in each trial were not completely consistent, and this may induce bias in results. And there was disparity in the definition of some outcome events among the enrolled trials (myocardial infarction and MACE). Secondly, in most of the included trials, it was hard to delineate the two separate subtypes of stroke clearly (hemorrhagic and ischemic), because of a lack of clinical information in detail. So we did not include this important indicator. Thirdly, heterogeneity caused by different factors is an unavoidable limitation. Fortunately, the heterogeneities of clinical outcomes in our meta-analysis can be identified, and did not influence our overall conclusion.
Conclusions
In this updated analysis, new intravenous P2Y12 inhibitor (cangrelor) is associated with a reduced risk for MACE and stent thrombosis in patients with CAD, at the expense of increased minor bleeding.
Disclosure of conflict of interest
None.
References
- 1.Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without st-segment elevation. N Engl J Med. 2001;345:494–502. doi: 10.1056/NEJMoa010746. [DOI] [PubMed] [Google Scholar]
- 2.Chen ZM, Jiang LX, Chen YP, Xie JX, Pan HC, Peto R, Collins R, Liu LS COMMIT (ClOpidogrel and Metoprolol in Myocardial Infarction Trial) collaborative group. Addition of clopidogrel to aspirin in 45, 852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet. 2005;366:1607–1621. doi: 10.1016/S0140-6736(05)67660-X. [DOI] [PubMed] [Google Scholar]
- 3.Lotrionte M, Castagno D, Agostoni P, Abbate A, Sangiorgi G, Sheiban I, Biondi-Zoccai GG. Long-term effect of chronic oral anticoagulation: focus on coronary artery disease. Future Cardiol. 2009;5:259–271. doi: 10.2217/fca.09.6. [DOI] [PubMed] [Google Scholar]
- 4.Serebruany VL, Steinhubl SR, Berger PB, Malinin AI, Bhatt DL, Topol EJ. Variability in platelet responsiveness to clopidogrel among 544 individuals. J Am Coll Cardiol. 2005;45:246–251. doi: 10.1016/j.jacc.2004.09.067. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, Zhang Y, Tang Y, Huang X, Xie Y. High-maintenance-dose clopidogrel in patients undergoing percutaneous coronary intervention: a systematic review and meta-analysis. PLoS One. 2013;8:e78549. doi: 10.1371/journal.pone.0078549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehta SR, Tanguay JF, Eikelboom JW, Jolly SS, Joyner CD, Granger CB, Faxon DP, Rupprecht HJ, Budaj A, Avezum A, Widimsky P, Steg PG, Bassand JP, Montalescot G, Macaya C, Di Pasquale G, Niemela K, Ajani AE, White HD, Chrolavicius S, Gao P, Fox KA, Yusuf S CURRENT-OASIS trial investigators. Double-dose versus standard-dose clopidogrel and high-dose versus low-dose aspirin in individuals undergoing percutaneous coronary intervention for acute coronary syndromes (currentoasis 7): a randomised factorial trial. Lancet. 2010;376:1233–1243. doi: 10.1016/S0140-6736(10)61088-4. [DOI] [PubMed] [Google Scholar]
- 7.Price MJ, Berger PB, Teirstein PS, Tanguay JF, Angiolillo DJ, Spriggs D, Puri S, Robbins M, Garratt KN, Bertrand OF, Stillabower ME, Aragon JR, Kandzari DE, Stinis CT, Lee MS, Manoukian SV, Cannon CP, Schork NJ, Topol EJ GRAVITAS Investigators. Standard-vs high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention: the gravitas randomized trial. JAMA. 2011;305:1097–1105. doi: 10.1001/jama.2011.290. [DOI] [PubMed] [Google Scholar]
- 8.Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann FJ, Ardissino D, De Servi S, Murphy SA, Riesmeyer J, Weerakkody G, Gibson CM, Antman EM TRITON-TIMI Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–2015. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 9.Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, Mahaffey KW, Scirica BM, Skene A, Steg PG, Storey RF, Harrington RA PLATO Investigators. Freij A, Thorsén M. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- 10.Bhatt DL, Lincoff AM, Gibson CM, Stone GW, McNulty S, Montalescot G, Kleiman NS, Goodman SG, White HD, Mahaffey KW, Pollack CV Jr, Manoukian SV, Widimsky P, Chew DP, Cura F, Manukov I, Tousek F, Jafar MZ, Arneja J, Skerjanec S, Harrington RA CHAMPION PLATFORM Investigators. Intravenous platelet blockade with cangrelor during pci. N Engl J Med. 2009;361:2330–2341. doi: 10.1056/NEJMoa0908629. [DOI] [PubMed] [Google Scholar]
- 11.Bhatt DL, Stone GW, Mahaffey KW, Gibson CM, Steg PG, Hamm CW, Price MJ, Leonardi S, Gallup D, Bramucci E, Radke PW, Widimský P, Tousek F, Tauth J, Spriggs D, McLaurin BT, Angiolillo DJ, Généreux P, Liu T, Prats J, Todd M, Skerjanec S, White HD, Harrington RA CHAMPION PHOENIX Investigators. Effect of platelet inhibition with cangrelor during pci on ischemic events. N Engl J Med. 2013;368:1303–1313. doi: 10.1056/NEJMoa1300815. [DOI] [PubMed] [Google Scholar]
- 12.White HD, Chew DP, Dauerman HL, Mahaffey KW, Gibson CM, Stone GW, Gruberg L, Harrington RA, Bhatt DL. Reduced immediate ischemic events with cangrelor in pci: a pooled analysis of the champion trials using the universal definition of myocardial infarction. Am Heart J. 2012;163:182–190. doi: 10.1016/j.ahj.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, Steg PG, Morel MA, Mauri L, Vranckx P, McFadden E, Lansky A, Hamon M, Krucoff MW, Serruys PW Academic Research Consortium. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344–2351. doi: 10.1161/CIRCULATIONAHA.106.685313. [DOI] [PubMed] [Google Scholar]
- 14.Chesebro JH, Knatterud G, Roberts R, Borer J, Cohen LS, Dalen J, Dodge HT, Francis CK, Hillis D, Ludbrook P, et al. Thrombolysis in Myocardial Infarction (TIMI) Trial, Phase I: A comparison between intravenous tissue plasminogen activator and intravenous streptokinase. Clinical findings through hospital discharge. Circulation. 1987;76:142–154. doi: 10.1161/01.cir.76.1.142. [DOI] [PubMed] [Google Scholar]
- 15.Higgins JP, Green S. The Cochrane Collaboration. 2013. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1. 0 [updated March 2011] Retrieved onpublished at whilst December year 1111 from www.cochrane-handbook.org. [Google Scholar]
- 16.Leonardi S, Mahaffey KW, White HD, Gibson CM, Stone GW, Steg GW, Hamm CW, Price MJ, Todd M, Dietrich M, Gallup D, Liu T, Skerjanec S, Harrington RA, Bhatt DL. Rationale and design of the Cangrelor versus standard therapy to achieve optimal management of platelet inhibition phoenix trial. Am Heart J. 2012;163:768–776. doi: 10.1016/j.ahj.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 17.Harrington RA, Stone GW, McNulty S, White HD, Lincoff AM, Gibson CM, Pollack CV Jr, Montalescot G, Mahaffey KW, Kleiman NS, Goodman SG, Amine M, Angiolillo DJ, Becker RC, Chew DP, French WJ, Leisch F, Parikh KH, Skerjanec S, Bhatt DL. Platelet inhibition with cangrelor in patients undergoing PCI. N Engl J Med. 2009;361:2318–2329. doi: 10.1056/NEJMoa0908628. [DOI] [PubMed] [Google Scholar]
- 18.Ferguson AD, Dokainish H, Lakkis N. Aspirin and clopidogrel response variability. Tex Heart Inst J. 2008;35:313–320. [PMC free article] [PubMed] [Google Scholar]
- 19.Bonello L, Tantry US, Marcucci R, Blindt R, Angiolillo DJ, Becker R, Bhatt DL, Cattaneo M, Collet JP, Cuisset T, Gachet C, Montalescot G, Jennings LK, Kereiakes D, Sibbing D, Trenk D, Van Werkum JW, Paganelli F, Price MJ, Waksman R, Gurbel PA Working Group on High On-Treatment Platelet Reactivity. Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J Am Coll Cardiol. 2010;56:919–933. doi: 10.1016/j.jacc.2010.04.047. [DOI] [PubMed] [Google Scholar]
- 20.Ren H, Li M, Feng L, Jiang J, Zhang Y, Zhang Y, Zhu X. Effects of clopidogrel on vascular proliferation and apoptosis in an atherosclerotic rabbit model. J Cardiovasc Pharmacol. 2010;55:617–624. doi: 10.1097/FJC.0b013e3181dc98dc. [DOI] [PubMed] [Google Scholar]
- 21.Heitzer T, Rudolph V, Schwedhelm E, Karstens M, Sydow K, Ortak M, Tschentscher P, Meinertz T, Böger R, Baldus S. Clopidogrel improves systemic endothelial nitric oxide bioavailability in patients with coronary artery disease: evidence for antioxidant and antiinflammatory effects. Arterioscler Thromb Vasc Biol. 2006;26:1648–1652. doi: 10.1161/01.ATV.0000225288.74170.dc. [DOI] [PubMed] [Google Scholar]
- 22.CURRENT-OASIS Investigators. Mehta SR, Bassand JP, Chrolavicius S, Diaz R, Eikelboom JW, Fox KA, Granger CB, Jolly S, Joyner CD, Rupprecht HJ, Widimsky P, Afzal R, Pogue J, Yusuf S. Dose comparisons of clopidogrel and aspirin in acute coronary syndromes. N Engl J Med. 2010;363:930–942. doi: 10.1056/NEJMoa0909475. [DOI] [PubMed] [Google Scholar]
- 23.Biondi-Zoccai G, Lotrionte M, Agostoni P, Abbate A, Romagnoli E, Sangiorgi G, Angiolillo DJ, Valgimigli M, Testa L, Gaita F, Sheiban I. Adjusted indirect comparison meta-analysis of prasugrel versus ticagrelor for patients with acute coronary syndromes. Int J Cardiol. 2011;150:325–331. doi: 10.1016/j.ijcard.2010.08.035. [DOI] [PubMed] [Google Scholar]
- 24.Cannon CP, Husted S, Harrington RA. Safety, tolerability, and initial efficacy of AZD6140, the first reversible oral adenosine diphosphate receptor antagonist, compared with clopidogrel, in patients with non-ST-segment elevation acute coronary syndrome: primary results of the DISPERSE-2 trial. J Am Coll Cardiol. 2007;50:1844–1851. doi: 10.1016/j.jacc.2007.07.053. [DOI] [PubMed] [Google Scholar]
- 25.Steg PG, Bhatt DL, Hamm CW, Stone GW, Gibson CM, Mahaffey KW, Leonardi S, Liu T, Skerjanec S, Day JR, Iwaoka RS, Stuckey TD, Gogia HS, Gruberg L, French WJ, White HD, Harrington RA CHAMPION Investigators. Effect of cangrelor on periprocedural outcomes in percutaneous coronary interventions: a pooled analysis of patient-level data. Lancet. 2013;382:1981–1992. doi: 10.1016/S0140-6736(13)61615-3. [DOI] [PubMed] [Google Scholar]
- 26.Agrawal K, Bhatt DL. Antiplatelet therapy: does prasugrel or ticagrelor suffice in patients with STEMI? Nat Rev Cardiol. 2013;10:121–122. doi: 10.1038/nrcardio.2012.199. [DOI] [PubMed] [Google Scholar]
- 27.Alexopoulos D, Xanthopoulou I, Gkizas V, Kassimis G, Theodoropoulos KC, Makris G, Koutsogiannis N, Damelou A, Tsigkas G, Davlouros P, Hahalis G. Randomized Assess ment of ticagrelor versus prasugrel antiplatelet eff ects in patients with ST-segment-elevation myocardial infarction. Circ Cardiovasc Interv. 2012;5:797–804. doi: 10.1161/CIRCINTERVENTIONS.112.972323. [DOI] [PubMed] [Google Scholar]
- 28.Angiolillo DJ, Schneider DJ, Bhatt DL, French WJ, Price MJ, Saucedo JF, Shaburishvili T, Huber K, Prats J, Liu T, Harrington RA, Becker RC. Pharmacodynamic effects of cangrelor and clopidogrel: the platelet function substudy from the Cangrelor versus Standard Therapy to Achieve Optimal Management of Platelet Inhibition (CHAMPION) trials. J Thromb Thrombolysis. 2012;34:44–55. doi: 10.1007/s11239-012-0737-3. [DOI] [PubMed] [Google Scholar]