Abstract
The Angiotensin-converting enzyme (ACE) I/D polymorphism has been indicated to be correlated with peripheral neuropathy (PN) susceptibility, but study results are still debatable. Thus, a meta-analysis was conducted. Databases including PubMed, Embase and CNKI were searched. Data were extracted and pooled odds ratios (OR) with 95% confidence intervals (CI) were calculated. Eight studies with 1430 cases and 1873 controls were included in this meta-analysis. The association between ACE I/D polymorphism and PN risk was significant (OR = 1.25; 95% CI 1.05-1.48; P = 0.01). When stratified by ethnicity, the significantly increased PN risk was observed in Caucasians (OR = 1.24; 95% CI 1.05-1.47; P = 0.01). In conclusion, this meta-analysis suggested that ACE I/D polymorphism was a risk factor for PN.
Keywords: Angiotensin-converting enzyme, genetics, peripheral neuropathy, meta-analysis
Introduction
Diabetes is becoming a global public health threat, largely due to an increase in type 2 diabetes. The prevalence among adults is expected to rise from 371 million in 2012 to 552 million by the year 2030 [1]. Diabetes is a heterogeneous disorder that is manifested in the form of hyperglycemia and glucose intolerance due to relative insulin deficiency, impaired effectiveness of insulin action, or both. Approximately 50% of patients with diabetes develop clinically detectable peripheral neuropathy (PN) [2].
Angiotensin-converting enzyme (ACE) is an important component of the renin-angiotensin system (RAS) that converts angiotensin (Ang) I to Ang II [3]. It has been recommended that high levels of Ang II may play a key role in glucose and insulin regulation, and may increase the risk of diabetes [4]. It is also suggested that the tissue levels of Ang II increase with hyperglycemia, and the impacts of Ang II on endothelial damage and oxidative stressmay affect the development of PN [5]. Serum and tissue ACE activity are influenced by the presence of an insertion (I) or deletion (D) of a 287-base pair (bp) fragment in intron 16 of the ACE gene resulting in a common variant, with the D allele being associated with higher ACE activity [6,7]. A few papers investigated the association between this polymorphism and PN risk. However, the results remained inconclusive [8-15]. Meta-analysis is a useful method for investigating associations between genetic factors and diseases, because a quantitative approach is used to combine the results from different studies on the same topic, thereby providing more reliable conclusions. Thus, we performed a meta-analysis to clarify the association of ACE I/D polymorphism with PN risk. To our knowledge, this is the first meta-analysis of the association between ACE I/D polymorphism and the risk of PN.
Materials and methods
Publication search
In our meta-analysis, we searched the articles using the search terms “Angiotensin-converting enzyme”, “ACE”, “type 2 diabetes” and “peripheral neuropathy” in the PubMed, Embase and CNKI databases, and the last search updated on July 2014. Additional studies were identified by a hand search of references of original studies or review articles. No publication date or language restriction was imposed.
Study selection
The following inclusion criteria were used: (1) the study should have evaluated the association between the ACE I/D polymorphism and the risk of PN; (2) the study should have had a case-control or cohort design; (3) sufficient data should have been provided in order to calculate odds ratios (OR) and 95% confidence intervals (CI). The following exclusion criteria were used: (1) irrelevant PN, ACE, or ACE I/D polymorphism and PN risk; (2) abstract or review; (3) genotype frequencies were not reported; (4) non-clinical study; (5) studies were repeated or publications overlapped.
Data extraction
Two investigators independently extracted data and reached consensus on the following characteristics of the selected studies: the first author’s name, year of publication, ethnicity of the study population, age, gender, number of cases and controls with genotype numbers.
Statistical analysis
OR and 95% CI we reemployed to evaluate the strength of the association between ACE I/D polymorphism and PN risk. ORs were calculated for the genotypes: DD vs. II (OR1), ID vs. II (OR2), and DD vs. ID (OR3) for the I/D polymorphism. These pairwise differences were used to indicate the most appropriate genetic model as follows: if OR1 = OR3 ≠ 1 and OR2 = 1, then a recessive model was suggested; if OR1 = OR2 ≠ 1 and OR3 = 1, then a dominant model was suggested; if OR2 = 1/OR3 ≠ 1 and OR1 = 1, then a complete overdominant model was suggested; if OR1>OR2>1 and OR1>OR3>1 (or OR1<OR2<1 and OR1<OR3<1), then a codominant model was suggested [16]. Once the best genetic model was identified, this model was used to collapse the three genotypes into two groups (except in the case of a codominant model) and to pool the results again.
The Q statistic and the I2 statistic were used to assess the degree of heterogeneity among the studies included in the meta-analysis. The random-effects model was used to estimate the pooled OR (the DerSimonian and Laird method). Subgroup analyses were carried out by ethnicity. We did cumulative meta-analysis by undertaking sequential random effects pooling, starting with the earliest studies. Each successive meta-analysis then summarized all the trials in the preceding years. Results were presented as a series of mini meta-analyses, which were ordered chronologically in a forest plot to show the consequence of adding studies on the effect size. The potential publication bias was tested using Egger’s test [17].
All statistical tests were performed using STATA 11.0 software (Stata Corporation, College Station, TX, USA). A P value < 0.05 was considered statistically significant.
Results
Eligible studies
A total of 8 case-control studies (Figure 1) with 1430 cases and 1873 controls on the association between ACE I/D polymorphism and PN risk were included for this meta-analysis [8-15]. There was1 study of Asian population and 7 studies of Caucasian population. The characteristics of each study and the genotype in each study are presented in Table 1.
Figure 1.
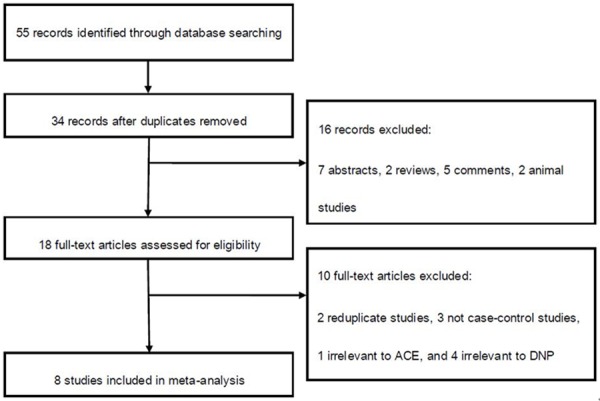
Flow of study identification, inclusion, and exclusion.
Table 1.
Characteristics of the studies
| First author/Year | Ethnicity | Age | Gender | Case | Control | Case genotype | Control genotype | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| DD | ID | II | DD | ID | II | ||||||
| Ito/2002 | Asian | 66.0 | Mixed | 63 | 21 | 10 | 27 | 26 | 1 | 6 | 14 |
| Degirmenci/2005 | Caucasian | 57.6 | Mixed | 65 | 208 | 21 | 38 | 6 | 49 | 113 | 46 |
| Costacou/2006 | Caucasian | 49.5 | Mixed | 114 | 256 | NA | NA | NA | NA | NA | NA |
| Stephens/2006 | Caucasian | 69.9 | Female | 173 | 399 | 61 | 87 | 25 | 125 | 199 | 78 |
| Jurado/2012 | Caucasian | 69.8 | Mixed | 82 | 201 | NA | NA | NA | NA | NA | NA |
| Mansoor/2012 | Caucasian | NA | NA | 496 | 276 | 56 | 161 | 59 | 105 | 230 | 161 |
| Inanir/2013 | Caucasian | 57.2 | Mixed | 235 | 281 | 48 | 49 | 24 | 37 | 42 | 21 |
| Settin/2014 | Caucasian | 55.5 | Mixed | 202 | 231 | NA | NA | NA | NA | NA | NA |
NA, not available.
Meta-analysis
The estimatedOR1, OR2 and OR3 were 1.57 (P = 0.005), 1.68 (P = 0.0002), and 0.99 (P = 0.97), respectively (Table 2). These estimates suggested a dominant genetic model, and therefore DD and ID were compared with II. The pooled OR in this analysis was 1.25 (95% CI 1.05-1.48; P = 0.01) (Figure 2). This result suggested that the DD and ID genotypes were significantly associated with PN risk. In the subgroup analysis by ethnicity, a significant association was found among Caucasians (OR = 1.24; 95% CI 1.05-1.47; P = 0.01).With regard to the cumulative meta-analysis, the evidence was observed to support a significant association of the ACE I/D polymorphism with the susceptibility to PN (Figure 3). Egger’s test showed no evidence of publication bias (P = 0.132).
Table 2.
Determination of the genetic effect of ACE I/D polymorphism on diabetic peripheral neuropathy and subgroup analysis
| Comparison | Study | Sample size | No. of studies | Test of association | Model | Heterogeneity | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||||
| case | control | OR (95% CI) | Z | P Value | χ2 | P Value | I2 (%) | ||||
| DD vs. II | Overall | 336 | 637 | 5 | 1.57 (1.15-2.14) | 2.83 | 0.005 | R | 4.29 | 0.37 | 7 |
| ID vs. II | Overall | 502 | 910 | 5 | 1.68 (1.28-2.19) | 3.78 | 0.0002 | R | 4.22 | 0.38 | 5 |
| DD vs. ID | Overall | 558 | 907 | 5 | 0.99 (0.79-1.25) | 0.04 | 0.97 | R | 3.42 | 0.49 | 0 |
| DD+ID vs. II | Overall | 1430 | 1873 | 8 | 1.25 (1.05-1.48) | 2.58 | 0.01 | R | 5.58 | 0.59 | 0 |
| DD+ID vs. II | Caucasian | 1367 | 1852 | 7 | 1.24 (1.05-1.47) | 2.49 | 0.01 | R | 4.53 | 0.61 | 0 |
vs., versus; R, random-effects model.
Figure 2.
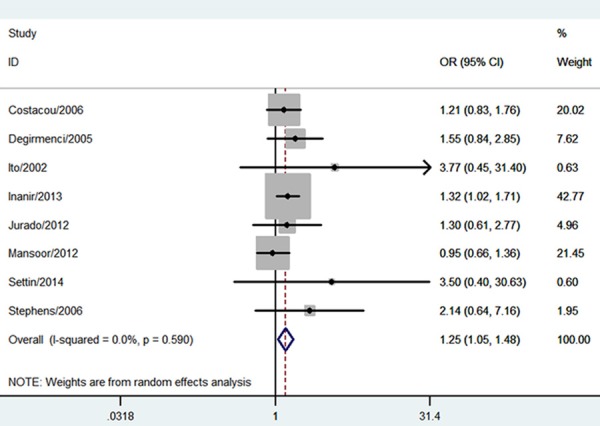
Meta-analysis for the association between the ACE I/D polymorphisma nd DPN risk.
Figure 3.
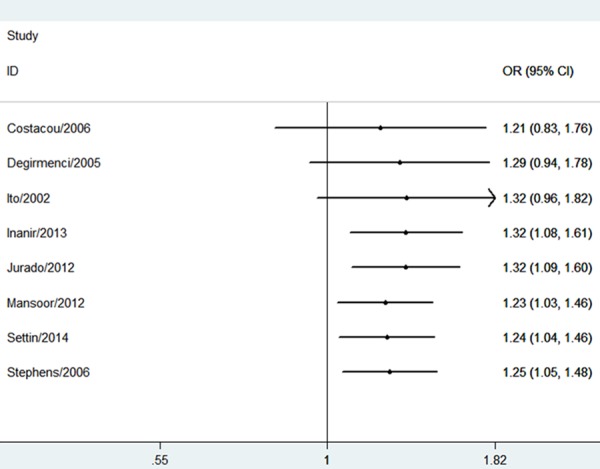
Cumulative meta-analysis for association between the ACE I/D polymorphism and DPN risk.
Discussion
This present meta-analysis investigated the relationship between ACE I/D polymorphism and risk of PN. Eight case-control studies with a total of 3303 subjects were eligible. At the overall analysis, the ACE I/D polymorphism was significantly associated with PN risk. In the subgroup analysis by ethnicity, we noted that Caucasians carrying the DD and ID genotypes had an increased PN risk. Moreover, to investigate the stability of the result, we performed cumulative meta-analysis. The cumulative meta-analysis showed a trend of significant association between this polymorphism and the risk of PN as data accumulated each year. This procedure proved that our result was robust. Thus, results from this meta-analysis suggested that ACE I/D polymorphism was associated with PN risk. Because PN is a major cause of morbidity related to foot ulceration and amputation, it is important to recognize diabetic patients with DD and ID genotypes, who are prone to develop PN.
Previous reports suggest that inhibition of the tissue RAS improves microvascular complications, such as diabetic nephropathy [18]. However, little is known regarding the therapeutic potential of ACE inhibitors and/or Ang II receptor antagonists in the treatment of diabetic neuropathy. The ACE DD genotype is associated with higher serum ACE levels and the findings of the present study suggest a protective role for the II genotype against the development of PN; thus, ACE inhibitor therapy might be a potential treatment in protecting against the development of PN. This important issue should be investigated in the future studies.
Some possible limitations should be acknowledged. First, only published studies that were included in the selected electronic databases were identified; it is possible that some relevant published or unpublished studies may have been missed. Second, the effect of gene-gene and gene-environment interactions was not addressed in this meta-analysis, because of limited available data. Third, our meta-analysis was based on unadjusted OR estimates because not all published studies presented adjusted ORs.
Conclusion
This meta-analysis suggests that ACE I/D polymorphism may be associated with PN development. Further studies can assess the possible gene-environmental and gene-gene interactions in the association between this polymorphism and PN risk.
Disclosure of conflict of interest
None.
References
- 1.Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94:311–21. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 2.Thomas PK. Diabetic peripheral neuropathies: their cost to patient and society and the value of knowledge of risk factors for development of interventions. Eur Neurol. 1999;41(Suppl 1):35–43. doi: 10.1159/000052078. [DOI] [PubMed] [Google Scholar]
- 3.Tiret L, Blanc H, Ruidavets JB, Arveiler D, Luc G, Jeunemaitre X, Tichet J, Mallet C, Poirier O, Plouin PF, Cambien F. Gene polymorphisms of the renin-angiotensin system in relation to hypertension and parental history of myocardial infarction and stroke: the PEGASE study. Projet d’Etude des Gènes de l’Hypertension Artérielle Sévère à modérée Essentielle. J Hypertens. 1998;16:37–44. doi: 10.1097/00004872-199816010-00007. [DOI] [PubMed] [Google Scholar]
- 4.Zhou D, Ruiter R, Zhang J, Zhou M, Liu H, Liu W, Wang S. Angiotensin-converting enzyme I/D polymorphism is not associated with type 2 diabetes in a Chinese population. J Renin Angiotensin Aldosterone Syst. 2012;13:372–8. doi: 10.1177/1470320311435535. [DOI] [PubMed] [Google Scholar]
- 5.Nickenig G. Central role of the AT1-receptor in atherosclerosis. J Hum Hypertens. 2002;16:S26–S33. doi: 10.1038/sj.jhh.1001436. [DOI] [PubMed] [Google Scholar]
- 6.Hubert C, Houot AM, Corvol P, Soubrier F. Structure of the angiotensin I-converting enzyme gene. Two alternate promoters correspond to evolutionary steps of a duplicated gene. J Biol Chem. 1991;266:15377–83. [PubMed] [Google Scholar]
- 7.Rigat B, Hubert C, Alhenc-Gelas F, Cambien F, Corvol P, Soubrier F. An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Invest. 1990;86:1343–6. doi: 10.1172/JCI114844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ito H, Tsukui S, Kanda T, Utsugi T, Ohno T, Kurabayashi M. Angiotensin-converting enzyme insertion/deletion polymorphism and polyneuropathy in type 2 diabetes without macroalbuminuria. J Int Med Res. 2002;30:476–82. doi: 10.1177/147323000203000502. [DOI] [PubMed] [Google Scholar]
- 9.Degirmenci I, Kebapci N, Basaran A, Efe B, Gunes HV, Akalin A, Kurt H, Urhan M, Demirustu C. Frequency of angiotensin-converting enzyme gene polymorphism in Turkish type 2 diabetic patients. Int J Clin Pract. 2005;59:1137–42. doi: 10.1111/j.1368-5031.2005.00586.x. [DOI] [PubMed] [Google Scholar]
- 10.Costacou T, Chang Y, Ferrell RE, Orchard TJ. Identifying genetic susceptibilities to diabetes-related complications among individuals at low risk of complications: An application of tree-structured survival analysis. Am J Epidemiol. 2006;164:862–72. doi: 10.1093/aje/kwj287. [DOI] [PubMed] [Google Scholar]
- 11.Stephens JW, Dhamrait SS, Acharya J, Humphries SE, Hurel SJ. A common variant in the ACE gene is associated with peripheral neuropathy in women with type 2 diabetes mellitus. J Diabetes Complications. 2006;20:317–21. doi: 10.1016/j.jdiacomp.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Jurado J, Ybarra J, Romeo JH, Garcia M, Zabaleta-Del-Olmo E. Angiotensin-converting enzyme gene single polymorphism as a genetic biomarker of diabetic peripheral neuropathy: longitudinal prospective study. J Diabetes Complications. 2012;26:77–82. doi: 10.1016/j.jdiacomp.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 13.Mansoor Q, Javaid A, Bilal N, Ismail M. Angiotensin-converting enzyme (ACE) gene II genotype protects against the development of diabetic peripheral neuropathy in type 2 diabetes mellitus. J Diabetes. 2012;4:257–61. doi: 10.1111/j.1753-0407.2012.00205.x. [DOI] [PubMed] [Google Scholar]
- 14.Inanir A, Basol N, Karakus N, Yigit S. The importance of association between angiotensin-converting enzyme (ACE) Gene I/D polymorphism and diabetic peripheral neuropathy. Gene. 2013;530:253–6. doi: 10.1016/j.gene.2013.07.051. [DOI] [PubMed] [Google Scholar]
- 15.Settin A, El-Baz R, Ismaeel A, Tolba W, Allah WA. Association of ACE and MTHFR genetic polymorphisms with type 2 diabetes mellitus: Susceptibility and complications. J Renin Angiotensin Aldosterone Syst. 2014 doi: 10.1177/1470320313516172. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Thakkinstian A, McElduff P, D’Este C, Duffy D, Attia J. A method for meta-analysis of molecular association studies. Stat Med. 2005;24:1291–1306. doi: 10.1002/sim.2010. [DOI] [PubMed] [Google Scholar]
- 17.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruddy MC. Angiotensin II receptor blockade in diabetic nephropathy. Am J Hypertens. 2002;15:468–71. doi: 10.1016/s0895-7061(01)02320-2. [DOI] [PubMed] [Google Scholar]


