Abstract
To further understand the molecular mechanism of lymphocytes B cells in postmenopausal women osteoporosis. Microarray data (GSE7429) were downloaded from Gene Expression Omnibus, in which B cells were separated from the whole blood of postmenopausal women, including 10 with high bone mineral density (BMD) and 10 with low BMD. Differentially expressed genes (DEGs) between high and low BMD women were identified by Student’s t-test, and P < 0.01 was used as the significant criterion. Functional enrichment analysis was performed for up- and down-regulated DEGs using KEGG, REACTOME, and Gene Ontology (GO) databases. Protein-protein interaction network (PPI) of up- and down-regulated DEGs was respectively constructed by Cytoscape software using the STRING data. Total of 169 up-regulated and 69 down-regulated DEGs were identified. Functional enrichment analysis indicated that the genes (ITPA, ATIC, UMPS, HPRT1, COX10 and COX15) might participate in metabolic pathways, MAP3K10 and MAP3K9 might participate in the activation of JNKK activity, COX10 and COX15 might involve in mitochondrial electron transport, and ATIC, UMPS and HPRT1 might involve in transferase activity. MAPK3, ITPA, ATIC, UMPS and HPRT1 with a higher degree in PPI network were identified. MAPK3, MAP3K10, MAP3K9, COX10, COX15, ATIC, UMPS and HPRT1 might participate in the pathogenesis of osteoporosis.
Keywords: Osteoporosis, function enrichment, pathway enrichment, PPI network
Introduction
Osteoporosis is a common disease for postmenopausal women and characterized by the reduction in the density of bone tissue, and subsequently the increase of bone fragility [1]. The old bone is replaced by new bone tissue through continuous remodeling dynamics, including bone resorption and bone formation [2]. The imbalance between bone resorption and formation may result in the decrease of bone density [3]. The activation of hematopoietic precursors to become osteoclast (OC) contributes to the bone remodeling in OC, osteoblast (OB) and osteocytes [4]. T cells, acted as a regulator of OC and OB formation, participate in the development of rheumatoid arthritis [5], bone metastasis [6] and osteoporosis [7]. T cells may contribute to osteoclastogenesis by the overexpression of receptor activator of nuclear factor-kappa B ligand (RANKL) and tumor necrosis factor-alpha (TNF-alpha) [8]. The expression of TNF increases the numbers of T cells, and induces the bone loss due to estrogen deficiency [9]. Furthermore, Onal et al. suggested that the expression of RANKL can be increased in T cells and B cells and may finally lead to the bone loss [10]. However, few studies showed that B cells participate in the development of osteoporosis. So it is an interesting study to identify the correlation of B cells and osteoporosis.
In 2008, Xiao et al. [11] analyzed the gene expression profile in B cells of postmenopausal osteoporosis patients and identified that down-regulation of estrogen receptor 1 (ESR1) and mitogen activated protein kinase 3 (MAPK3) in B cells regulates secretion of factors, resulting in increased osteoclastogenesis or decreased osteoblastogenesis. To obtain the more genes in B cells correlated with the osteoporosis, differentially expressed genes (DEGs) between high BMD (normal) and low BMD (osteoporosis) were screened by the cut-off point of P < 0.01, but not the Benjamini and Hochberg (BH) adjusted P ≤ 0.05 as the work of Xiao et al. [11]. Furthermore, we also explored the underlying function of DEGs by KEGG, REACTOME pathway and Gene Ontology (GO) enrichment analyses. Protein-protein interaction network (PPI) was also constructed to obtain the crucial genes that are involved in osteoporosis by regulating and influencing the other genes.
Methods
Microarray data
Microarray data (GSE7429) [11] were downloaded from Gene Expression Omnibus (GEO, http://www.ncbi.nlm.nih.gov/geo/). A total of 20 samples were available. B cells were isolated from the whole blood of 20 unrelated postmenopausal women 54 to 60 years of age, including 10 with high BMD and 10 with low BMD. The microarray platform of GSE7429 was GPL96 [HG-U133A] Affymetrix Human Genome U133A Array.
Data preprocessing and identification of DEGs
The data were preprocessed by Affy package [12] in Bioconductor and Affymetrix annotation files from Brain Array Lab. The background correction, quartile data normalization and probe summarization were performed by the Robust Multiarray Average (RMA) algorithm [13] to obtain the gene expression matrix. DEGs were identified by Student’s t-test. P < 0.01 was used as the significant criterion.
Functional enrichment of DEGs
Pathway enrichment analysis in KEGG database based on conserved sub-pathways information [14]. However, REACTOME database based on open-source open-data resource of human pathways and reactions [15]. So we performed the pathway enrichment analysis for up- and down-regulated DEGs using the two databases with the threshold of P < 0.01. In addition, DEGs were also enriched in the biological process (BP), cellular component (CC) and molecular function (MF) categories of GO [16] database, and P < 0.01 was chosen as cut-off criteria.
Construction of PPI network
PPI network of up- and down-regulated DEGs was respectively constructed using the STRING (Search Tool for the Retrieval of Interacting Genes) data [17], and the confidence score > 0.4 was used as cut-off criterion. The PPI network was visualized by Cytoscape software [18].
Results
DEGs analysis
Total of 235 DEGs between the high BMD group and low BMD group were identified, of which 169 DEGs were up-regulated and 69 DEGs were down-regulated.
Pathway enrichment analysis
The two enriched KEGG pathway of up-regulated DEGs were prion diseases pathway (P = 0.006874007) and p53 signaling pathway (P = 0.006924881) (Table 1). MAPK3 involved in the prion diseases pathway. The six enriched KEGG pathway of down-regulated DEGs included metabolic pathways (P = 0.000226044), nucleotide excision repair (P = 0.001525797), drug metabolism-other enzymes (P = 0.002474417), Glycosylphosphatidylinositol (GPI)-anchor biosynthesis (P = 0.007508316), mRNA surveillance pathway (P = 0.009212606) and purine metabolism (P = 0.009837667) (Table 1). The genes (ITPA, ATIC, UMPS, HPRT1, COX10 and COX15) participated in metabolic pathways.
Table 1.
Enriched KEGG pathways for up- and down-regulated DEGs
| DEGs | KEGG pathway | Gene counts | P-value | Gene |
|---|---|---|---|---|
| Up-regulated | Prion diseases | 3 | 0.006874007 | C1QB, C7, MAPK3 |
| p53 signaling pathway | 4 | 0.006924881 | CHEK1, PIDD, TNFRSF10B, TP53I3 | |
| Down-regulated | Metabolic pathways | 15 | 0.000226044 | ATIC, CMAS, COX10, COX15, DPM1, GBE1, HPRT1, ITPA, ODC1, PIGC, PIGK, PIP5K1B, POLE3, SUCLG2, UMPS |
| Nucleotide excision repair | 3 | 0.001525797 | GTF2H1, POLE3, RFC4 | |
| Drug Metabolism-other enzymes | 3 | 0.002474417 | HPRT1, ITPA, UMPS | |
| Glycosylphosphatidylinositol (GPI)-anchor biosynthesis | 2 | 0.007508316 | PIGC, PIGK | |
| mRNA surveillance pathway | 3 | 0.009212606 | CPSF6, CSTF2T, PPP2CA | |
| Purine metabolism | 4 | 0.009837667 | ATIC, HPRT1, ITPA, POLE3 |
KEGG: Kyoto Encyclopedia of Genes and Genomes; DEGs: differentially expressed genes.
The two enriched REACTOME pathway of up-regulated DEGs were complement cascade (P = 0.00757477) and synthesis of PE (P = 0.00772870) (Table 2). The REACTOME pathway enrichment of down-regulated DEGs showed that the genes (ATIC, HPRT1, ITPA and UMPS) involved in metabolism of nucleotides (P = 0.00059424). The genes (COX10 and COX15) participated in the two pathways, including heme biosynthesis (P = 0.00124899) and metabolism of porphyrins (P = 0.00602279) (Table 2).
Table 2.
Enriched REACTOME pathways for up- and down-regulated DEGs
| DEGs | REACTOME pathway | Gene counts | P-value | Adjust p-value | Gene |
|---|---|---|---|---|---|
| Up-regulated | Complement cascade | 3 | 0.00757477 | 1 | C1QB, C7, GZMM |
| Synthesis of PE | 2 | 0.00772870 | 1 | CHKA, PISD | |
| Down-regulated | Post-translational modification | 3 | 0.00025911 | 0.39591249 | DPM1, PIGC, PIGK |
| Metabolism of nucleotides | 4 | 0.00058424 | 0.89271608 | ATIC, HPRT1, ITPA, UMPS | |
| Heme biosynthesis | 2 | 0.00124899 | 1 | COX10, COX15 | |
| Metabolism of porphyrins | 2 | 0.00602279 | 1 | COX10, COX15 |
DEGs: differentially expressed genes.
GO enrichment analysis
GO enrichment analysis of up-regulated DEGs showed that 44, 6 and 32 terms were respectively enriched in BP, CC and MF category, and the top five terms in BP, CC and MF category were listed (Table 3). The top three enriched GO terms in BP category of up-regulated DEGs were the activation of JNKK activity (P = 0.000916844), humoral immune response (P = 0.001738278) and response to stilbenoid (P = 0.001900954) (Table 3). MAP3K10 and MAP3K9 involved in activation of JNKK activity. The top three enriched GO terms in MF category were alcohol dehydrogenase (NADP+) activity (P = 0.000150784), JUN kinase kinase kinase activity (P = 0.000243013) and aldo-keto reductase (NADP) activity (P = 0.000537427) (Table 3). MAP3K10 and MAP3K9 involved in JUN kinase kinase kinase activity.
Table 3.
Top five GO terms were respectively enriched in BP, CC and MF category for up-regulated DEGs
| GO ID | Cate gory | Term | Gene counts | P-value | Genes |
|---|---|---|---|---|---|
| GO: 0007256 | BP | activation of JNKK activity | 2 | 0.000916844 | MAP3K10, MAP3K9 |
| GO: 0006959 | BP | humoral immune response | 6 | 0.001738278 | C1QB, C7, FOXJ1, IFNG, TREM2, ZP3 |
| GO: 0035634 | BP | response to stilbenoid | 2 | 0.001900954 | FGL1, SLC22A7 |
| GO: 0048609 | BP | multicellular organismal reproductive process | 15 | 0.002142066 | DAZ1, DAZ2, DAZ3, DAZ4, EIF2B4 |
| GO: 0046683 | BP | response to organophosphorus | 5 | 0.002641967 | AKR1C1, ALDH3A1, AQP8, DMTN, VGF |
| GO: 0034703 | CC | cation channel complex | 6 | 0.001553268 | CNGB1, KCNA6, KCNG1, KCNJ5, SCN4A, SCNN1B |
| GO: 0005887 | CC | integral to plasma membrane | 21 | 0.003272558 | AGTR2, ABCC1, AQP8, ART3, C7, CNGB1 |
| GO: 0044459 | CC | plasma membrane part | 29 | 0.003560869 | MAPK3, ABCC1, AGTR2, AQP8, ART3 |
| GO: 0031226 | CC | intrinsic to plasma membrane | 21 | 0.005042495 | AGTR2, ABCC1, AQP8, ART3, C7 |
| GO: 0005796 | CC | Golgi lumen | 4 | 0.006999886 | MUC3A, MUC3B, MUC5AC, WNT7A |
| GO: 0008106 | MF | alcohol dehydrogenase (NADP +) activity | 3 | 0.000150784 | AKR1C1, ALDH3A1, DHRS3 |
| GO: 0004706 | MF | JUN kinase kinase kinase activity | 2 | 0.000243013 | MAP3K10, MAP3K9 |
| GO: 0004033 | MF | aldo-keto reductase (NADP) activity | 3 | 0.000537427 | AKR1C1, ALDH3A1, DHRS3 |
| GO: 0022838 | MF | substrate-specific channel activity | 11 | 0.000700403 | AQP8, CNGB1, FXYD7, KCNA6, KCNG1 |
| GO: 0005261 | MF | cation channel activity | 9 | 0.000949791 | CNGB1, KCNA6, KCNG1, KCNJ5, KCNK7 |
GO: Gene Ontology; BP: biological process; CC: cellular component; MF: molecular function; DEGs: differentially expressed genes.
GO enrichment analysis of down-regulated DEGs showed that 67, 8 and 14 terms were respectively enriched in BP, CC and MF category, and the top five terms in BP, CC and MF category were listed (Table 4). The top three enriched GO terms in BP category showed that the genes (COX10 and COX15) involved in the three GO terms, such as mitochondrial electron transport (P = 1.66E-05), heme a biosynthetic process (P = 1.66E-05) and heme a metabolic process (P = 1.66E-05) (Table 4). The enriched GO terms in MP category was transferase activity (P = 0.000211892) involving in ATIC, HPRT1 and UMPS, cytochrome-c oxidase activity (P = 0.004120099) including in COX10 and COX15, and heme-copper terminal oxidase activity (P = 0.004120099) involving in COX10 and COX15 (Table 4).
Table 4.
Top five GO terms were respectively enriched in BP, CC and MF category for down-regulated DEGs
| GO ID | Cate gory | Term | Gene counts | P-value | Genes |
|---|---|---|---|---|---|
| GO: 0006123 | BP | mitochondrial electron transport, cytochrome c to oxygen | 2 | 1.66E-05 | COX10, COX15 |
| GO: 0006784 | BP | heme a biosynthetic process | 2 | 1.66E-05 | COX10, COX15 |
| GO: 0046160 | BP | heme a metabolic process | 2 | 1.66E-05 | COX10, COX15 |
| GO: 0006501 | BP | C-terminal protein lipidation | 3 | 0.000142394 | DPM1, PIGC, PIGK |
| GO: 0018410 | BP | C-terminal protein amino acid modification | 3 | 0.000247707 | DPM1, PIGC, PIGK |
| GO: 0005622 | CC | intracellular | 57 | 0.000964678 | ATIC, CPSF6, HPRT1, ITPA, POLE3, RFC4, UMPS, ADORA2A, ASH2L, ATP5S |
| GO: 0044464 | CC | cell part | 62 | 0.001191597 | ATIC, CPSF6, HPRT1, ITPA, POLE3, RFC4, UMPS, ADORA2A, ASH2L, CCNE1 |
| GO: 0005623 | CC | cell | 62 | 0.001196158 | ATIC, CPSF6, HPRT1, ITPA, POLE3, RFC4, UMPS, ADORA2A, ASH2L, CCNE1 |
| GO: 0005671 | CC | Ada2/Gcn5/Ada3 transcription activator complex | 2 | 0.001560069 | POLE3, MBIP |
| GO: 0044424 | CC | intracellular part | 56 | 0.001932115 | ATIC, CPSF6, HPRT1, ITPA, POLE3, RFC4, UMPS, ADORA2A, ASH2L, CD1A |
| GO: 0016740 | MF | transferase activity | 17 | 0.000211892 | ATIC, HPRT1, POLE3, UMPS, ASH2L, CCNE1, CMAS, COX10, DPM1, EIF2B3 |
| GO: 0001664 | MF | G-protein coupled receptor binding | 5 | 0.000925208 | ADORA2A, CREB3, NPFF, ROR2, YARS |
| GO: 0016757 | MF | transferase activity, transferring glycosyl groups | 5 | 0.003521176 | UMPS, DPM1, GBE1, HPRT1, PIGC |
| GO: 0004129 | MF | cytochrome-c oxidase activity | 2 | 0.004120099 | COX10, COX15 |
| GO: 0015002 | MF | heme-copper terminal oxidase activity | 2 | 0.004120099 | COX10, COX15 |
GO: Gene Ontology; BP: biological process; CC: cellular component; MF: molecular function; DEGs: differentially expressed genes.
PPI network analysis
The genes/proteins with the degree in PPI network of up-regulated DEGs were MAPK3 (degree = 3) and AGTR2 (degree = 3) (Figure 1). The genes/proteins with the degree in PPI network of down-regulated DEGs were ITPA (degree = 4), RFC4 (degree = 3), ATIC (degree = 3), UMPS (degree = 2) and HPRT1 (degree = 2) (Figure 2).
Figure 1.
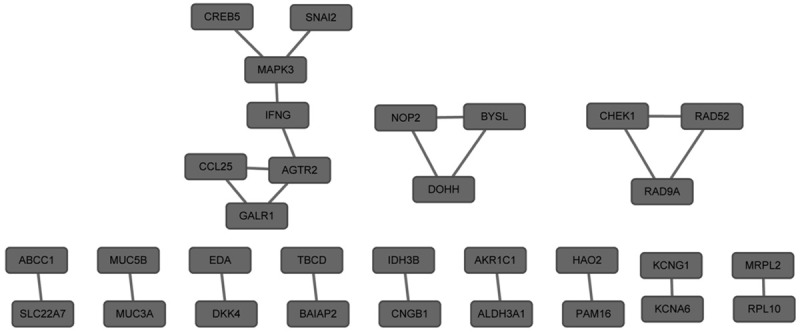
Protein-protein interaction network of up-regulated differentially expressed genes. The nodes represented up-regulated differentially expressed genes.
Figure 2.
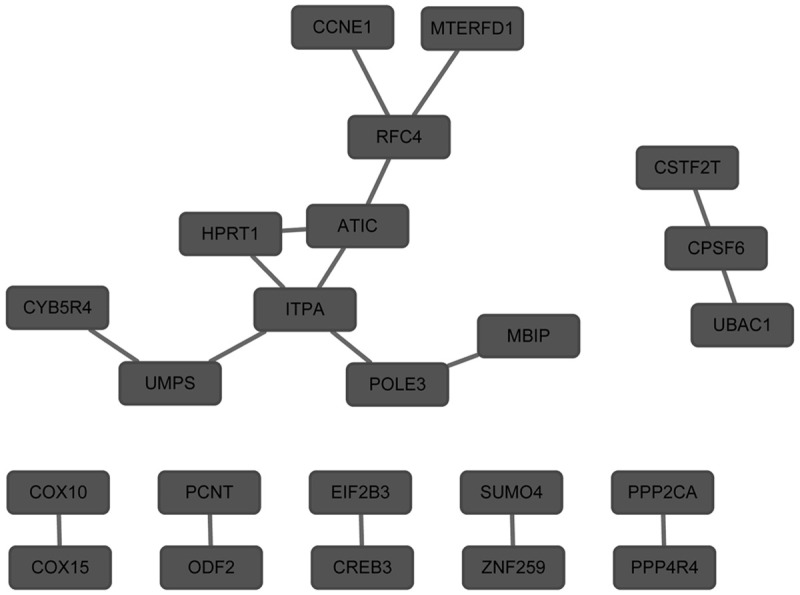
Protein-protein interaction network of down-regulated differentially expressed genes. The nodes represented down-regulated differentially expressed genes.
Discussion
In this study, 235 DEGs between the high BMD group and low BMD group were identified, including 169 up-regulated DEGs and 69 down-regulated DEGs. Functional enrichment analysis showed that MAPK3 involved in the prion diseases pathway, MAP3K10 and MAP3K9 involved in the activation of JNKK activity, COX15 and COX10 involved in mitochondrial electron transport and heme a biosynthetic process, and ATIC, UMPS and HPRT1 involved in transferase activity.
MAPK3 belongs to the Mitogen-Activated Protein Kinase (MAPK) family, which also known as the extracellular signal-regulated kinase (ERK) [19]. According to the work of Park et al., hydrogen peroxide (H2O2)-induced cell apoptosis of osteoblasts may be mediated by the ERK signaling pathway [20]. Osteocyte apoptosis could be inhibited by estrogens via the activation of the ERKs signaling pathway [21]. ERK activity played an important role in the serum-induced osteoblast proliferation, and the up-regulated expression of MKP-1, acted as dual-specificity MAPK phosphatases, could be induced by the glucocorticoid which decreased the numbers of osteoblasts [22]. MAPK3 may participate in the etiology of osteoporosis via the ERK/MAPK signaling pathway [11]. Although our findings were consistent with the previous results that MAPK3 involved in the development of osteoporosis, MAPK3 participated in the prion diseases in this study. Prion diseases and Alzheimer disease (AD) share similar pathogenic mechanisms, including generation of oxidative stress molecules and complement activation [23]. Reactive oxygen species (ROS) involve in the pathogenesis of osteoarthritis which are induced by pro-inflammatory cytokines, such as interleukin-1 (IL-1) and tumour necrosis factor alpha (TNFalpha) [24,25]. The results implied that MAPK3 also involved in the development of osteoporosis via the prion diseases pathway, which was a new identified pathway in this study.
MAP3K10 (Mitogen-Activated Protein Kinase Kinase Kinase 10), MAP3K9 (Mitogen-Activated Protein Kinase Kinase Kinase 9) and MAP3K11 (Mitogen-Activated Protein Kinase Kinase Kinase 11) activate the JNK signaling cascade [26]. In addition, miR-155, targeting MAP3K10 (Mitogen-Activated Protein Kinase Kinase Kinase 10) [27,28], involves in the regulation of MAPK pathway, which included extracellular signal-regulated kinases (ERKs) pathway, c-Jun N-terminal kinase (JNK) pathway, p38 MAPK pathway and ERK5 pathway [29]. miR-155 also regulates the release of IL-6 and TNF-α [30]. The production of cytokines, including IL-1β, IL-6 and TNF-α, are higher in osteoporotic postmenopausal women than in healthy women [31]. Based on these results, we could speculate that MAPK3, MAP3K10 and MAP3K9 participated in the etiology of osteoporosis through the MAPK pathway.
According to the above reports, ROS involves in the development of osteoporosis. The impairment of mitochondrial electron transport chain causes the increase of intracellular ROS, and finally results in the diseases related to mitochondria [32]. Guo et al. report that mitochondrial DNA participated in the pathogenesis of osteoporosis, age-related diseases [33]. According to the work of Petruzzella et al., COX15 and COX10 involved in the formation of mitochondrial respiratory chain [34]. COX15, along with SCO1, involve in COX deficiency, and the expression of COX15 increases heme A products and activates the COX [35]. SCO1 and COX10 are involved in the Cytochrome c oxidase (COX) assembly, and COX defects are determined in mitochondrial disorders [36]. Our results, which COX15 and COX10 involved in mitochondrial electron transport and heme a biosynthetic process, were consistent with the previous reports. So we could speculate that COX15 and COX10 involved in the pathology of osteoarthritis via mitochondrial respiratory chain.
In this study, the genes (ATIC, UMPS and HPRT1) involved in transferase activity. Gamma-glutamyl transferase contributes to the generation of free radical species, and oxidative stress has harmful effects on bone metabolism [37]. Isomura et al. also report that oxidative stress participated in the pathogenesis of osteoporosis by analysis of the correlation of oxidative stress and bone metabolism using bone metabolic markers and cytokines, including serum osteopontin and TGF-β1 [38]. The distinct metabolic changes, including lipid, energy and amino acid metabolism changed, in osteoporotic ovariectomized rats have been determined, and altered metabolites participate in the oxidative defense system [39]. Based on these findings, we could speculate that ATIC, UMPS and HPRT1 might regulate the bone metabolism to involve in the development of osteoporosis via the process of the transferase activity.
Conclusion
In this study, 235 DEGs between the high and low BMD group were identified, of which 169 DEGs were up-regulated and 69 DEGs were down-regulated. MAPK3 and the genes (MAP3K10 and MAP3K9) might involve in the pathogenesis of osteoporosis via the prion diseases pathway (or the MAPK signaling pathway) and the MAPK signaling pathway, respectively. COX10 and COX15 might participate in the pathogenesis of osteoporosis via the mitochondrial electron transport chain. ATIC, UMPS and HPRT1 might involve in the development of osteoporosis via the process of the transferase activity. The results implied that B cells may be participated in the mechanism of postmenopausal women osteoarthritis. However, the results need to be further confirmed by experiments.
Disclosure of conflict of interest
None.
References
- 1.Bonnick S, De Villiers T, Odio A, Palacios S, Chapurlat R, Dasilva C, Scott BB, Le Bailly De Tilleghem C, Leung AT, Gurner D. Effects of odanacatib on BMD and safety in the treatment of osteoporosis in postmenopausal women previously treated with alendronate: a randomized placebo-controlled trial. J Clin Endocrinol Metab. 2013;98:4727–4735. doi: 10.1210/jc.2013-2020. [DOI] [PubMed] [Google Scholar]
- 2.Karsenty G, Oury F. The central regulation of bone mass, the first link between bone remodeling and energy metabolism. J Clin Endocrinol Metab. 2010;95:4795–4801. doi: 10.1210/jc.2010-1030. [DOI] [PubMed] [Google Scholar]
- 3.Recker R, Lappe J, Davies KM, Heaney R. Bone remodeling increases substantially in the years after menopause and remains increased in older osteoporosis patients. J Bone Miner Res. 2004;19:1628–1633. doi: 10.1359/JBMR.040710. [DOI] [PubMed] [Google Scholar]
- 4.Faienza MF, Ventura A, Marzano F, Cavallo L. Postmenopausal osteoporosis: the role of immune system cells. Clinical and Developmental Immunology Clin Dev Immunol. 2013;2013:575936. doi: 10.1155/2013/575936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kotake S, Udagawa N, Hakoda M, Mogi M, Yano K, Tsuda E, Takahashi K, Furuya T, Ishiyama S, Kim KJ. Activated human T cells directly induce osteoclastogenesis from human monocytes: possible role of T cells in bone destruction in rheumatoid arthritis patients. Arthritis Rheum. 2001;44:1003–1012. doi: 10.1002/1529-0131(200105)44:5<1003::AID-ANR179>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 6.Roato I, Grano M, Brunetti G, Colucci S, Mussa A, Bertetto O, Ferracini R. Mechanisms of spontaneous osteoclastogenesis in cancer with bone involvement. FASEB J. 2005;19:228–230. doi: 10.1096/fj.04-1823fje. [DOI] [PubMed] [Google Scholar]
- 7.D’amelio P, Grimaldi A, Di Bella S, Brianza SZ, Cristofaro MA, Tamone C, Giribaldi G, Ulliers D, Pescarmona GP, Isaia G. Estrogen deficiency increases osteoclastogenesis up-regulating T cells activity: a key mechanism in osteoporosis. Bone. 2008;43:92–100. doi: 10.1016/j.bone.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 8.Brunetti G, Colucci S, Pignataro P, Coricciati M, Mori G, Cirulli N, Zallone A, Grassi FR, Grano M. T cells support osteoclastogenesis in an in vitro model derived from human periodontitis patients. J Periodontol. 2005;76:1675–1680. doi: 10.1902/jop.2005.76.10.1675. [DOI] [PubMed] [Google Scholar]
- 9.Roggia C, Gao Y, Cenci S, Weitzmann MN, Toraldo G, Isaia G, Pacifici R. Up-regulation of TNF-producing T cells in the bone marrow: a key mechanism by which estrogen deficiency induces bone loss in vivo. Proc Natl Acad Sci U S A. 2001;98:13960–13965. doi: 10.1073/pnas.251534698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Onal M, Xiong J, Chen X, Thostenson JD, Almeida M, Manolagas SC, O’brien CA. Receptor activator of nuclear factor kappaB ligand (RANKL) protein expression by B lymphocytes contributes to ovariectomy-induced bone loss. J Biol Chem. 2012;287:29851–29860. doi: 10.1074/jbc.M112.377945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiao P, Chen Y, Jiang H, Liu YZ, Pan F, Yang TL, Tang ZH, Larsen JA, Lappe JM, Recker RR. In Vivo Genome-Wide Expression Study on Human Circulating B Cells Suggests a Novel ESR1 and MAPK3 Network for Postmenopausal Osteoporosis. J Bone Miner Res. 2008;23:644–654. doi: 10.1359/JBMR.080105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gautier L, Cope L, Bolstad BM, Irizarry RA. affy-analysis of Affymetrix GeneChip data at the probe level. Bioinformatics. 2004;20:307–315. doi: 10.1093/bioinformatics/btg405. [DOI] [PubMed] [Google Scholar]
- 13.Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 14.Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Croft D, Mundo AF, Haw R, Milacic M, Weiser J, Wu G, Caudy M, Garapati P, Gillespie M, Kamdar MR. The Reactome pathway knowledgebase. Nucleic Acids Res. 2014;42:D472–D477. doi: 10.1093/nar/gkt1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT. Gene Ontology: tool for the unification of biology. Nat Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franceschini A, Szklarczyk D, Frankild S, Kuhn M, Simonovic M, Roth A, Lin J, Minguez P, Bork P, Von Mering C. STRING v9. 1: protein-protein interaction networks, with increased coverage and integration. Nucleic Acids Res. 2013;41:D808–D815. doi: 10.1093/nar/gks1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smoot ME, Ono K, Ruscheinski J, Wang PL, Ideker T. Cytoscape 2.8: new features for data integration and network visualization. Bioinformatics. 2011;27:431–432. doi: 10.1093/bioinformatics/btq675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dineley KT, Westerman M, Bui D, Bell K, Ashe KH, Sweatt JD. β-Amyloid activates the mitogen-activated protein kinase cascade via hippocampal α7 nicotinic acetylcholine receptors: in vitro and in vivo mechanisms related to Alzheimer’s disease. J Neurosci. 2001;21:4125–4133. doi: 10.1523/JNEUROSCI.21-12-04125.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park BG, Yoo CI, Kim HT, Kwon CH, Kim YK. Role of mitogen-activated protein kinases in hydrogen peroxide-induced cell death in osteoblastic cells. Toxicology. 2005;215:115–125. doi: 10.1016/j.tox.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Plotkin LI, Aguirre JI, Kousteni S, Manolagas SC, Bellido T. Bisphosphonates and estrogens inhibit osteocyte apoptosis via distinct molecular mechanisms downstream of extracellular signal-regulated kinase activation. J Biol Chem. 2005;280:7317–7325. doi: 10.1074/jbc.M412817200. [DOI] [PubMed] [Google Scholar]
- 22.Engelbrecht Y, De Wet H, Horsch K, Langeveldt C, Hough F, Hulley P. Glucocorticoids induce rapid up-regulation of mitogen-activated protein kinase phosphatase-1 and dephosphorylation of extracellular signal-regulated kinase and impair proliferation in human and mouse osteoblast cell lines. Endocrinology. 2003;144:412–422. doi: 10.1210/en.2002-220769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Unterberger U, Höftberger R, Gelpi E, Flicker H, Budka H, Voigtländer T. Endoplasmic reticulum stress features are prominent in Alzheimer disease but not in prion diseases in vivo. J Neuropathol Exp Neurol. 2006;65:348–357. doi: 10.1097/01.jnen.0000218445.30535.6f. [DOI] [PubMed] [Google Scholar]
- 24.Wang M-X, Wei A, Yuan J, Trickett A, Knoops B, Murrell GA. Expression and regulation of peroxiredoxin 5 in human osteoarthritis. FEBS Lett. 2002;531:359–362. doi: 10.1016/s0014-5793(02)03511-1. [DOI] [PubMed] [Google Scholar]
- 25.Wu X, Kondragunta V, Kornman K, Wang H, Duff G, Renner J, Jordan J. IL-1 receptor antagonist gene as a predictive biomarker of progression of knee osteoarthritis in a population cohort. Osteoarthritis Cartilage. 2013;21:930–938. doi: 10.1016/j.joca.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katoh M. WNT/PCP signaling pathway and human cancer (review) Oncol Rep. 2005;14:1583. [PubMed] [Google Scholar]
- 27.Zhu J, Chen T, Yang L, Li Z, Wong MM, Zheng X, Pan X, Zhang L, Yan H. Regulation of microRNA-155 in atherosclerotic inflammatory responses by targeting MAP3K10. PLoS One. 2012;7:e46551. doi: 10.1371/journal.pone.0046551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhong J, Kyriakis JM. Dissection of a signaling pathway by which pathogen-associated molecular patterns recruit the JNK and p38 MAPKs and trigger cytokine release. J Biol Chem. 2007;282:24246–24254. doi: 10.1074/jbc.M703422200. [DOI] [PubMed] [Google Scholar]
- 29.Bai XC, Lu D, Bai J, Zheng H, Ke ZY, Li XM, Luo SQ. Oxidative stress inhibits osteoblastic differentiation of bone cells by ERK and NF-κB. Biochem Biophys Res Commun. 2004;314:197–207. doi: 10.1016/j.bbrc.2003.12.073. [DOI] [PubMed] [Google Scholar]
- 30.Ceppi M, Pereira PM, Dunand-Sauthier I, Barras E, Reith W, Santos MA, Pierre P. MicroRNA-155 modulates the interleukin-1 signaling pathway in activated human monocyte-derived dendritic cells. Proc Natl Acad Sci U S A. 2009;106:2735–2740. doi: 10.1073/pnas.0811073106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zheng S, Vrindts Y, Lopez M, De Groote D, Zangerlé PF, Collette J, Franchimont N, Geenen V, Albert A, Reginster JY. Increase in cytokine production (IL-1β, IL-6, TNF-α but not IFN-γ, GM-CSF or LIF) by stimulated whole blood cells in postmenopausal osteoporosis. Maturitas. 1997;26:63–71. doi: 10.1016/s0378-5122(96)01080-8. [DOI] [PubMed] [Google Scholar]
- 32.Indo HP, Davidson M, Yen HC, Suenaga S, Tomita K, Nishii T, Higuchi M, Koga Y, Ozawa T, Majima HJ. Evidence of ROS generation by mitochondria in cells with impaired electron transport chain and mitochondrial DNA damage. Mitochondrion. 2007;7:106–118. doi: 10.1016/j.mito.2006.11.026. [DOI] [PubMed] [Google Scholar]
- 33.Guo Y, Yang TL, Liu YZ, Shen H, Lei SF, Yu N, Chen J, Xu T, Cheng Y, Tian Q. Mitochondria-Wide Association Study of Common Variants in Osteoporosis. Ann Hum Genet. 2011;75:569–574. doi: 10.1111/j.1469-1809.2011.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petruzzella V, Tiranti V, Fernandez P, Ianna P, Carrozzo R, Zeviani M. Identification and characterization of human cDNAs specific to BCS1, PET112, SCO1, COX15, and COX11, five genes involved in the formation and function of the mitochondrial respiratory chain. Genomics. 1998;54:494–504. doi: 10.1006/geno.1998.5580. [DOI] [PubMed] [Google Scholar]
- 35.Antonicka H, Mattman A, Carlson CG, Glerum DM, Hoffbuhr KC, Leary SC, Kennaway NG, Shoubridge EA. Mutations in COX15 Produce a Defect in the Mitochondrial Heme Biosynthetic Pathway, Causing Early-Onset Fatal Hypertrophic Cardiomyopathy. Am J Hum Genet. 2003;72:101–114. doi: 10.1086/345489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valnot I, Von Kleist-Retzow JC, Barrientos A, Gorbatyuk M, Taanman JW, Mehaye B, Rustin P, Tzagoloff A, Munnich A, Rötig A. A mutation in the human heme A: farnesyltransferase gene (COX10) causes cytochrome c oxidase deficiency. Hum Mol Genet. 2000;9:1245–1249. doi: 10.1093/hmg/9.8.1245. [DOI] [PubMed] [Google Scholar]
- 37.Kim BJ, Baek S, Ahn SH, Kim SH, Jo MW, Bae SJ, Kim HK, Park GM, Kim YH, Lee SH. A higher serum gamma-glutamyl transferase level could be associated with an increased risk of incident osteoporotic fractures in Korean men aged 50 years or older. Endocr J. 2014;61:257–263. doi: 10.1507/endocrj.ej13-0463. [DOI] [PubMed] [Google Scholar]
- 38.Isomura H, Fujie K, Shibata K, Inoue N, Iizuka T, Takebe G, Takahashi K, Nishihira J, Izumi H, Sakamoto W. Bone metabolism and oxidative stress in postmenopausal rats with iron overload. Toxicology. 2004;197:92–99. doi: 10.1016/j.tox.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 39.Xue L, Wang Y, Liu L, Zhao L, Han T, Zhang Q, Qin L. A 1HNMR-based metabonomics study of postmenopausal osteoporosis and intervention effects of er-xian decoction in ovariectomized rats. Int J Mol Sci. 2011;12:7635–7651. doi: 10.3390/ijms12117635. [DOI] [PMC free article] [PubMed] [Google Scholar]


