Abstract
Systemic lupus erythematosus (SLE) involves multiple factors, which result in the breakdown of self-tolerance and development of autoimmunity with organ damage. Bone marrow mesenchymal stem cells (BMMSCs) from the patients with SLE showed an impaired proliferative capacity compared with that from normal controls. In this study, we isolated BMMSCs from the patients with SLE and found that Vitamin D analog EB1089 could induce BMMSCs proliferation and mineralization deposition. Furthermore, we found that the expression of p-Smad 1/5/8 was promoted in BMMSCs with EB1089 treatment. In conclusion, our results support the notion that EB1089 promoted proliferation and osteogenic differentiation of BMMSCs by Smad 1/5/8 signaling pathway.
Keywords: Systemic lupus erythematosus, bone marrow mesenchymal stem cells, EB1089, proliferation, Smad signaling pathway
Introduction
Adult mesenchymal stem cells (MSCs) were first discovered by Friedenstein and associates, who identified an adherent, fibroblast-like population that could regenerate rudiments of normal bone in vivo [1]. The multilineage potential of MSCs plays an important role in wound healing and tissue regeneration through differentiation and the release of important growth factors and cytokines [2]. Bone marrow-derived mesenchymal stem cells (BM-derived MSCs) have therapeutic potential in a wide range of diseases [3,4]. Previous studies suggest that autoimmune diseases may be described as a stem cell disorder. For example, Perez-Simon et al. [5] reported that the BMMSCs from chronic primary immune thrombocytopenia (ITP) patients showed an impaired proliferative capacity compared with that from normal controls. MSCs derived bone marrow in systemic lupus erythematosus (SLE) showed evidence of growth retardation in vitro [6].
1,25 (OH)2VD3 has been found to induce a multiple-step differentiation of promyelocytes into mature osteoclasts [7], to suppress parathyroid hormone expression and parathyroid cell growth [8], and to inhibit the growth and stimulate differentiation of keratinocytes [9]. EB1089 is an analogue of VD3 where the side chain has been altered by the addition of two double bonds [10]. This analog has been shown to be 50-200 times more potent than VD3 in anti-proliferative and differentiating activities on cancer cells [11,12].
However, to our knowledge, no studies showed the effects of EB1089 on the defective bone marrow-derived mesenchymal stem cells. So in this paper, we studied the biological character changes of BM-derived MSCs from SLE patients after EB1089 treatment. The objective was to explore the role EB1089 in repairing defective BM-derived MSCs.
Materials and methods
Patients
Bone marrow samples were obtained from 19 patients with SLE. Written informed consent was obtained from all the participants. This study was approved by the ethics committee of China Medical University.
Isolation of MSCs from bone marrow and cell culture
Bone marrow mononuclear cells from patients were isolated by Ficoll gradient and cultured at an initial density of 5 × 104 cells/cm2 in Dulbecco’s Modified Eagle Medium: Nutrient Mixture F-12 (DMEM/F-12; Gibco, Carlsbad, CA, USA), supplemented with 10% fetal calf serum (Gibco), 2 mM L-glutamine, 100 U/ml penicillin/streptomycin, 10 ng/ml epidermal growth factor (EGF; PeproTech, Rocky Hill, NJ, USA), 2 ng/ml basic-fibroblast growth factor (bFGF; PeproTech), 1 × insulin-transferrin-selenium (ITS; Gibco). The cultures were maintained at 37°C in a 5% CO2 incubator, and the medium was changed after 48 hours and then every three days.
Phenotype assay
Cells from the patients with SLE were washed twice using PBS, stained for 30 min at 4°C using fluorchrome labeled antibodies against CD3, CD11b, CD14, CD19, CD31, CD34, CD105, CD106, CD133, CD25, CD44, CD45, CD73, CD80, CD86, CD90, Flk-1, c-Kit, Sca-1, MHC class I and MHC class II or with fluorochrome-matched control antibodies (Becton Dickinson, San Diego, CA, USA).
Chemicals
EB1089 (No. 3993/1) was purchased from R&D systems China (Shanghai, China) and supplied as a solution diluted in isopropanol at a concentration of 4 × 103 μM. As the methods of Wang et al. [13], dilutions were performed in absolute ethanol to obtain stock solutions of 100 μM. The aliquots of stock solutions were stored at -20°C and protected from light.
Cellular proliferation MTT assay
Cellular proliferation was assessed using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Cells (2 × 103 per well) were plated in 96-well microtiter plates and allowed to adhere. After 24 h, cells were treated with various concentrations of EB1089 (e.g. 0, 25, 50, 100, 200 nM for each). After 48 h, MTT was added to each well at a final concentration of 500 μg/ml. The mixture was further incubated for 1 hour at 37°C, and the liquid in the wells was removed. Four hours later, cells were lysed with dimethyl sulfoxide (DMSO) and absorbance rates were measured at 550-560 nm using a microplate reader (Bio-Rad, Hercules, CA, USA).
In vitro mineralization assay
Cells were seeded in 6-well plates in triplicate at the density of 3 × 103/cm2. Alizarin Red S staining, which detects calcium deposition, was used as an indicator of mineralization. The cells were rinsed in PBS, and fixed in 70% ice-cold ethanol prior to staining with 40 mM Alizarin Red S (pH = 4.2, Sigma-Aldrich, Carlsbad, CA, USA) for 10 min at room temperature. Calcium content was quantified by measuring the amount of Alizarin Red S staining, which was bound to the mineralizing nodules. Alkaline phosphatase (ALP) staining was performed as previously described [14].
Western blot
Cells were washed once with phosphate-buffered saline, lysed for 30 min in lysis buffer (50 mM Tris-HCl, pH = 7.5, 150 mM NaCl, 1% Nonidet P-40) containing protease inhibitors (Cocktail; Roche, Basel, Switzerland) and phosphatase inhibitors (1 mM NaF and 1 mM Na3VO4), and centrifuged at 15,000 × g at 4°C for 15 min. Proteins were resolved by 10% SDS-PAGE, transferred to a nitrocellulose membrane, and detected using the following antibodies: anti-Smad 1 (Sigma), anti-Smad 5, anti-Smad 8, anti-phospho-Smad 1/5/8 (pSer463/Ser465). Immunostaining was detected using an enhanced chemiluminescence (ECL) system (Amersham Biosciences; Westborough, MA, USA).
Statistical analysis
Values are presented as mean and standard deviation (SD) for these experiments (mean ± SD). Statistical significance was calculated with a Mann-Whitney-Wilcoxon statistical test (GraphPad 5.0). Statistical significance was set to a P-value less than 0.05. Each experiment was repeated three times.
Results
Bone marrow-derived MSC populations (BMMSCs)
Plastic adherent bone marrow stromal cells were isolated from pooled bone marrow from the patients with SLE, propagated for 1 week, hematopoietic cell depleted, and expanded further in vitro for 2 weeks. Flow cytometry analysis indicated that the majority of cells expressed the surface markers CD44, CD73, CD90, CD105, and MHC class I and didn’t express CD45, CD11b, CD14, CD34, MHC class II and CD31 (Figure 1).
Figure 1.
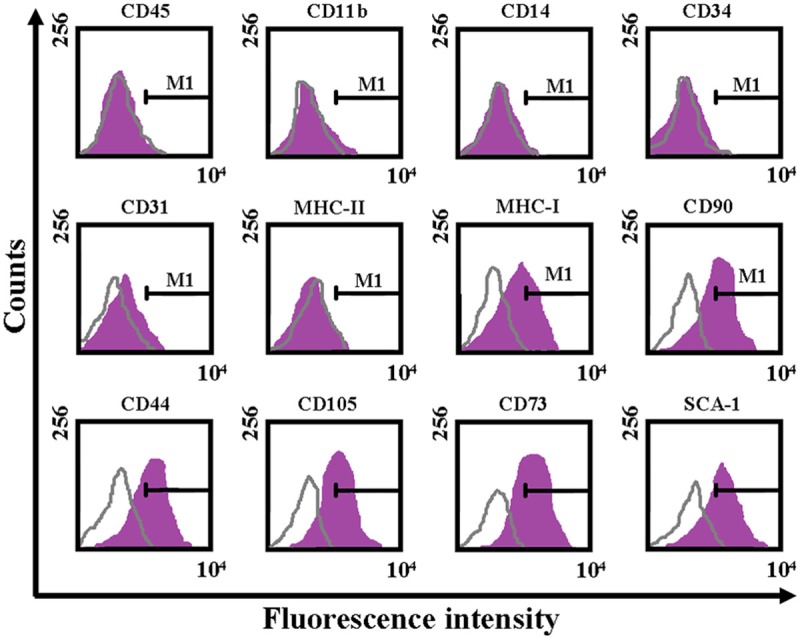
Immunophenotypic characterization of BMMSCs by flow cytometry (fluorescence-activated cell sorting).
The proliferation of BMMSCs was regulated by EB1089
Compared to a control group with no EB1089, we found that increasing EB1089 in fixed increments (25-50-100-200 nM) significantly increased the proliferation rate of BMMSCs (P < 0.05, Figure 2A). ALP activities rates increased from 1.5 ± 0.35 times to 4.4 ± 0.27 times in BMMSCs with EB1089 treatment respect to the control ones (P < 0.05, Figure 2B). After osteogenic induction of BMMSCs with EB1089 treatment for 3 weeks, mineralization nodules were observed with Alizarin Red S staining (P < 0.05, Figure 2C).
Figure 2.
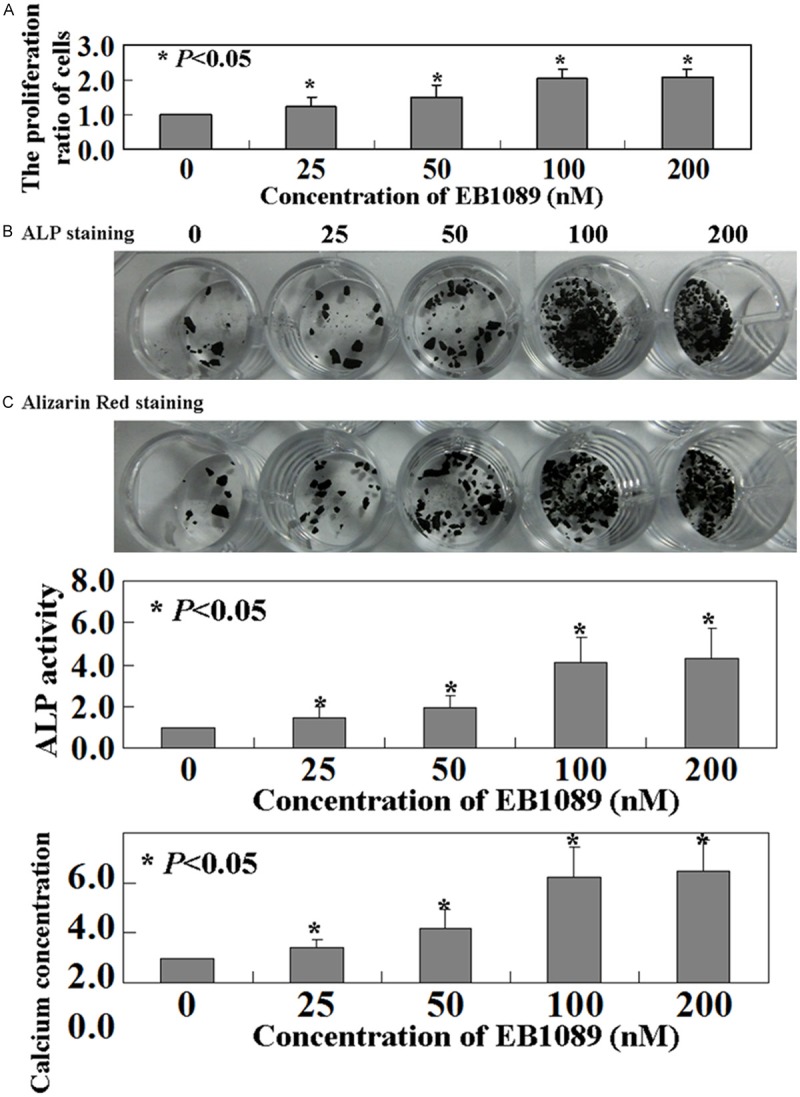
Proliferation of BMMSCs was regulated by EB1089. A. Cell proliferation was assessed by MTT assay. B. ALP activity and C. mineralization were measured by ALP staining and Alizarin Red staining, respectively.
EB1089 improved BMMSCs viability via the Smad 1/5/8 pathway
To determine the effect of EB1089 on Smad 1/5/8 signaling, we performed Western blot analysis on BMMSCs. As shown by Western blot (P < 0.05, Figure 3), the phosphorylation of Smad1/5/8 was activated by EB1089 treatment in BMMSCs, while the total levels of Smad 1/5/8 showed no changes (P > 0.05, Figure 3).
Figure 3.
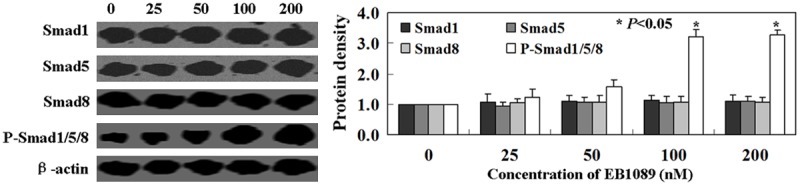
The mechanism of EB1089-induced proliferation in BMMSCs. Western blot analysis was performed using Smad 1, Smad 5, Smad 8, and p-Smad 1/5/8. β-actin was used as an internal control.
Discussion
SLE involves multiple factors, which result in the breakdown of self-tolerance and development of autoimmunity with organ damage [15]. Sun et al. [6] found that bone marrow derived MSCs in patient with SLE are defective and may thus play an important role in the disease. More and more evidence showed the inhibitory effects of EB1089 on many cancer cells, such as gastric cancer [13], hepatocellular cancer [16], and prostate cancer [17]. van den Bemd et al. [18] found that both 1,25-(OH)2VD3 and the analogs stimulated the growth of rat osteoblast-like cells (ROS 17/2.8). In this study, we isolated BMMSCs from the patients with SLE and found that Vitamin D analog EB1089 could induce BMMSCs proliferation and mineralization deposition.
The Smad signaling pathway is critical for mediating TGF-β superfamily signals from the cell surface to the nucleus [19]. P-Smad1/5/8 proteins translocate to the nucleus, bind to target genes, and regulate the transcription [20]. The level of Smad 1/5/8 activity was quantified in this study to explore the mechanism through which EB1089 induced-proliferation in BMMSCs. Our results showed that the expression of p-Smad 1/5/8 was promoted in BMMSCs with EB1089 treatment.
In conclusion, our results support the notion that EB1089 promoted proliferation and osteogenic differentiation of BMMSCs by Smad1/5/8 signaling pathway.
Acknowledgements
We would like to acknowledge the excellent technical support by Miss Yu Miao (China Medical University).
Disclosure of conflict of interest
None.
References
- 1.Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6:230–247. [PubMed] [Google Scholar]
- 2.Gharaibeh B, Lavasani M, Cummins JH, Huard J. Terminal differentiation is not a major determinant for the success of stem cell therapy-cross-talk between muscle-derived stem cells and host cells. Stem Cell Res Ther. 2011;2:31. doi: 10.1186/scrt72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Minguell J, Erices A. Mesenchymal stem cells and the treatment of cardiac disease. Exp Biol Med. 2006;231:39–49. doi: 10.1177/153537020623100105. [DOI] [PubMed] [Google Scholar]
- 4.Mctaggart S, Atkinson K. Mesenchymal stem cells: immunobiology and therapeutic potential in kidney disease. Nephrology. 2007;12:44–52. doi: 10.1111/j.1440-1797.2006.00753.x. [DOI] [PubMed] [Google Scholar]
- 5.Pérez-Simón JA, Tabera S, Sarasquete ME, Díez-Campelo M, Canchado J, Sánchez-Abarca LI, Blanco B, Alberca I, Herrero-Sánchez C, Cañizo C, San Miguel JF. Mesenchymal stem cells are functionally abnormal in patients with immune thrombocytopenic purpura. Cytotherapy. 2009;11:698–705. doi: 10.3109/14653240903051558. [DOI] [PubMed] [Google Scholar]
- 6.Sun LY, Zhang HY, Feng XB, Hou YY, Lu LW, Fan LM. Abnormality of bone marrow-derived mesenchymal stem cells in patients withsystemic lupus erythematosus. Lupus. 2007;16:121–128. doi: 10.1177/0961203306075793. [DOI] [PubMed] [Google Scholar]
- 7.Abe E, Miyaura C, Sakagami H, Takeda M, Konno K, Yamazaki T, Yoshiki S, Suda T. Differentiation of mouse myeloid leukemia cells induced by 1 alpha, 25-dihydroxyvitamin D3. Proc Natl Acad Sci U S A. 1981;78:4990–4994. doi: 10.1073/pnas.78.8.4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silver J, Russell J, Sherwood LM. Regulation by vitamin D metabolites of messenger ribonucleic acid for preproparathyroid hormone in isolated bovine parathyroid cells. Proc Natl Acad Sci U S A. 1985;82:4270–4273. doi: 10.1073/pnas.82.12.4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hosomi J, Hosoi J, Abe E, Suda T, Kuroki T. Regulation of terminal differentiation of cultured mouse epidermal cells by 1 alpha, 25-dihydroxyvitamin D3. Endocrinology. 1983;113:1950–1957. doi: 10.1210/endo-113-6-1950. [DOI] [PubMed] [Google Scholar]
- 10.Hansen CM, Hamberg KJ, Binderup E, Binderup L. Seocalcitol (EB 1089): A vitamin D analogue of anticancer potential. Background, design, synthesis, preclinical and clinical evaluation. Curr Pharm Design. 2000;6:803–808. doi: 10.2174/1381612003400371. [DOI] [PubMed] [Google Scholar]
- 11.Mathiasen IS, Lademann U, Jaattela M. Apoptosis induced by vitamin D compounds in breast cancer cells is inhibited by Bcl-2 but does not involve known caspases or p53. Cancer Res. 1999;59:4848–4856. [PubMed] [Google Scholar]
- 12.Hansen CM, Maenpaa PH. EB, a novel vitamin D analog with strong antiproliferative and differentiation-inducing effects on target cells. Biochem Pharmacol. 1997;54:1173–1179. doi: 10.1016/s0006-2952(97)00181-0. [DOI] [PubMed] [Google Scholar]
- 13.Wang W, Zhao CH, Zhang N, Wang J. Vitamin D analog EB1089 induces apoptosis in a subpopulation of SGC-7901 gastric cancer cells through a mitochondrial-dependent apoptotic pathway. Nutr Cancer. 2013;65:1067–1075. doi: 10.1080/01635581.2013.811273. [DOI] [PubMed] [Google Scholar]
- 14.Qi L, Zhang Y. The microRNA 132 regulates fluid shear stress-induced differentiation in periodontal ligament cells through mTOR signaling pathway. Cell Physiol Biochem. 2014;33:433–445. doi: 10.1159/000358624. [DOI] [PubMed] [Google Scholar]
- 15.Kamalaraj N, Tsai T, Massasso D. Abdominal pain in systemic lupus erythematosus: lupus enteritis, mesenteric thrombosis, or median arcuate ligament syndrome? Scand J Rheumatol. 2014;7:1–2. doi: 10.3109/03009742.2014.965741. [DOI] [PubMed] [Google Scholar]
- 16.Zhang J, Zhang H, Zhang X, Yu Z. Synergistic effect of retinoic acid and vitamin D analog EB1089-induced apoptosis of hepatocellular cancer cells. Cytotechnology. 2013;65:457–465. doi: 10.1007/s10616-012-9500-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhatia V, Saini MK, Shen X, Bi LX, Qiu S, Weigel NL, Falzon M. EB1089 inhibits the parathyroid hormone-related protein-enhanced bone metastasis and xenograft growth of human prostate cancer cells. Mol Cancer Ther. 2009;8:1787–1798. doi: 10.1158/1535-7163.MCT-09-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van den Bemd GJ, Pols HA, Birkenhäger JC, Kleinekoort WM, van Leeuwen JP. Differential effects of 1, 25-dihydroxyvitamin D3-analogs on osteoblast-like cells and on in vitro bone resorption. J Steroid Biochem Mol Biol. 1995;55:337–346. doi: 10.1016/0960-0760(95)00218-9. [DOI] [PubMed] [Google Scholar]
- 19.Tang MR, Wang YX, Guo S, Han SY, Wang D. CSMD1 exhibits antitumor activity in A375 melanoma cells through activation of the Smad pathway. Apoptosis. 2012;17:9279–37. doi: 10.1007/s10495-012-0727-0. [DOI] [PubMed] [Google Scholar]
- 20.Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004;22:233–241. doi: 10.1080/08977190412331279890. [DOI] [PubMed] [Google Scholar]


