Abstract
Objective: Pulmonary metastasis of hepatocellular carcinoma (HCC) could be defined as advanced HCC and systematic treatment is the main therapeutic modality. However, local therapy of intrahepatic tumor, which is significantly associated with the prognosis of HCC, remains important for advanced HCC. Methods: Twenty-six HCC patients with pulmonary metastasis underwent intrahepatic transcatheter arterial chemoembolization (TACE). We investigated the progression of lung metastastic tumors, overall survival and risk factors related to survival of these patients. Results: Of the 26 patients who underwent TACE for one to four times, 10 patients achieved complete remission (CR) of intrahepatic tumors and among these 10 patients, 4 patients successfully received hepatic artery-venous shunt embolization combined with TACE. The lung metastasis lesions also achieved CR and the survival time was significantly longer than the other 22 patients. The lung metastastic lesions of the other 6 patients of intrahepatic tumors achieved stable disease (SD). Six patients acquired partial remission (PR) of intrahepatic tumors after TACE, while the lung metastastic lesions showed SD or progress disease (PD). Patients who showed CR and PR of intrahepatic tumors had longer survival time than patients with SD and PD. Portal vein tumor thrombus and size of the lung metastastic lesions were significant prognostic factors in these advanced HCC patients. Conclusions: With respect to HCC patients with lung metastasis, TACE was an effective and important therapeutic tool to control pulmonary metastatic tumor growth, and prolong the survival of advanced HCC patients, especially patients with hepatic artery-venous shunt.
Keywords: Transcatheter arterial chemoembolization therapy, intrahepatic tumor, pulmonary metastasis, hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors and has the second highest mortality rate among all types of cancers [1,2]. In Asia, HCC is particularly common due to the prevalence of chronic hepatitis [3]. Regular testing (imaging inspection and detection of tumor markers) of high-risk groups helps increase the detection rate of early HCC, leading to radical treatment and long-term survival of HCC patients [4]. Unfortunately, 10-20% of patients receiving radical treatment suffer from extra-hepatic metastases 5-10 years after surgery [5]. As for patients whose HCC cannot be cured with radical treatment, there is even a higher occurrence of extra-hepatic metastasis after palliative treatment [6]. Moreover, some patients are diagnosed as having superinduced extra-hepatic metastases even during the first visit of palliative care. Nowadays, the most common extrahepatic metastasis is pulmonary metastasis [7].
The occurrence of pulmonary metastasis indicates advanced HCC. In accordance with the protocol of Barcelona Clinic Liver Cancer, systemic therapy should be adopted as the main treatment for pulmonary metastasis using the molecular targeting drug sorafenib as recommended [2]. Recent reports suggest that radical treatment such as surgical resection or radiofrequency ablation for isolated pulmonary metastases significantly prolonged patient survival in appropriate cases [8-10]. However, instead of isolated lung tumors, most HCC patients with pulmonary metastasis have both liver and lung tumors.
Transcatheter arterial chemoembolization (TACE) is currently the most widely used treatment for non-early HCC [2]. In this study, we reviewed the data of representative HCC patients and analyzed the impact of TACE therapy on pulmonary metastasis and survival time for HCC patients with pulmonary metastases.
Materials and methods
Patients
From July 2008 to July 2013, a total of 26 HCC patients with pulmonary metastasis were treated with pure TACE therapy for intrahepatic tumors in our department. The pulmonary metastases were left untreated. Diagnosis of HCC was based on pathological confirmation (postoperative pathological examination or tumor biopsy) and typical radiographic evidence (significantly enhanced tumor in the arterial phase and rapidly cleared contrast agents in the portal venous phase). Pulmonary metastases were diagnosed by lung X-ray or enhanced computed tomography (CT) scan. In the 26 cases, intrahepatic tumors of 21 patients treated with topical therapy (surgery, ablation, TACE, etc.) were fully controlled (marked as complete remission, CR), but intrahepatic tumor recurrence and pulmonary metastasis were observed in a later follow-up. The other 5 patients were diagnosed to have pulmonary metastases at the first visit. All patients showed liver and lung tumors at the visit, and none had pulmonary metastasis with well-controlled intrahepatic tumors. Six patients suffered from respiratory symptoms including cough, hemoptysis and dyspnea. All patients treated with TACE therapy had no metastasis to other organs, and the patients were in good general condition with adequate hepatic reserve function and Child-pugh classification of Class A-B. Clinical characteristics of the 26 HCC patients are shown in Table 1. All procedures were approved by the Ethics Committee of Shandong Cancer Hospital. Informed consents were obtained from all patients or their families.
Table 1.
Demographic and baseline characteristics of HCC patients with pulmonary metastasis
| Variables | No. of patients | Proportion (%) |
|---|---|---|
| Age (median, range, years) | 56 (34-76) | |
| Sex | ||
| Male | 21 | 80.7 |
| Female | 5 | 19.3 |
| Cause of HCC | ||
| Hepatitis B virus | 23 | 88.5 |
| Hepatitis C virus | 2 | 7.7 |
| Other | 1 | 3.8 |
| Child-pugh classfication | ||
| Child class A | 19 | |
| Child class B | 7 | 73.1 |
| Cirrhosis | 26.7 | |
| Presence | 20 | 76.9 |
| Absence | 6 | 23.1 |
| ECOG performance status | ||
| 0 | 16 | 61.6 |
| 1 | 9 | 34.6 |
| 2 | 1 | 3.8 |
| AFP | ||
| < 100 | 16 | 61.5 |
| ≥ 100 | 12 | 38.5 |
| Prior therapy | ||
| No | 5 | 19.2 |
| Yes | 21 | 80.8 |
| Intrahepatic lesion | ||
| Maximum tumor diameter (cm) | 8.74 ± 3.44 | |
| Tumor number | ||
| Solitary | 11 | 42.3 |
| Multiple | 15 | 57.7 |
| Portal vein tumor thrombus | ||
| Absence | 23 | 88.4 |
| Presence | 3 | 11.6 |
| Hepatic vein tumor thrombus | ||
| Absence | 22 | 84.6 |
| Presence | 4 | 15.4 |
| Pulmonary metastasis | ||
| Number of metastasis | ||
| Solitary | 5 | 19.2 |
| Multiple | 21 | 80.8 |
| Maximum metastasis diameter (cm) | 1.99 ± 0.79 | |
| < 2 cm | 12 | 46.1 |
| ≥ 2 cm | 14 | 53.9 |
Note: AFP: alpha fetoprotein; ECOG: Eastern Cooperative Oncology Group; CR (Complete Response) represents the complete disappearance of all target lesions; PR (Partial Response) means 30% decrease in the longest diameter of target lesions; PD (Progressive Disease) corresponds to at least 20% increase in the longest diameter of target lesions; SD (Stable Disease) indicates neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD.
TACE therapy
Puncture with Seldinger technique was routinely performed with a 5F catheter being placed into the celiac aorta. Further catheterization was performed in the feeding artery of the intrahepatic tumor after angiography. Next, the intrahepatic tumor was treated with TACE therapy in which iodized oil emulsion was injected into the tumor artery before gelatin sponge particles to completely embolize the tumor feeding artery. Some patients were found to have intrahepatic artery-hepatic vein shunt or artery-portal venous shunt during digital subtraction angiography. In this case, shunt embolization was first completed followed by further tumor artery embolization. Routine liver enhanced CT scans were performed to determine the effects of embolization between 4 and 6 weeks after the first TACE treatment. If enhanced CT of the liver indicated that the tumor had not been fully embolized after four times of TACE therapy, TACE treatment would not be continued.
Response evaluation criteria in solid tumors (RECIST) evaluation
Enhanced CT scans of the abdomen and chest were routinely performed during 4-6 weeks after treatment to observe changes of intrahepatic tumors and pulmonary metastases. To objectively judge the efficacy of the treatment, we applied mRECIST evaluation criteria to describe the intrahepatic tumor’s response to the treatment. Complete response (CR) represents the complete disappearance of all target lesions. Partial response (PR) means 30% decrease in the longest diameter of target lesions, and progressive disease (PD) corresponds to at least 20% increase in the longest diameter of target lesions. Stable disease (SD) indicates neither sufficient decrease to qualify for PR nor sufficient increase to qualify for PD [13].
Statistical analysis
The survival time of patients was counted from the initial treatment after the diagnosis of pulmonary metastases. The survival curves of the 26 patients were calculated by the Kaplan-Meier method. Survival differences caused by various pathological factors were evaluated using log-rank test, and Cox’s proportional hazard regression model was used to perform multivariate analysis to determine the postoperative factors that affected the survival of patients. Statistical analysis was performed using SPSS 19.0 software (IBM, USA). P < 0.05 was considered statistically significant.
Results
Nearly all HCC patients died within 40 months with a median survival of 11.0 months
The survival time of patients was recorded during follow-ups. The median survival time of all patients was 11.0 months with a range from 3.5 to 40 months. Two patients lost contact during the follow-up, and all other patients died. The Kaplan-Meier survival curve of the patients is shown in Figure 1. Among these patients, only two died of airway obstruction caused by pulmonary metastase oppression, and the other 24 patients died of intrahepatic tumors or complications related to the liver. Sixteen patients died of liver failure, 4 patients died of upper gastrointestinal bleeding, 2 patients died of kidney failure, 1 died of sudden liver rupture, and 1 patient died of spontaneous bacterial peritonitis (Table 2). This data indicated that nearly all HCC patients died within 40 months with the median survival time being 11.0 months.
Figure 1.
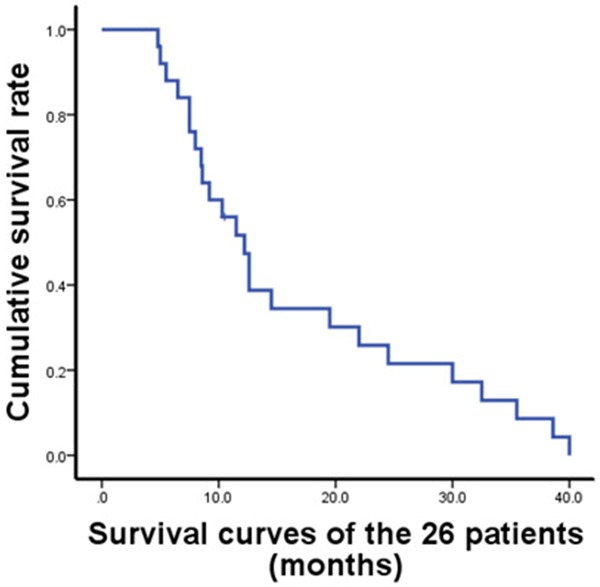
Survival curves of the 26 patients. A total of 26 patients diagnosed to have HCC with pulmonary metastasis were treated with pure TACE therapy against intrahepatic tumors with pulmonary metastases being left untreated. Diagnosis of HCC was based on pathological confirmation (postoperative pathological examination or tumor biopsy) and typical radiographic evidence. Pulmonary metastases were diagnosed by lung X-ray or enhanced computed tomography (CT) scan.
Table 2.
Causes of death
| Causes of death | Frequency, n=26 (%) |
|---|---|
| Liver failure | 16 (61.5) |
| Upper gastrointestinal bleeding | 4 (15.4) |
| Kidney failure | 2 (7.7) |
| Liver rupture | 1 (3.8) |
| Spontaneous bacterial peritonitis | 1 (3.8) |
Portal vein tumor thrombus shortens the survival time of patients with pulmonary metastasis and affects prognosis according to univariate and multivariate analyses
Four of the 26 patients (15.4%) were found to have hepatic vein tumor thrombi. This percentage was higher than that of regular HCC patients, indicating the association of pulmonary metastasis with hepatic vein tumor thrombi. Univariate analysis indicated that the survival time of patients with hepatic vein tumor thrombi was not significantly different from that of patients without it (12.8 vs. 8.5 months, P = 0.44). However, patients with portal vein tumor thrombi had significantly shorter survival time compared with patients without portal vein tumor thrombi, according to univariate and multivariate analyses (12.6 vs. 4.8 months, P = 0.01). Once portal vein tumor thrombi emerged, it would significantly affect the survival time of patients with pulmonary metastasis. In HCC patients without pulmonary metastasis, portal vein tumor thrombus is also the main factor impacting prognosis [14]. Furthermore, our study demonstrated that patients with pulmonary metastase size < 2 cm had a significantly longer survival time than patients with pulmonary metastase size ≥ 2 cm (P = 0.03). However, multivariate analysis showed no significant difference between them. Univariate analysis of other clinical-pathological factors such as gender, age (≤ 50 vs. > 50), the underlying cause of liver cancer (HBV vs. Non-HBC), Child-pugh classification (grade A vs. grade B), cirrhosis (presence vs. absence), alpha fetoprotein value (< 100 ng/ml vs. ≥ 100 ng/ml), liver lesion size (< 10 cm vs. ≥ 10 cm), number of intrahepatic tumors (single vs. multiple), number of pulmonary metastases (single vs. multiple) showed no significant difference between the respective groups (Table 3). This data suggests that portal vein tumor thrombi shorten the survival time of patients with pulmonary metastasis and affect prognosis.
Table 3.
Comparison of median survival time stratified by potential factors
| Stratification | Median survival time (95% CI) | P | Stratification | Median survival time (95% CI) | P |
|---|---|---|---|---|---|
| Portal vein tumor thrombi | Abdominal dropsy | ||||
| Yes vs. No | 8.0 (6.0 ~ 10.0) VS. 22.0 (9.0 ~ 38.0) | 0.001 | Presence vs. Absence | 7.0 (3.0 ~ 13.0) VS. 11.5 (8.0 ~ 22.0) | 0.078 |
| Pulmonary metastasis size | AFP level | ||||
| < 2 vs. ≥ 2 cm | 8.0 (6.0 ~ 30.0) VS. 13.0 (10.0 ~ 22.0) | 0.830 | < 100 vs. ≥ 100 ng/ml | 12.0 (6.0 ~ 22.0) VS. 10.0 (5.0 ~ 24.0) | 0.726 |
| Gender | Liver lesion size | ||||
| Male vs. Female | 10.0 (8.0 ~ 20.0) VS. 11.0 (5.0 ~ 30.0) | 0.537 | < 10 vs. ≥ 10 cm | 12.0 (6.0~20.0) VS. 10.0 (7.0 ~ 22.0) | 0.801 |
| Age | Intrahepatic tumors | ||||
| ≤ 50 vs. > 50 | 10.5 (7.0 ~ 13.0) VS. 12.0 (3.0 ~ 38.0) | 0.570 | Solitary vs. Multiple | 9.0 (5.0 ~ 14.0) VS. 11.0 (7.0 ~ 24.0) | 0.447 |
| Cause of HCC | Pulmonary metastases | ||||
| HBV vs. Non-HBC | 10.5 (8.0 ~ 20.0) VS. 9.5 (5.0 ~ 40.0) | 0.734 | Solitary vs. Multiple | 14.0 (7.0 ~ 38.0) VS. 10.0 (6.0 ~ 13.0) | 0.378 |
| Child-pugh classification | Intrahepatic tumor response | ||||
| Grade A vs. Grade B | 12.0 (7.0 ~ 22.0) VS. 8.0 (3.0 ~ 13.0) | 0.150 | CR + PR VS. SD + PD | 17.0 (10.0 ~ 30.0) VS. 6.5 (3.0 ~ 9.0) | < 0.001 |
Note: AFP, alpha fetoprotein; ECOG, Eastern Cooperative Oncology Group; CR (Complete Response) represents the complete disappearance of all target lesions; PR (Partial Response) means 30% decrease in the longest diameter of target lesions; PD (Progressive Disease) corresponds to at least 20% increase in the longest diameter of target lesions; SD (Stable Disease) indicates neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD.
TACE treatment
To evaluate the effect of TACE therapy, all 26 HCC patients underwent 1 to 4 TACE treatments for intrahepatic tumor. After treatment, a total of 10 patients obtained CR based on the response of tumor. Among these patients, 4 patients were found to suffer from intrahepatic artery-hepatic vein shunt at the initial digital subtraction angiography. Through orificium fistulae after shunt embolization, TACE therapy for intrahepatic tumors was successful and the tumors exhibited CR. Meanwhile, intrapulmonary tumors of the four patients also had CR, and the patients had significantly prolonged survival time than the other 22 patients (35.5 vs. 10.3 months, P = 0.001). Six patients bearing intrahepatic tumors with CR underwent TACE treatments for intrahepatic tumors more than two times, and no artery-venous shunt was found in the liver. After intrahepatic tumors showed CR, pulmonary tumors showed PR or SD. Six patients held PR intrahepatic tumors after TACE therapy, and their intrapulmonary tumors were SD or PD. Together, the 16 HCC patients with intrahepatic tumors under control (CR + PR) showed significantly longer survival time than those with tumors that were not under satisfactory control (SD + PD) (12.6 vs. 7.5 months, P = 0.01) (Figure 2).
Figure 2.
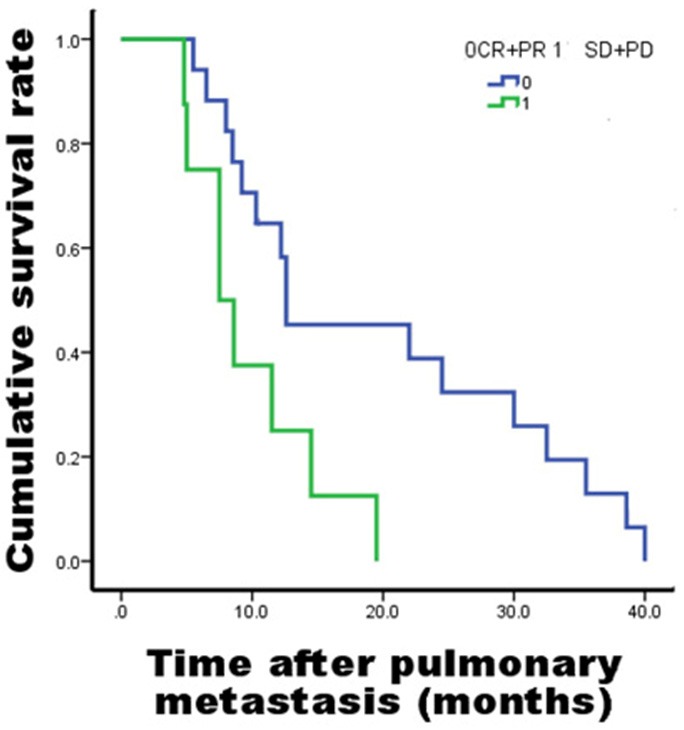
Survival curve of intrahepatic tumor patients with different TACE results. To objectively judge the efficacy of the treatment, mRECIST evaluation criteria were applied to describe the treatment response of the intrahepatic tumor. Complete response (CR) represents the complete disappearance of all target lesions. Partial response (PR) means 30% decrease in the longest diameter of target lesions, and progressive disease (PD) corresponds to at least 20% increase in the longest diameter of target lesions. Stable disease (SD) indicates neither sufficient decrease to qualify for PR nor sufficient increase to qualify for PD.
Discussion
For patients with advanced HCC, TACE is commonly used as a topical treatment modality. For HCC patients with lung metastases, controlling intrahepatic cancer is the main focus of treatment. Sung et al. treated 183 extra hepatic metastasis patients using intrahepatic cancer therapy or intrahepatic cancer therapy combined with systemic therapy and found that 24 patients receiving CR or PR treatment had significantly prolonged survival time compared to those who had objective tumor response (OR) [12]. Their research also confirmed that controlling intrahepatic tumor played a major role in the treatment of advanced HCC.
Joachim Huainan reported that multiple lung metastases in a HCC patient were spontaneously regressive after treatment by TACE. This might be due to antigen release after tumor necrosis or reduced immunosuppression and activated body immune system after the reduction of tumor volume. However, the mechanism is not clear yet [14]. Hobo et al. and Mochizuki et al. reported a case that after a HCC patient with bone metastasis was treated by radiation therapy, the intrahepatic tumor became regressive. They found significantly increased levels of TNF-α in patients after radiation therapy, demonstrating that TNF-α was involved in spontaneous tumor regression process. TNF-α, whose concentration is affected by tumor necrosis products, significantly increases the activity of NK cells [15,16]. Rae et al. reported that after two HCC patients with multiple lung metastases were treated by RFA for a single metastasis, multiple metastases disappeared spontaneously. This finding suggested that after ablation therapy, the degradation products in tumors might induce an immune response, thus, controlling distant tumors through immunoreaction [15]. Other ablation treatments such as microwave therapy and cryoablation also induced similar immunoreactions [17,18].
These HCC patients were treated by TACE therapy in the liver. Embolization of tumor vessels causes tumor necrosis, releasing antigens and inducing an immune response. Notably, the four patients whose lung metastases disappeared completely had completely embolized tumor vessels and radical tumor necrosis. This finding suggests that complete embolization of the tumor might prompt intensive immune response and significantly relieve immunosuppression. In addition, when hepatic chemoembolization is performed, part of the chemotherapeutic drug outflows from the hepatic vein will pass through the lungs and exert therapeutic effects. However, effective anti-tumor concentration of the drugs in the lungs is difficult to achieve due to its metabolism in the liver. Therefore, the effect of chemotherapeutic drugs on metastatic tumors is secondary. These 25 patients were not similar to common HCC patients with lung metastasis. Hepatic arteriovenous shunting occurred in 9 of the 26 patients, which was significantly higher than that of common patients. Once hepatic arteriovenous shunting occurs, it is easy for HCC cells to access hepatic vein from the fistula. The cells enter the circulatory system, and reach the lungs. Then, the cells are retained in peripheral vessels in the lungs followed by planting, growing, and forming metastases. This explains why lung metastasis is prone to occur in patients with arteriovenous shunting. When TACE is performed in patients with arteriovenous shunting, embolization at the shunt must be first performed to reduce or close the shunt to avoid emboli from moving through the shunt and inferior vena cava to the pulmonary vein, causing pulmonary embolism. In addition, this also ensures that tumors in the liver are embolized properly. When the shunt is blocked, the probability of tumor cells reaching the lungs through fistula decreases. However, the reason why lung tumors disappear or decrease is still unclear. In 4 of the 26 patients, lung metastases disappeared completely. Their common feature is the presence and complete embolization of hepatic arteriovenous shunting. In 4-6 weeks after the surgery, no enhancement was found in enhanced CT scan and the iodized oil was completely deposited, suggesting CR. This explains the effect of embolizing hepatic arteriovenous shunting on lung metastasis. In addition, the metastatic tumor disappears or decreases only when hepatic tumor shows CR, indicating the importance of controlling hepatic tumor in the cure to lung metastasis. Moreover, among the four patients whose lung metastases completely disappeared, the diameter of their metastatic tumors was all < 2 cm, suggesting a stronger therapeutic effect when the metastatic tumor was small.
For HCC patients with pulmonary metastases combined with hepatic arteriovenous shunting, if the shunt was completely blocked and liver tumor showed CR, the metastases in lung disappeared completely. For HCC patients with pulmonary metastases, TACE treatment in the liver affects the growth of the metastatic tumor and prolongs survival. Therefore, it is worth considering using TACE in the liver as a treatment for HCC patients.
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Ni S, Liu L, Shu Y. Sequential transcatheter arterial chemoembolization, three-dimensional conformal radiotherapy, and high-intensity focused ultrasound treatment for unresectable hepatocellular carcinoma patients. J Biomed Res. 2012;26:260–267. doi: 10.7555/JBR.26.20120016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hao XS, Wang PP, Chen KX, Li Q, He M, Yu SB, Guo ZY, Perruccio A, Rohan T. Twenty-year trends of primary liver cancer incidence rates in an urban Chinese population. Eur J Cancer Prev. 2003;12:273–279. doi: 10.1097/00008469-200308000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Maluccio M, Covey A. Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. CA Cancer J Clin. 2012;62:394–399. doi: 10.3322/caac.21161. [DOI] [PubMed] [Google Scholar]
- 5.Kanda M, Tateishi R, Yoshida H, Sato T, Masuzaki R, Ohki T, Imamura J, Goto T, Yoshida H, Hamamura K, Obi S, Kanai F, Shiina S, Omata M. Extrahepatic metastasis of hepatocellular carcinoma: incidence and risk factors. Liver Int. 2008;28:1256–1263. doi: 10.1111/j.1478-3231.2008.01864.x. [DOI] [PubMed] [Google Scholar]
- 6.Lin SC, Shih SC, Kao CR, Chou SY. Transcatheter arterial embolization treatment in patients with hepatocellular carcinoma and risk of pulmonary metastasis. World J Gastroenterol. 2003;9:1208–1211. doi: 10.3748/wjg.v9.i6.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katyal S, Oliver JH 3rd, Peterson MS, Ferris JV, Carr BS, Baron RL. Extrahepatic metastases of hepatocellular carcinoma. Radiology. 2000;216:698–703. doi: 10.1148/radiology.216.3.r00se24698. [DOI] [PubMed] [Google Scholar]
- 8.Han KN, Kim YT, Yoon JH, Suh KS, Song JY, Kang CH, Sung SW, Kim JH. Role of Surgical Resection for Pulmonary Metastasis of Hepatocellular Carcinoma. Lung Cancer. 2010;70:295–300. doi: 10.1016/j.lungcan.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 9.Kitano K, Murayama T, Sakamoto M, Nagayama K, Ueno K, Murakawa T, Nakajima J. Outcome and Survival Analysis of Pulmonary Metastasectomy for Hepatocellular Carcinoma. Eur J Cardiothorac Surg. 2012;41:376–82. doi: 10.1016/j.ejcts.2011.05.052. [DOI] [PubMed] [Google Scholar]
- 10.Lee CY, Bae MK, Park IK, Kim DJ, Lee JG, Choi JS, Han KH, Chung KY. Surgical Resection for Pulmonary Metastasis from Hepatocellular Carcinoma: Analysis of Prognosis in Relation to Primary Control. J Surg Oncol. 2010;101:239–43. doi: 10.1002/jso.21487. [DOI] [PubMed] [Google Scholar]
- 11.Uka K, Aikata H, Takaki S, Shirakawa H, Jeong SC, Yamashina K, Hiramatsu A, Kodama H, Takahashi S, Chayama K. Clinical features and prognosis of patients with extrahepatic metastases from hepatocellular carcinoma. World J Gastroenterol. 2007;13:414–420. doi: 10.3748/wjg.v13.i3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jung SM, Jang JW, You CR, Yoo SH, Kwon JH, Bae SH, Choi JY, Yoon SK, Chung KW, Kay CS, Jung HS. Role of Intrahepatic Tumor Control in the Prognosis of Patients with Hepatocellular Carcinoma and Extrahepatic Metastases. J Gastroenterol Hepatol. 2012;27:684–9. doi: 10.1111/j.1440-1746.2011.06917.x. [DOI] [PubMed] [Google Scholar]
- 13.Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX, Sherman M, Schwartz M, Lotze M, Talwalkar J, Gores GJ Panel of Experts in HCC-Design Clinical Trials. Design and Endpoints of Clinical Trials in Hepatocellular Carcinoma. J Natl Cancer Inst. 2018;100:698–711. doi: 10.1093/jnci/djn134. [DOI] [PubMed] [Google Scholar]
- 14.Heianna J, Miyauchi T, Suzuki T, Ishida H, Hashimoto M, Watarai J. Spontaneous regression of multiple lung metastases following regression of hepatocellular carcinoma after transcatheter arterial embolization. A case report. Hepatogastroenterology. 2007;54:1560–2. [PubMed] [Google Scholar]
- 15.Ohba K, Omagari K, Nakamura T, Ikuno N, Saeki S, Matsuo I, Kinoshita H, Masuda J, Hazama H, Sakamoto I, Kohno S. Abscopal regression of hepatocellular carcinoma after radiotherapy for bone metastasis. Gut. 1998;43:575–577. doi: 10.1136/gut.43.4.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mochizuki T, Takehara Y, Nishimura T, Takahashi M, Kaneko M. Regression of hepatocellular carcinoma. AJR Am J Roentgenol. 1991;156:868–869. doi: 10.2214/ajr.156.4.1848389. [DOI] [PubMed] [Google Scholar]
- 17.Dong BW, Zhang J, Liang P, Yu XL, Su L, Yu DJ, Ji XL, Yu G. Sequential pathological and immunologic analysis of percutaneous microwave coagulation therapy of hepatocellular carcinoma. Int J Hyperth. 2003;19:119–133. doi: 10.1080/0265673021000017154. [DOI] [PubMed] [Google Scholar]
- 18.Ng KK, Lam CM, Poon RT, Shek TW, To JY, Wo YH, Ho DW, Fan ST. Comparison of systemic responses of radiofrequency ablation, cryotherapy, and surgical resection in a porcine liver model. Ann Surg Oncol. 2004;11:650–657. doi: 10.1245/ASO.2004.10.027. [DOI] [PubMed] [Google Scholar]


