Abstract
SIRT1 is the homologue of sir2 in mammals, which is a nicotinamide adenine dinucleotide (NAD+) dependent histone deacetylase. SIRT1 is involved in many physiological processes, such as metabolism, senescence, inflammatory response, neuroprotection, and tumorigenesis by acetylating histones and multiple transcription factors. However, the exact role of SIRT1 in tumor is still under controversial. Immunohistochemistry and Western blot were performed to investigate the expressions and subcellular localizations of SIRT1 and Phospho-SIRT1 in colorectal cancer tissues and adjacent normal tissues. The relationship between SIRT1 or Phospho-SIRT1 and clinicopathological characteristics was also analyzed. Real-Time PCR was performed to investigate the transcriptional level of SIRT1 mRNA in colorectal cancer tissues and adjacent normal tissues. SIRT1 and Phospho-SIRT1 were both localized in the nucleus. The expressions of SIRT1 and Phospho-SIRT1 were higher in colorectal cancer tissues than normal tissues. SIRT1 expression in cancer tissues was associated with patient age, TNM stage and mutant P53 loss. Phospho-SIRT1 expression in cancer tissues was associated with Ki67. SIRT1 and Phospho-SIRT1 were highly correlated in cancer tissues and normal tissues. The ratios of Phospho-SIRT1 and SIRT1 expression in cancer tissues were higher than normal tissues. SIRT1 mRNA level was no significant difference in cancer tissues and normal tissues. SIRT1 have a dual character in colorectal cancer, and Phospho-SIRT1 may determine the role of SIRT1 in colorectal cancer formation.
Keywords: SIRT1, Phospho-SIRT1, colorectal cancer, Ki67, P53
Introduction
Silent information regulator 2 (Sir2) is an anti-aging gene discovered in budding yeast originally, which encodes a protein with NAD+ dependent histone deacetylase activity [1]. SIRT1 is one of seven homologs of Sir2 in mammals [2], which involved in cell energy metabolism, proliferation, senescence, multiple inflammatory processes, neuroprotection, tumorigenesis and so on [3,4]. However, there are many controversies on the role of SIRT1 in tumors [5].
SIRT1 maybe is a tumor promoter. SIRT1 overexpression was observed in prostate cancer [6], colon cancer [7], ovarian cancer [8], breast cancer [9], gastric cancer [10], pancreatic cancer [11], hepatocellular cancer [12], acute myeloid leukemia [13] and diffuses large B cell lymphoma [14], which associated with low grade, advanced TNM stage, lymph node metastasis and short overall survival. SIRT1 represses cellular apoptosis by deacetylating P53 [15], KU70 [16], FOXO [17] and other substrates, and promotes cellular metastasis by deacetylating Cortactin and ZEB1 [18-20]. Researchers reported that shRNA or SIRT1 inhibition could reverse drug resistance [21-23].
SIRT1 maybe is a tumor suppressor. Some studies have found low expression of SIRT1 in colon cancer, breast cancer, hepatocellular cancer, prostate cancer, ovarian cancer and bladder cancer, which associated with short overall survival [24,25]. SIRT1 manipulates deacetylate of NF-κB [26], β-catenin [27], Survivin [28] and c-Myc [29] to promote cellular apoptosis. In mouse fibroblast cell lines MEFs, SIRT1 activator resveratrol can reduce the inflammatory response induced by TNF-α [30]. In addition, Wang et al. 2008b observed early mortality and chromatin instability in mutant SIRT1-/-mouse [25].
SIRT1 was originally thought to be a nucleoprotein [31]. More and more studies have found that SIRT1 subcellular localization was diversity-completely localized in the nucleus [9,10,12,24], or completely localized in the cytoplasm [32,33], or both localized in the nucleus and cytoplasm [8,18,34]. It has been demonstrated that SIRT1 contains two nuclear localization signals and two nuclear export signals, and subcellular localization of SIRT1 was due to the nucleocytoplasmic shuttling [32,35].
SIRT1 is regulated by series of factors at transcription, translation and post-translational modification stages. Studies had pointed out that SIRT1 protein level was not associated with SIRT1 mRNA level, indicating that phosphorylation played an important role in regulating the activity of SIRT1 deacetylation and nucleocytoplasmic shuttling [36,37]. However, researches on Phospho-SIRT1 in tumors are less common.
In this research, we observed the expressions and subcellular localizations of SIRT1 and Phospho-SIRT1 in colorectal cancer tissues and adjacent normal tissues, and investigated the correlation between the expressions and clinicopathological characteristics. We also observed SIRT1 mRNA in colorectal cancer tissues and adjacent normal tissues to determine the role of phosphorylation.
Materials and methods
Samples collection and reagents
50 cases of colorectal cancer paraffin tissues and 20 cases of adjacent normal paraffin tissues were collected in Liaocheng People’s Hospital from January 2012 to December 2013. 20 cases of fresh cancer tissues and adjacent normal tissues were collected immediately after surgery, and stored at -80°C. Adjacent normal tissue refer to the tissue that have more than 5 cm distance from the cancerous tissue, and no pathologically confirmed invasive cancer. All samples have clear diagnosis and complete pathological data. Preoperative chemotherapy was not carried out. Ethics committees have given the approval for the use of all samples.
50 cases of colorectal cancer paraffin tissues were shown as follows: 32 males, 18 females; aged ≥ 60 years 29 cases, 21 cases less than 60 years old; well differentiated type 3 cases, moderate differentiated type 41 cases, poorly differentiated-type 6 cases; I of 13 cases, II of 17 cases, III of 19 cases, IV of 1 cases; lymph node metastasis 19 cases; distant metastasis 1 cases.
Antibodies
Antibodies for immunohistochemistry (IHC): SIRT1 (1104-1, Epitomics, 1:100), Phospho-SIRT1 (pS47) (2381-1, Epitomics, 1:1000), p53 (MAB-0142, maixin, China, 1:150), Ki67 (MAB-0542, maixin, China, 1:200). EliVisionTM plus detection Kit (KIT-9903, maixin, China).
Antibodies for Western blot (WB): SIRT1 (1104-1, Epitomics, 1:2000), Phospho-SIRT1 (pS47) (2381-1, Epitomics, 1:2000), β-actin (AA128, Beyotime, China, 1:1000), H3 (AH433, Beyotime, China, 1:1000), goat anti-rabbit HRP-conjugated IgG (H + L) (A0208, Beyotime, China, 1:1000), goat anti-mouse HRP-conjugated IgG (H + L) (A0216, Beyotime, China, 1:1000).
Immunohistochemical analysis
SIRT1, Phospho-SIRT1, p53 and Ki67 were detected according to the procedure of EliVision plus detection Kit. Briefly, 3 μm paraffin slides were heated at 65°C for 30 mins. Antigen retrieval was performed for 2 mins in Pressure Cooker containing sodium citrate buffer after deparaffinization and hydration. Inactivate endogenous peroxidase with 3% hydrogen peroxide for 10 mins and block slides with 5% BSA for 1 hour. Primary antibodies were incubated for 1 hour at room temperature. HRP-conjugated secondary antibodies were applied to each slides and incubated for 30 mins at room temperature.
Evaluation of IHC staining
Two independent pathologists read the slides in case of unknown clinical data. Final score was dependent on both staining density and staining area. The staining density was graded as follows: 0, no staining; 1, weak staining; 2, moderate staining; 3, intense staining. Percentage area was graded as follows: 0, < 5%; 1, 5% to 25%; 2, 26% to 50%; 3, 51% to 75%; 4, > 75%. The final score of SIRT1 and Phospho-SIRT1 was scored by multiplying the staining density by the staining area, and positive was regarded if final scores ≥ 4. P53 was considered positive if ≥ 30% tumor cells were stained. Ki67 was considered positive if ≥ 50% tumor cells were stained.
Western blot analysis
Fresh frozen tissues were extracted respectively for whole protein, cytoplasm protein and nucleus protein by RIPA buffer and nucleus-cytoplasm protein extraction kit. Protein concentration was determined by BCA assay and boiled at 100°C for 5 min mixed with loading buffer. Proteins were separated on SDS-PAGE gel and transferred to PVDF membrane. PVDF membrane was blocked by 5% non-fat milk for 1.5 hours at room temperature. Primary antibodies were incubated overnight at 4°C and HRP-conjugated secondary antibodies were incubated for 1h at room temperature. Immuno-reactive signals were detected by ECL. Cytoplasm protein were normalized with β-actin, nucleus protein were normalized with H3.
Real Time transcription-polymerase chain reaction (Real-Time PCR)
Total RNA was isolated from colorectal cancer tissues and adjacent normal tissues with Trizol reagent (Beyotime, China). cDNA was synthesized by QuantScript RT Kit (KR103, TIANGEN, China). Prepare the reaction system according to SuperReal PreMix Plus (SYBR Green) (FP205, TIANGEN, China). GAPDH was selected as an internal control gene. The primer sequences were as follows: SIRT1 forward 5’-CCGGATTTGAAGAATGTTGG-3’, SIRT1 reverse 5’-ATCTGCTCCTTTGCCACTCT-3’, GAPDH forward 5’-GGTATCGTCGAAGGACTCATGAC-3’, GAPDH reverse 5’-ATGCCAGTGAGCTTCCCGTTCAGC-3’.
Statistical analysis
SPSS 19.0 software was used to analysis the data. Mann-Whitney U test was used to analysis expression differences of SIRT1 or Phospho-SIRT1 in cancer tissues and normal tissues. The χ2 test or Fisher’s exact test was used to evaluate the differences between SIRT1 or Phospho-SIRT1 expression and clinicopathological characteristics in colorectal cancer tissues. Spearman correlation analysis was used to examine the expression correlation between Phospho-SIRT1 and SIRT1. SIRT1 mRNA expression differences in cancer tissues and normal tissues were analyzed using the comparative CT method. All values were expressed as mean ± standard error (Mean ± SE). P value < 0.05 was considered statistically significance.
Results
SIRT1 and Phospho-SIRT1 were both localized in the nucleus and expressed in colorectal cancer tissues.
IHC revealed that SIRT1 and Phospho-SIRT1 were both localized in the nucleus of cancer tissues and normal tissues (Figure 1A). SIRT1 expression in cancer tissues and normal tissues were 60% and 25%, respectively. Phospho-SIRT1 expression in cancer tissues and normal tissues were 38% and 15%, respectively. The average score of SIRT1 in cancer tissues and normal tissues were 4.780 ± 0.353 (Mean ± SE) and 2.450 ± 0.373, respectively. The average score of Phospho-SIRT1 in cancer tissues and normal tissues were 3.020 ± 0.228 and 1.900 ± 0.261, respectively (Figure 1B). Statistical analysis showed that SIRT1 and Phospho-SIRT1 were higher expressed in cancer tissues than normal tissues (P < 0.05 for SIRT1, P = 0.008 for Phospho-SIRT1).
Figure 1.
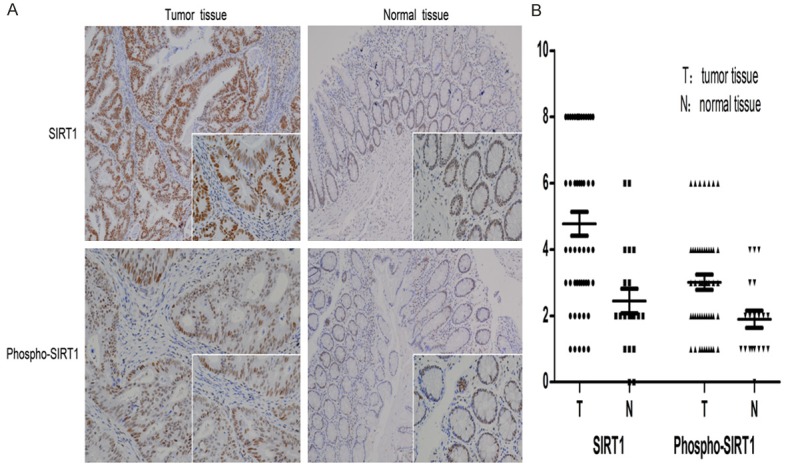
SIRT1 and Phospho-SIRT1 were detected by IHC (100 × and 400 × magnification). Mann-Whitney U test was used to analysis expression difference of SIRT1 or Phospho-SIRT1 in cancer tissues and normal tissues. A. Brown staining was seen localized to the nucleus of cells. SIRT1 expression in cancer tissues and normal tissues were 60% and 25%, respectively, Phospho-SIRT1 expression were 38% and 15%, respectively. B. Final IHC staining score of each slide was scored by multiplying the staining density by the staining area, and staining scores were shown as a scatter plot. The average score of SIRT1 in cancer tissues and normal tissues were 4.780 ± 0.353 and 2.450 ± 0.373, respectively (P < 0.05), the average scores of Phospho-SIRT1 were 3.020 ± 0.228 and 1.900 ± 0.261, respectively (P < 0.05).
WB revealed that SIRT1 and Phospho-SIRT1 were both localized in the nucleus of cancer tissues and normal tissues (Figure 2A). SIRT1 expression was scored as 1.351 ± 0.020 in cancer tissues and 0.825 ± 0.015 in normal tissues. Phospho-SIRT1 expression was scored as 0.747 ± 0.022 in cancer tissues and 0.381 ± 0.015 in normal tissues (Figure 2B). Statistical analysis showed that, the expressions of SIRT1 and Phospho-SIRT1 were higher in cancer tissues than in normal tissues (P < 0.05 for SIRT1, P < 0.05 for Phospho-SIRT1).
Figure 2.
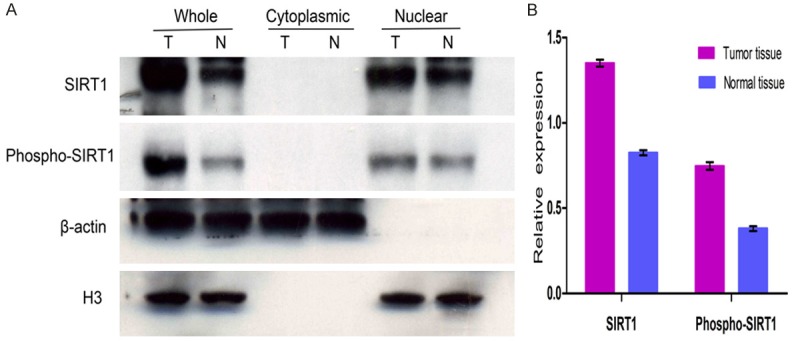
SIRT1 and Phospho-SIRT1 were detected by WB. Mann-Whitney U test was used to analysis expression differences of SIRT1 or Phospho-SIRT1 in cancer tissues and normal tissues. A. Tumor tissues and normal tissues were both extracted for whole protein, cytoplasmic protein and nuclear protein (T = tumor tissue, N = normal tissue). Cytoplasm protein was normalized with β-actin and nucleus protein was normalized with H3. SIRT1 and Phospho-SIRT1 were both localized in the nucleus of cancer tissues and normal tissues. B. Relative expression of SIRT1 and Phospho-SIRT1 in tumor tissues and normal tissues. SIRT1 expression in cancer tissues and normal tissues were 1.351 ± 0.020 and 0.825 ± 0.015, respectively (P < 0.05), Phospho-SIRT1 expression were 0.747 ± 0.022 and 0.381 ± 0.0148, respectively (P < 0.05).
SIRT1 and Phospho-SIRT1 expression in tumor tissues were both related with clinicopathological characteristics
We used the results of IHC to analysis the relationships of SIRT1 and Phospho-SIRT1 expression with clinicopathological characteristics (Table 1). Our experiment results showed that SIRT1 expression correlated with younger age (P = 0.047), advanced TNM stage (P = 0.018) and mutant P53 loss (P = 0.015). Phospho-SIRT1 expression correlated with Ki67 (P = 0.011). Our research demonstrated that SIRT1 may be as both favorable and unfavorable factors and Phospho-SIRT1 may play an important role in colorectal cancer formation.
Table 1.
The expression of SIRT1 and Phospho-SIRT1 in colorectal cancer
| Characteristics | n | Expression of SIRT1 | Expression of P-SIRT1 | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Positive | Negative | P value | Positive | Negative | P value | ||
| Gender | 0.470 | 0.190 | |||||
| Male | 32 | 18 | 14 | 10 | 22 | ||
| Female | 18 | 12 | 6 | 9 | 9 | ||
| Age (year) | 0.047 | 0.547 | |||||
| < 60 | 21 | 16 | 5 | 9 | 12 | ||
| ≥ 60 | 29 | 14 | 15 | 10 | 19 | ||
| Grade | 0.929 | 0.802 | |||||
| High-Middle | 44 | 27 | 17 | 17 | 27 | ||
| Low | 6 | 3 | 3 | 2 | 4 | ||
| TNM stage | 0.018 | 0.405 | |||||
| I-II | 30 | 14 | 16 | 10 | 20 | ||
| III-IV | 20 | 16 | 4 | 9 | 11 | ||
| LN metastasis | 0.341 | 0.285 | |||||
| Absent | 31 | 17 | 14 | 10 | 21 | ||
| Present | 19 | 13 | 6 | 9 | 10 | ||
| Distant metastasis | NS | NS | |||||
| Absent | 49 | 30 | 20 | 19 | 31 | ||
| Present | 1 | 0 | 0 | 0 | 0 | ||
| P53 mutation | 0.015 | 0.186 | |||||
| Positive | 27 | 12 | 15 | 8 | 19 | ||
| Negative | 23 | 18 | 5 | 11 | 12 | ||
| Ki67 | 0.108 | 0.011 | |||||
| Positive | 34 | 23 | 11 | 17 | 17 | ||
| Negative | 16 | 7 | 9 | 2 | 14 | ||
P-SIRT1, Phospho-SIRT1; TNM, tumor node metastasis; LN, lymph node. The χ2 test or Fisher’s exact test was used to evaluate the association between SIRT1 or Phospho-SIRT1 expression and clinicopathological characteristics. P value < 0.05 was considered statistically significant.
Phosphorylation of SIRT1 was higher in colorectal cancer tissues than in normal tissues
To investigate the role of Phosphorylation in colorectal cancer, we examined the correlations of Phospho-SIRT1 and SIRT1 in cancer tissues and normal tissues (Figure 3). Our experiment results showed that Phospho-SIRT1 and SIRT1 were positively correlated in cancer tissues and normal tissues, and the ratios of Phospho-SIRT1 and SIRT1 were higher in cancer tissues than normal tissues, suggesting that phosphorylation would promote the colorectal cancer formation.
Figure 3.
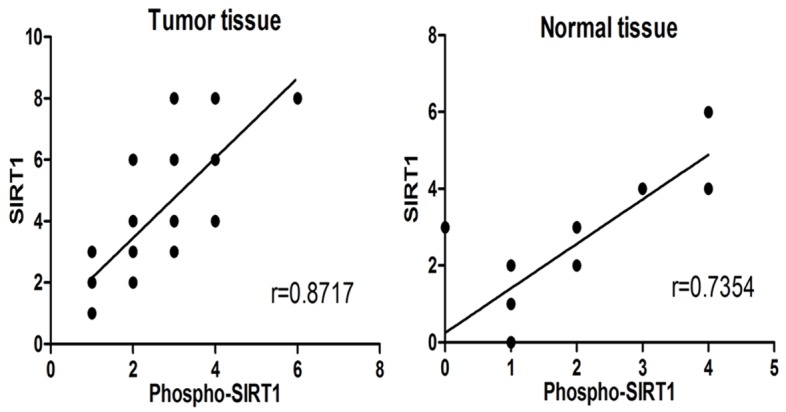
Spearman correlation analysis was used to examine the expression correlation between Phospho-SIRT1 and SIRT1. Phospho-SIRT1 and SIRT1 had a highly positive correlation in both tumor tissues (r = 0.872) and normal tissues (r = 0.735). The ratios of Phospho-SIRT1 and SIRT1 expression in tumor tissues were higher than normal tissues.
SIRT1 mRNA was no significant difference in colorectal cancer tissues and normal tissues
To determine whether SIRT1 expression was at transcriptional level or at post-transcriptional level, we examined SIRT1 mRNA level in colorectal cancer tissues and normal tissues using Real-Time PCR (Table 2). Results showed that the fold change in expression of the SIRT1 gene in cancer tissues compared to the normal tissues is 1.1, suggesting that SIRT1 mRNA has no significant difference in the cancer tissues and normal tissues. We inference that post-transcription may affect the expression of SIRT1 in colorectal cancer tissues.
Table 2.
Relative expression of SIRT1 mRNA in tumor tissues and normal tissue
| Tissue | Avg. SIRT1 CT | Avg. GAPDH CT | ΔCT | ΔΔCT | Normalized SIRT1 amount relative to normal 2-ΔΔCt |
|---|---|---|---|---|---|
| Tumor | 27.187 ± 0.165 | 25.265 ± 0.287 | 1.922 ± 0.259 | 0.178 ± 0.259 | 1.1 (0.7-1.1) |
| Normal | 26.725 ± 0.119 | 24.981 ± 0.321 | 1.744 ± 0.299 | 0.000 ± 0.299 | 1.0 (0.8-1.2) |
The comparative CT method was used to analyze the expression of SIRT1 mRNA. Avg., Average; ΔCT = Avg; SIRT1 CT - Avg; GAPDH CT; ΔΔCT = Avg; ΔCT,Tumor - Avg. ΔCT,Normal. Fold change = 2-ΔΔCt.
Discussion
In this study, we examined the expression and subcellular localization of SIRT1 and Phospho-SIRT1 in colorectal cancer tissues and corresponding normal tissues. SIRT1 mRNA level was also examined in cancer tissues and normal tissues using Real-Time PCR. The main results are that: 1. SIRT1 and Phospho-SIRT1 were both localized in the nucleus and overexpressed in cancer tissues. 2. SIRT1 overexpression was associated with younger age, advanced TNM stage and mutant P53 loss, Phospho-SIRT1 was associated with Ki67. 3. Phosphorylation of SIRT1 was higher in colorectal cancer tissues than normal tissues. 4. SIRT1 mRNA level was no significant difference in cancer tissues and normal tissues.
There are many controversies on SIRT1: SIRT1 may be as a tumor promoter or tumor suppressor, SIRT1 may localize in the nucleus or cytoplasm. Even in the same tumors, such as colorectal cancer, SIRT1 results were various. Walter Stünkel’s found that SIRT1 was highly expressed in cancer tissues, and mainly localized in the cytoplasm [7]. Si-Hyong Jang demonstrated that SIRT1 was low expression in colon cancer and located in the nucleus by IHC [24]. Qihuang Jin revealed that SIRT1 in LoVo cell lines might partially localized in the cytoplasm and proved that cytoplasm localization of SIRT1 was transferred from the nucleus [38]. In our study, we observed overexpression and nuclear localization of SIRT1 in colorectal cancer.
Studies have shown that SIRT1 protein level was associated with the mitotic activity regardless of their mRNA level [39]. It also has been demonstrated that phosphorylation might affect the activity and subcellular localization of SIRT1. We hypothesis that whether the phosphorylation status of SIRT1 more directly determine the role of SIRT1 in tumors? Whether the subcellular localization of Phospho-SIRT1 reflects the nucleocytoplasmic shuttling of SIRT1? However, until now, researches on Phospho-SIRT1 in tumors are rare. It is known that SIRT1 have 13 phosphorylation sites and Ser47 is a common one, so we applied Phospho-SIRT1 (pS47) antibody to analysis in detail the expression and subcellular localization of Phospho-SIRT1 in colorectal cancer.
First, to determine whether SIRT1 in colorectal cancer was overexpressed at the transcriptional level or the post-transcriptional level, we evaluated SIRT1 mRNA level using Real-Time PCR. Our data revealed that SIRT1 mRNA level in colorectal cancer tissues and normal tissues was no significant difference, suggesting that post-transcriptional level plays an important role on the regulation of SIRT1 expression.
Results of IHC and WB showed that Phospho-SIRT1 was highly expressed in colorectal cancer and located in the nucleus. After analysis of its relationship with clinicopathological characteristics, we found Phospho-SIRT1 was positively related with Ki67 and had no significant correlation with lymph node metastasis, TNM stage. As we all know, Ki67 was closely related with mitosis and reflects the proliferation of cells, so we believe that Phospho-SIRT1 may promote the tumorigenesis in colorectal cancer.
To confirm the role of Phospho-SIRT1 in cancer, we analyzed the correlation between Phospho-SIRT1 and SIRT1 in cancer tissues and normal tissues. We found that Phospho-SIRT1 and SIRT1 was positively correlated in both cancer tissues and normal tissues, the ratios of Phospho-SIRT1 and SIRT1 were higher in cancer tissues. The results demonstrated that phosphorylation might promote the formation of colorectal cancer.
Phosphorylation has been demonstrated to promote the transfer of SIRT1 to the nucleus. We speculate that SIRT1 may have a dual position of nucleus and cytoplasm, Phospho-SIRT1 may represent the shuttling portion of SIRT1 from the cytoplasm to the nucleus. To our surprise, our results showed that SIRT1 and Phospho-SIRT1 were both located in the nucleus and not in cytoplasmic. We couldn’t explain the phenomenon. Maybe SIRT1 localization has a complex regulation. Therefore, we cannot come to the view that phospho-SIRT1 may affect the subcellular localization of SIRT1.
Our experiments have some limitations, such as small sample size, Phospho-SIRT1 antibody selected only for certain sites which is not enough to represent all phosphorylation status of SIRT1, and so on. We cannot exclude the tumor heterogeneity and staining scoring method to interfere the experiment. In short, we need further study on the roles of Phospho-SIRT1 in cancers.
Acknowledgements
The authors thank Dr. Junlong Xu (pathologist from the Department of Pathology, Liaocheng People’s Hospital, Liaocheng, China) for his expert suggestions and technical assistance. This work was supported by China Postdoctoral Science Fund (No. 2011M500531 and No. 2014M561937) and Science and Technology development Planning of Liaocheng city (No. 2012NS11).
Disclosure of conflict of interest
None.
References
- 1.Fessel MR, Lira CB, Giorgio S, Ramos CH, Cano MI. Sir2-Related Protein 1 from Leishmania amazonensis is a glycosylated NAD+-dependent deacetylase. Parasitology. 2011;138:1245–1258. doi: 10.1017/S0031182011001077. [DOI] [PubMed] [Google Scholar]
- 2.Imai S, Guarente L. Ten years of NAD-dependent SIR2 family deacetylases: implications for metabolic diseases. Trends Pharmacol Sci. 2010;31:212–220. doi: 10.1016/j.tips.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Michan S, Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem J. 2007;404:1–13. doi: 10.1042/BJ20070140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baur JA, Ungvari Z, Minor RK, Le Couteur DG, de Cabo R. Are sirtuins viable targets for improving healthspan and lifespan? Nat Rev Drug Discov. 2012;11:443–461. doi: 10.1038/nrd3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song NY, Surh YJ. Janus-faced role of SIRT1 in tumorigenesis. Ann N Y Acad Sci. 2012;1271:10–19. doi: 10.1111/j.1749-6632.2012.06762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huffman DM, Grizzle WE, Bamman MM, Kim JS, Eltoum IA, Elgavish A, Nagy TR. SIRT1 is significantly elevated in mouse and human prostate cancer. Cancer Res. 2007;67:6612–6618. doi: 10.1158/0008-5472.CAN-07-0085. [DOI] [PubMed] [Google Scholar]
- 7.Stunkel W, Peh BK, Tan YC, Nayagam VM, Wang X, Salto-Tellez M, Ni B, Entzeroth M, Wood J. Function of the SIRT1 protein deacetylase in cancer. Biotechnol J. 2007;2:1360–1368. doi: 10.1002/biot.200700087. [DOI] [PubMed] [Google Scholar]
- 8.Jang KY, Kim KS, Hwang SH, Kwon KS, Kim KR, Park HS, Park BH, Chung MJ, Kang MJ, Lee DG, Moon WS. Expression and prognostic significance of SIRT1 in ovarian epithelial tumours. Pathology. 2009;41:366–371. doi: 10.1080/00313020902884451. [DOI] [PubMed] [Google Scholar]
- 9.Sung JY, Kim R, Kim JE, Lee J. Balance between SIRT1 and DBC1 expression is lost in breast cancer. Cancer Sci. 2010;101:1738–1744. doi: 10.1111/j.1349-7006.2010.01573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng AN, Zhang LH, Fan XS, Huang Q, Ye Q, Wu HY, Yang J. Expression of SIRT1 in gastric cardiac cancer and its clinicopathologic significance. Int J Surg Pathol. 2011;19:743–750. doi: 10.1177/1066896911412181. [DOI] [PubMed] [Google Scholar]
- 11.Zhao G, Cui J, Zhang JG, Qin Q, Chen Q, Yin T, Deng SC, Liu Y, Liu L, Wang B, Tian K, Wang GB, Wang CY. SIRT1 RNAi knockdown induces apoptosis and senescence, inhibits invasion and enhances chemosensitivity in pancreatic cancer cells. Gene Ther. 2011;18:920–928. doi: 10.1038/gt.2011.81. [DOI] [PubMed] [Google Scholar]
- 12.Chen HC, Jeng YM, Yuan RH, Hsu HC, Chen YL. SIRT1 promotes tumorigenesis and resistance to chemotherapy in hepatocellular carcinoma and its expression predicts poor prognosis. Ann Surg Oncol. 2012;19:2011–2019. doi: 10.1245/s10434-011-2159-4. [DOI] [PubMed] [Google Scholar]
- 13.Sonnemann J, Gruhn B, Wittig S, Becker S, Beck JF. Increased activity of histone deacetylases in childhood acute lymphoblastic leukaemia and acute myeloid leukaemia: support for histone deacetylase inhibitors as antileukaemic agents. Br J Haematol. 2012;158:664–666. doi: 10.1111/j.1365-2141.2012.09187.x. [DOI] [PubMed] [Google Scholar]
- 14.Jang KY, Hwang SH, Kwon KS, Kim KR, Choi HN, Lee NR, Kwak JY, Park BH, Park HS, Chung MJ, Kang MJ, Lee DG, Kim HS, Shim H, Moon WS. SIRT1 expression is associated with poor prognosis of diffuse large B-cell lymphoma. Am J Surg Pathol. 2008;32:1523–1531. doi: 10.1097/PAS.0b013e31816b6478. [DOI] [PubMed] [Google Scholar]
- 15.Inoue Y, Iemura S, Natsume T, Miyazawa K, Imamura T. Suppression of p53 activity through the cooperative action of Ski and histone deacetylase SIRT1. J Biol Chem. 2011;286:6311–6320. doi: 10.1074/jbc.M110.177683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geng YQ, Li TT, Liu XY, Li ZH, Fu YC. SIRT1 and SIRT5 activity expression and behavioral responses to calorie restriction. J Cell Biochem. 2011;112:3755–3761. doi: 10.1002/jcb.23315. [DOI] [PubMed] [Google Scholar]
- 17.Xia N, Strand S, Schlufter F, Siuda D, Reifenberg G, Kleinert H, Forstermann U, Li H. Role of SIRT1 and FOXO factors in eNOS transcriptional activation by resveratrol. Nitric Oxide. 2013;32:29–35. doi: 10.1016/j.niox.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Zhang M, Dong H, Yong S, Li X, Olashaw N, Kruk PA, Cheng JQ, Bai W, Chen J, Nicosia SV, Zhang X. Deacetylation of cortactin by SIRT1 promotes cell migration. Oncogene. 2009;28:445–460. doi: 10.1038/onc.2008.388. [DOI] [PubMed] [Google Scholar]
- 19.Eades G, Yao Y, Yang M, Zhang Y, Chumsri S, Zhou Q. miR-200a regulates SIRT1 expression and epithelial to mesenchymal transition (EMT)-like transformation in mammary epithelial cells. J Biol Chem. 2011;286:25992–26002. doi: 10.1074/jbc.M111.229401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Byles V, Zhu L, Lovaas JD, Chmilewski LK, Wang J, Faller DV, Dai Y. SIRT1 induces EMT by cooperating with EMT transcription factors and enhances prostate cancer cell migration and metastasis. Oncogene. 2012;31:4619–4629. doi: 10.1038/onc.2011.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peck B, Chen CY, Ho KK, Di Fruscia P, Myatt SS, Coombes RC, Fuchter MJ, Hsiao CD, Lam EW. SIRT inhibitors induce cell death and p53 acetylation through targeting both SIRT1 and SIRT2. Mol Cancer Ther. 2010;9:844–855. doi: 10.1158/1535-7163.MCT-09-0971. [DOI] [PubMed] [Google Scholar]
- 22.Bourguignon LY, Xia W, Wong G. Hyaluronan-mediated CD44 interaction with p300 and SIRT1 regulates beta-catenin signaling and NFkappaB-specific transcription activity leading to MDR1 and Bcl-xL gene expression and chemoresistance in breast tumor cells. J Biol Chem. 2009;284:2657–2671. doi: 10.1074/jbc.M806708200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kojima K, Ohhashi R, Fujita Y, Hamada N, Akao Y, Nozawa Y, Deguchi T, Ito M. A role for SIRT1 in cell growth and chemoresistance in prostate cancer PC3 and DU145 cells. Biochem Biophys Res Commun. 2008;373:423–428. doi: 10.1016/j.bbrc.2008.06.045. [DOI] [PubMed] [Google Scholar]
- 24.Jang SH, Min KW, Paik SS, Jang KS. Loss of SIRT1 histone deacetylase expression associates with tumour progression in colorectal adenocarcinoma. J Clin Pathol. 2012;65:735–739. doi: 10.1136/jclinpath-2012-200685. [DOI] [PubMed] [Google Scholar]
- 25.Wang RH, Sengupta K, Li C, Kim HS, Cao L, Xiao C, Kim S, Xu X, Zheng Y, Chilton B, Jia R, Zheng ZM, Appella E, Wang XW, Ried T, Deng CX. Impaired DNA damage response, genome instability, and tumorigenesis in SIRT1 mutant mice. Cancer Cell. 2008;14:312–323. doi: 10.1016/j.ccr.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katto J, Engel N, Abbas W, Herbein G, Mahlknecht U. Transcription factor NFkappaB regulates the expression of the histone deacetylase SIRT1. Clin Epigenetics. 2013;5:11. doi: 10.1186/1868-7083-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Firestein R, Blander G, Michan S, Oberdoerffer P, Ogino S, Campbell J, Bhimavarapu A, Luikenhuis S, de Cabo R, Fuchs C, Hahn WC, Guarente LP, Sinclair DA. The SIRT1 deacetylase suppresses intestinal tumorigenesis and colon cancer growth. PLoS One. 2008;3:e2020. doi: 10.1371/journal.pone.0002020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang RH, Zheng Y, Kim HS, Xu X, Cao L, Luhasen T, Lee MH, Xiao C, Vassilopoulos A, Chen W, Gardner K, Man YG, Hung MC, Finkel T, Deng CX. Interplay among BRCA1, SIRT1, and Survivin during BRCA1-associated tumorigenesis. Mol Cell. 2008;32:11–20. doi: 10.1016/j.molcel.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuan J, Minter-Dykhouse K, Lou Z. A c-Myc-SIRT1 feedback loop regulates cell growth and transformation. J Cell Biol. 2009;185:203–211. doi: 10.1083/jcb.200809167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu X, Liu Q, Wang M, Liang M, Yang X, Xu X, Zou H, Qiu J. Activation of Sirt1 by resveratrol inhibits TNF-alpha induced inflammation in fibroblasts. PLoS One. 2011;6:e27081. doi: 10.1371/journal.pone.0027081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haigis MC, Sinclair DA. Mammalian sirtuins: biological insights and disease relevance. Annu Rev Pathol. 2010;5:253–295. doi: 10.1146/annurev.pathol.4.110807.092250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hisahara S, Chiba S, Matsumoto H, Tanno M, Yagi H, Shimohama S, Sato M, Horio Y. Histone deacetylase SIRT1 modulates neuronal differentiation by its nuclear translocation. Proc Natl Acad Sci U S A. 2008;105:15599–15604. doi: 10.1073/pnas.0800612105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanno M, Kuno A, Yano T, Miura T, Hisahara S, Ishikawa S, Shimamoto K, Horio Y. Induction of manganese superoxide dismutase by nuclear translocation and activation of SIRT1 promotes cell survival in chronic heart failure. J Biol Chem. 2010;285:8375–8382. doi: 10.1074/jbc.M109.090266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cha EJ, Noh SJ, Kwon KS, Kim CY, Park BH, Park HS, Lee H, Chung MJ, Kang MJ, Lee DG, Moon WS, Jang KY. Expression of DBC1 and SIRT1 is associated with poor prognosis of gastric carcinoma. Clin Cancer Res. 2009;15:4453–4459. doi: 10.1158/1078-0432.CCR-08-3329. [DOI] [PubMed] [Google Scholar]
- 35.Tanno M, Sakamoto J, Miura T, Shimamoto K, Horio Y. Nucleocytoplasmic shuttling of the NAD+-dependent histone deacetylase SIRT1. J Biol Chem. 2007;282:6823–6832. doi: 10.1074/jbc.M609554200. [DOI] [PubMed] [Google Scholar]
- 36.Sasaki T, Maier B, Koclega KD, Chruszcz M, Gluba W, Stukenberg PT, Minor W, Scrable H. Phosphorylation regulates SIRT1 function. PLoS One. 2008;3:e4020. doi: 10.1371/journal.pone.0004020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nasrin N, Kaushik VK, Fortier E, Wall D, Pearson KJ, de Cabo R, Bordone L. JNK1 phosphorylates SIRT1 and promotes its enzymatic activity. PLoS One. 2009;4:e8414. doi: 10.1371/journal.pone.0008414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jin Q, Yan T, Ge X, Sun C, Shi X, Zhai Q. Cytoplasm-localized SIRT1 enhances apoptosis. J Cell Physiol. 2007;213:88–97. doi: 10.1002/jcp.21091. [DOI] [PubMed] [Google Scholar]
- 39.Dixit D, Sharma V, Ghosh S, Mehta VS, Sen E. Inhibition of Casein kinase-2 induces p53-dependent cell cycle arrest and sensitizes glioblastoma cells to tumor necrosis factor (TNFalpha)-induced apoptosis through SIRT1 inhibition. Cell Death Dis. 2012;3:e271. doi: 10.1038/cddis.2012.10. [DOI] [PMC free article] [PubMed] [Google Scholar]


