Abstract
Objective: The aim of the present study was to improve the understanding of ureteral endometriosis, and remind the clinics to be highly suspicious of it in women of reproductive age with hydronephrosis without evidence of stones and malignancy. Methods: A retrospective analysis was performed on a database of 82 patients who underwent surgery for hydronephrosis due to ureteral endometriosis between Jan. 2007 and Apr. 2014. Results: All patients evaluated in this study were divided into three groups: Group A consisted of patients between 20-30 years (n = 12), Group B comprised of patients between 31-40 years (n = 29), Group C consisted of patients between 41-50 years (n = 41). Patients in Group C had a greater prevalence of pelvic pain compared with patients in Group A and Group B (P < 0.05). However there were no differences with respect to the prevalence of other non-specific genitourinary symptoms and the urinary symptoms. Infertility was found to occur more frequently in patients in Group A compared with patients in Group B and Group C (P < 0.05). Because of the lack of specific symptoms, ureteral endometriosis was diagnosed (20.1 ± 10.3) months on average after the patients suffered from mild hydronephrosis or mild loin pain. Preoperative examinations showed different degree of hydronephrosis, but lack of specificity. All patients underwent surgery by laparotomy or laparoscopy, such as ureterectomy with ureteroureterostomy or ureterocystoneostomy. The pathological examination confirmed the diagnosis of ureteral endometriosis. Conclusion: The diagnosis of ureteral endometriosis is elusive and relies heavily on clinical suspicion. Hence, women in the reproductive age, especially with infertility and pelvic pain, who have hydronephrosis without evidence of stones and malignance, should be adequately assessed via imaging techniques or diagnostic laparoscopy or cystoscopy to highly suspect the diagnosis of ureteral endometriosis.
Keywords: Ureteral endometriosis, hydronephrosis, infertility, pelvic pain
Introduction
Endometriosis is defined as the presence of endometrial-like tissue outside the endometrial cavity and uterine musculature [1]. It has been estimated to affect 10% to 20% of the general women, but approaching 30% to 40% in infertile women [2]. The main location of the endometrial tissue is in the pelvis, exceptionally can be located in urinary tract, however, extra-pelvic endometrial tissues have been found in nodes and gastrointestinal tract [3].
While endometriosis is a common disease, the urinary tract endometriosis is a rare entity, with a reported prevalence of less than 0.1% to 0.4% of endometriosis cases [4,5]. The relative frequencies of involvement of bladder, ureter, and kidney are 40:5:1, respectively [6]. It is most commonly diagnosed in women of reproductive age, with a peak age of 40 to 44 years [7]. In ureteral, ratio of left to right involvement is reported to be between 4:1, more commonly involving the distal segment of the left ureter [4].
Symptoms depend on the site of endometrial implantation, and severe disease can lead to pain and infertility due to extensive adhesions and distortion of anatomy. But the clinical characteristics of ureteral endometriosis is typically marked by non-specific symptoms, and as many as 50% of patients are often asymptomatic [8]. So ureteral endometriosis can potentially lead to serious consequences, such as urinary tract obstruction and finally silent loss of renal function. The risk of silent renal loss is reported to be as high as 25-50% [9]. Because of non-specific symptoms, insufficient preoperative evaluation, misinterpretation of imaging techniques or non-specific imaging findings, ureteral endometriosis is suspected before surgery in only 40% of patients [5,10].
The diagnosis of ureteral endometriosis is elusive and depends heavily on clinical suspicion as it can occur with both minimal and extensive disease. Therefore, the aim of our study was to arise the urologists and gynecologists attention to be highly suspicious of ureteral endometriosis in women of reproductive age with hydronephrosis without urolithiasis in order to make an early diagnosis and thus avoid renal loss.
Material and methods
A retrospective analysis was performed on a database of 82 patients who underwent surgery for hydronephrosis due to ureteral endometriosis between Jan. 2007 and Apr. 2014. Patient’ age, body mass index (BMI; weight/height²), presenting symptoms, history of previous pelvic surgery, site of involvement, imaging features, and type of treatment were obtained by review of the pathology reports and medical records when available.
Date management and statistics
The patients’ data were collected and entered into the SPSS V19.0 software package for Windows. Age, body mass index and the delay of diagnosis at least are expressed as mean ± SD. The results were compared using the following two-sided tests: analysis of variance, Kruskal-Wallis, chi-squared, Fisher exact, and the likelihood ratio test. For all tests, a P value of < 0.05 was considered to be statistically significant. The variables with statistical differences were adjusted in a multiple logistic regression.
Results
Table 1 shows the clinical characteristics of the patients. All patients evaluated in this study were divided into three groups: Group A consisted of patients between 21-30 years (n = 12), Group B comprised of patients between 31-40 years (n = 29), Group C consisted of patients between 41-50 years (n = 41). In group A, no patients had previous surgical history. In group B, one patient had hysterectomy because of uterine fibroids, while nine patients had undergone caesarean section. One patient suffered from gynecological endometriosis, which was treated by laparoscopic hysterectomy and bilateral salpingo-oophorectomy. In group C, six patients had previously been hysterectomised because of uterine fibroids, while ten patients had undergone caesarean section.
Table 1.
Clinical characteristics of the patients
| A 20-30 y (n = 12) | B 30-40 y (n = 29) | C 40-50 y (n = 41) | P value | |
|---|---|---|---|---|
| Age (mean ± SD) | 26.42 ± 2.84 | 35.93 ± 2.34 | 44.85 ± 2.56 | |
| BMI (mean ± SD) | 21.42 ± 3.21 | 22.31 ± 2.57 | 24.04 ± 2.86 | 1, 2, 3 > 0.05 |
| Site of involvement | ||||
| Left (n, %) | 9 (75) | 18 (62.1) | 27 (65.9) | 1, 2, 3 > 0.05 |
| Right (n, %) | 3 (25) | 10 (34.5) | 12 (29.3) | 1, 2, 3 > 0.05 |
| Bilateral (n,%) | 0 | 1 (3.4) | 1 (2.4) | 1, 2, 3 > 0.05 |
| Non-specific genitourinary symptoms | ||||
| Dysmenorrhea (n, %) | 2 (16.7) | 4 (13.8) | 9 (21.9) | 1, 2, 3 > 0.05 |
| Dyspareunia (n, %) | 0 | 3 (10.3) | 2 (4.9) | 1, 2, 3 > 0.05 |
| Pelvic pain (n, %) | 2 (16.7) | 12 (41.4) | 28 (68.3) | 1:0.027* |
| 2:0.048* | ||||
| 3:0.000* | ||||
| Urinary symptoms | ||||
| Dysuria (n %) | 0 | 2 (6.9) | 2 (4.9) | 1, 2, 3 > 0.05 |
| Hematuria (n, %) | 1 (8.3) | 2 (6.9) | 1 (2.4) | 1, 2, 3 > 0.05 |
| Urgency (n, %) | 0 | 1 (3.4) | 3 (7.3) | 1, 2, 3 > 0.05 |
| Loin pain (n, %) | 10 (83.3) | 21 (72.4) | 32 (78) | 1, 2, 3 > 0.05 |
| Infertility (n, %) | 8 (66.7) | 14 (48.3) | 8 (19.5) | 1 > 0.05 |
| 2:0.008* | ||||
| 3:0.049* | ||||
| No symptoms | 3 (25) | 15 (51.7) | 12 (29.3) | 1, 2, 3 > 0.05 |
| The delay of diagnosis at least (mean ± SD) | 13.50 ± 7.29 | 20.17 ± 9.22 | 21.52 ± 11.19 | 1, 2, 3 > 0.05 |
Note: comparisons: 1: A vs B. 2: B vs C. 3: A vs C.
P < 0.05.
Patients’mean age was 39 years (range 21-50), while their mean BMI was 23.6. In details, Group A comprised of 12 (14.6%) patients, Group B comprised of 29 (35.4%) and Group C comprised of 41 (50%) patients, respectively.90% of patients were between 35 to 48.8 years of age.
The affected side in 54 (65.9%) cases were found to be on the left side and on the right side in 25 (30.5%) cases, while bilateral involvement was found in 2 (2.4%) cases. In our cases, the ratio of left to right involvement is about 2.2:1. However, there was no differences with respect to the site of involvement among Group A-C.
At clinical presentation, 42 patients showed non-specific genitourinary symptoms (dysmenorrhea in 15 patients, dyspareunia in 5 patients, pelvic pain in 42 patients). Patients in Group B had a greater prevalence of pelvic pain (12 of 29, 41.4%) compared with patients in Group A (2 of 12, 16.7%) (P < 0.05). On the other hand, patients in Group C (28 of 41, 68.3%) had a greater prevalence of pelvic pain compared with patients in Group A (2 of 12, 16.7%) and with patients in Group B (12 of 29, 41.4%) (P < 0.05). However, there were no differences with respect to the prevalence of other non-specific genitourinary symptoms such as dysmenorrhea and dyspareunia between the three groups (P > 0.05). 6 patients showed urinary symptoms (dysuria in 4 cases, hematuria in 6 cases, urgency in 4 cases), chronologically related to the menstrual cycle. 63 (76.8%) patients suffered from loin pain, which was caused by different degree of hydronephrosis. No differences were found between the three groups with respect to the urinary symptoms (P > 05). Infertility was found to occur more frequently in patients in Group A (8 of 12, 66.7%) compared with patients in Group B (14 of 29, 48.3%) and with patients in Group C (8 of 41, 19.5%) (P < 0.05). It was also found to occur more frequently in patients in Group B (14 of 29, 48.3%) compared with patients in Group C (8 of 41, 19.5%) (P < 0.05). However, 30 (36.6%) patients had no symptoms.
On average ureteral endometriosis was diagnosed (20.1 ± 10.3) months after the patients suffered from mild hydronephrosis or mild loin pain resulting from it (range 3-38 months). However, no differences were found with respect to the delay of diagnosis at least between the three groups (P > 0.05).
In our cases, all urinalysis and urine cytology showed no abnormal findings. Blood analyses including CA125 were normal. All patients underwent abdominal ultrasonography which showed the different dilation of the upstream excretory tract, with no conclusive imaging evidence about the signs of the stenosis. Further examinations such as computed tomography, magnetic resonance imaging and magnetic resonance urography (Figure 1) were performed following the detection of positive findings of ultrasonography, which evaluated the pelvic spread of the disease. The emission computed tomography evaluated the renal function. It showed that the function of the diseased kidneys were decreased (about 20 to 50%) but reversible, and the overall kidney function were normal. Cystoscopy was performed in the cases with urinary symptoms, but no positive results were found.
Figure 1.
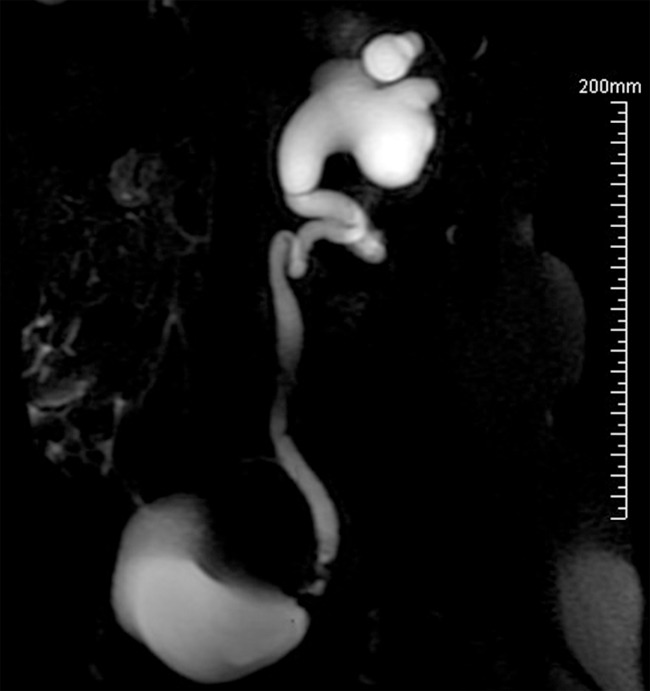
Magnetic resonance urography demonstrates severe left uretero-hydronephrosis and obstruction of the left distal ureter with proximal left ureter showing redundant kinking.
In our cases no patient was presumptively diagnosed preoperatively, but 10 (19.6%) patients were highly suspected of the diagnosis of ureteral endometriosis. All patients went surgery by laparotomy or laparoscopy, such as ureterectomy with ureteroureterostomy or ureterocystoneostomy, and the frozen section pathological examination of the excised ureteral tract in the surgery confirmed the diagnosis of ureteral endometriosis. The pathological examination (Figure 2) showed that the pathologic types were of the intrinsic type in 6 (7.3%) cases and the extrinsic type in 74 (90.2%) cases, while bilateral involvement was found in 2 (2.4%) cases. After discharge, all patients were recommended to go to gynecology department for further hormone therapy.
Figure 2.
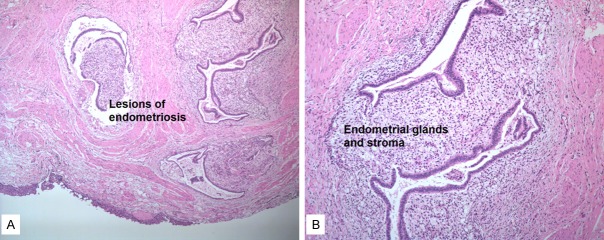
Extrinsic ureteral endometriosis. A. Reduced from ×50. B. Endometrial glands and stroma invading ureteral serosa. Reduced from ×100.
Discussion
Endometriosis is a fairly common gynecologic disease, which present as a biologically benign, albeit aggressive, pathology with high local recurrences [11]. It can exceptionally involve the urinary tract and the ureter in particular. Urinary tract endometriosis is located in the bladder, ureter, kidney and urethra in 84%, 10%, 4%, and 2% of cases, respectively [12]. Involvement of the genitourinary tract has been reported to have the peak age of incidence being between 40 and 44 years of age [7]. In our present cases, 90% of patients were between 35 to 48.8 years of age.
Two major pathological types exist: extrinsic and intrinsic ureteral endometriosis. In cases of intrinsic endometriosis, ectopic endometrial tissue is implanting within the muscularis propria, lamina propria or ureteral lumen, while the extrinsic occurs within the ureteral adventitia and adjacent soft tissues only. It causes a compression of the ureteric wall and inflammation and fibrosis. By this way, its normal anatomy is distorted. The extrinsic form occurs four times more often than intrinsic [5,8]. The most frequently affected side is the left one and it is readily explained by anatomic differences of the pelvis [13]. Our cases confirm this finding, highlighting the involvement of the left side in 65.9%.
Ureteral endometriosis is usually asymptomatic, while 25% of patients present with dysmenorrhea, dyspareunia, pelvic pain, infertility, dysuria, urgency, recurrent urinary tract infections, 15% with gross hematuria, and 50% of patients are often asymptomatic [5,8,14]. In the present sample, 36.6% (30 of 82) of patients were asymptomatic, and the patients of intrinsic ureteral endometriosis suffered from periodic urinary symptoms such as dysuria, hematuria and urgency. 51.2% (42 of 82) of patients suffered from pelvic pain, and there was a greater prevalence of pelvic pain among the three groups (P < 0.05), and the authors suggest that older patients suffer more often from pelvic pain than the younger patients. Endometriosis can cause infertility, but the theory of it remains elusive [15]. Harada reported that half of women with endometriosis suffer from infertility [16]. In the present cases, 36.6% (30 of 82) of patients suffered from infertility, and there was also a greater prevalence of infertility among the three groups (P < 0.05). Hence, the authors suggest that the earlier onset age of the ureteral endometriosis, the greater impact of on reproductive function.
Because of delayed diagnosis, the ureter can progressively narrow, with a consequent worsening of hydronephrosis and progressive deterioration of renal function. Tanuma reported that people with negative symptoms, 83.3% were referred to the clinics because of the existence of hydronephrosis [9,17]. And 30% patients have 25-50% loss of renal function and unknown number will then have loss of the kidney [18]. In our cases, due to its nonspecific or absent clinical signs, diagnosis was delayed for (20.1 ± 10.3) months from the beginning of symptoms. They all had different degrees of hydronephrosis. In order to achieve an early diagnosis of ureteral endometriosis, enough imaging techniques are necessary.
Ultrasonography should be used in the initial evaluation but it has low specificity. It may be confused with dermoid cysts, hemorrhagic cysts, or tumors. However, it can raise suspicion that could be confirmed by further investigations, such as urography, intravenous pyelogram, computerized tomography scans [7]. These imaging modalities just show stricture of the ureter and hydronephrosis but lack of specificity [19]. The emission computed tomography should be performed to assess the renal function [5,20]. Magnetic resonance imaging (MRI) is the most specific technique in precisely identifying the magnitude of the endometriosis lesions and their depth [3,9]. Moreover, magnetic resonance urography (MRU) can differentiate between intrinsic and extrinsic forms of ureteral involvement [10,19]. Radiological aspects of ureteral endometriosis are nonspecific, so imaging techniques are of limited value in providing a totally definite diagnosis of ureteral endometriosis. Because the absence of visible abnormalities, so doctors need cystoscopy or ureteroscopy or diagnostic laparoscopy to confirm the disease. In the intrinsic can use cystoscopy or ureteroscopy to have a direct observation of the urinary system, and get histologic material to confirm the diagnosis. In the extrinsic cases, laparoscopy not only allows direct localization of endometrial tissue around the ureter, and get samples of disease on a pathology specimen, but also allows immediate surgical correction if diagnosis of ureteral endometriosis is definitive.
The managements of ureteral endometriosis are numerous, including hormonal therapy alone or with double-J stent insertion, ureterolysis, segmental ureterectomy and end-to-end anastomosis, or segmental ureterectomy and uretro-cystoneostomy, and nephrectomy. The option is dependent on the extent of the disease, the degree of hydronephrosis and renal function compromise [6]. As an estrogen-dependent inflammatory disease, at early stage of the disease, before fibrosis occurs, about 80-90% of patient can gain relief with hormone therapy such as progestin, gonadotropin-releasing hormone (GnRH) agonists and danazol [21-25]. However, with the progress of the disease, once a fibrotic constricting band over the ureter is formed, the hormone therapy does not seem to alter the course of ureteric obstruction [7,26]. As for ureterolysis, it is the procedure of choice for the treatment of minimal, extrinsic, surroundings, and non-obstructive disease [27]. For patients who had obstruction and ureteric dilatation, the main stay of treatment is to remove the diseased ureter to release the ureteric obstruction [4]. In the present cases, all the patients had ureteric obstruction, but the renal damage was reversible. So we had to do segmental ureterectomy with ureteroureterostomy or ureterocystoneostomy. If the ureteric obstruction still exists, as many as 25% patients will sustain irreversible renal damage [26]. Once the renal damage is irreversible, nephrectomy is unavoidable. The postoperative hormone therapy is used to prevent recurrence [19]. Hence, the sooner the diagnosis of ureteral endometriosis, the easier the treatment, and the better the prognosis.
In conclusion, ureteral endometriosis is a rare and often silent disease which can lead to hydronephrosis and ultimately to renal failure. The diagnosis is elusive and relies heavily on clinical suspicion. Hence, women in the reproductive age, especially with infertility and pelvic pain, who have hydronephrosis without evidence of stones, should be adequately assessed via imaging techniques or diagnostic laparoscopy or cystoscopy to highly suspect the diagnosis of ureteral endometriosis. The option of the management is dependent on extent of the disease, the degree of hydronephrosis and the renal function compromise. Earlier diagnosis makes the treatment easier and the prognosis better.
Acknowledgements
I’d like to express my sincere thanks to all those who have lent me hands in the course of my writing this paper, such as my colleagues and people who do statistical analysis.
Disclosure of conflict of interest
None.
References
- 1.Dunselman GA, Vermeulen N, Becker C, Calhaz-Jorge C, D’Hooghe T, De Bie B, Heikinheimo O, Horne AW, Kiesel L, Nap A, Prentice A, Saridogan E, Soriano D, Nelen W. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29:400–412. doi: 10.1093/humrep/det457. [DOI] [PubMed] [Google Scholar]
- 2.Comiter CV. Endometriosis of the urinary tract. Urol Clin North Am. 2002;29:625–635. doi: 10.1016/s0094-0143(02)00065-4. [DOI] [PubMed] [Google Scholar]
- 3.Perez-Utrilla PM, Aguilera BA, Alonso DJM, Hernandez A, de Francisco MG, Martin HM, de Santiago J, de la Pena Barthel J. Urinary tract endometriosis: clinical, diagnostic, and therapeutic aspects. Urology. 2009;73:47–51. doi: 10.1016/j.urology.2008.08.470. [DOI] [PubMed] [Google Scholar]
- 4.Bosev D, Nicoll LM, Bhagan L, Lemyre M, Payne CK, Gill H, Nezhat C. Laparoscopic management of ureteral endometriosis: the Stanford University hospital experience with 96 consecutive cases. J Urol. 2009;182:2748–2752. doi: 10.1016/j.juro.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 5.Arrieta BS, Lopez CA, Hernandez GA, Rodriguez GR, de Santiago Garcia J. Complete loss of unilateral renal function secondary to endometriosis: a report of three cases. Eur J Obstet Gynecol Reprod Biol. 2013;171:132–137. doi: 10.1016/j.ejogrb.2013.08.022. [DOI] [PubMed] [Google Scholar]
- 6.Al-Khawaja M, Tan PH, MacLennan GT, Lopez-Beltran A, Montironi R, Cheng L. Ureteral endometriosis: clinicopathological and immunohistochemical study of 7 cases. Hum Pathol. 2008;39:954–959. doi: 10.1016/j.humpath.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 7.Yohannes P. Ureteral endometriosis. J Urol. 2003;170:20–25. doi: 10.1097/01.ju.0000054836.32660.9e. [DOI] [PubMed] [Google Scholar]
- 8.Gabriel B, Nassif J, Trompoukis P, Barata S, Wattiez A. Prevalence and management of urinary tract endometriosis: a clinical case series. Urology. 2011;78:1269–1274. doi: 10.1016/j.urology.2011.07.1403. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe Y, Ozawa H, Uematsu K, Kawasaki K, Nishi H, Kobashi Y. Hydronephrosis due to ureteral endometriosis treated by transperitoneal laparoscopic ureterolysis. Int J Urol. 2004;11:560–562. doi: 10.1111/j.1442-2042.2004.00828.x. [DOI] [PubMed] [Google Scholar]
- 10.Ponticelli C, Graziani G, Montanari E. Ureteral endometriosis: a rare and underdiagnosed cause of kidney dysfunction. Nephron Clin Pract. 2010;114:c89–93. doi: 10.1159/000254380. [DOI] [PubMed] [Google Scholar]
- 11.Antonelli A, Simeone C, Frego E, Minini G, Bianchi U, Cunico SC. Surgical treatment of ureteral obstruction from endometriosis: our experience with thirteen cases. Int Urogynecol J Pelvic Floor Dysfunct. 2004;15:407–412. doi: 10.1007/s00192-004-1171-7. [DOI] [PubMed] [Google Scholar]
- 12.Abrao MS, Dias JA Jr, Bellelis P, Podgaec S, Bautzer CR, Gromatsky C. Endometriosis of the ureter and bladder are not associated diseases. Fertil Steril. 2009;91:1662–1667. doi: 10.1016/j.fertnstert.2008.02.143. [DOI] [PubMed] [Google Scholar]
- 13.Chapron C, Chopin N, Borghese B, Foulot H, Dousset B, Vacher-Lavenu MC, Vieira M, Hasan W, Bricou A. Deeply infiltrating endometriosis: pathogenetic implications of the anatomical distribution. Hum Reprod. 2006;21:1839–1845. doi: 10.1093/humrep/del079. [DOI] [PubMed] [Google Scholar]
- 14.Seracchioli R, Mabrouk M, Montanari G, Manuzzi L, Concetti S, Venturoli S. Conservative laparoscopic management of urinary tract endometriosis (UTE): surgical outcome and long-term follow-up. Fertil Steril. 2010;94:856–861. doi: 10.1016/j.fertnstert.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 15.Abrao MS, Muzii L, Marana R. Anatomical causes of female infertility and their management. Int J Gynaecol Obstet. 2013;123(Suppl 2):S18–24. doi: 10.1016/j.ijgo.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Harada T. Dysmenorrhea and endometriosis in young women. Yonago Acta Med. 2013;56:81–84. [PMC free article] [PubMed] [Google Scholar]
- 17.Tanuma Y. Ureteral endometriosis: a case report and a review of the Japanese literature. Hinyokika Kiyo. 2001;47:573–577. [PubMed] [Google Scholar]
- 18.Moore JG, Hibbard LT, Growdon WA, Schifrin BS. Urinary tract endometriosis: enigmas in diagnosis and management. Trans Pac Coast Obstet Gynecol Soc. 1979;46:61–71. [PubMed] [Google Scholar]
- 19.Ghezzi F, Cromi A, Bergamini V, Bolis P. Management of ureteral endometriosis: areas of controversy. Curr Opin Obstet Gynecol. 2007;19:319–324. doi: 10.1097/GCO.0b013e328216f803. [DOI] [PubMed] [Google Scholar]
- 20.Jadoul P, Feyaerts A, Squifflet J, Donnez J. Combined laparoscopic and vaginal approach for nephrectomy, ureterectomy, and removal of a large rectovaginal endometriotic nodule causing loss of renal function. J Minim Invasive Gynecol. 2007;14:256–259. doi: 10.1016/j.jmig.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 21.Pezzuto A, Pomini P, Steinkasserer M, Nardelli GB, Minelli L. Patient with pelvic pains: retroperitoneal fibrosis or pelvic endometriosis? A case report and review of literature. Fertil Steril. 2009;92:1497, e9–12. doi: 10.1016/j.fertnstert.2009.07.982. [DOI] [PubMed] [Google Scholar]
- 22.Speroff L, Fritz MA. Clinical Gynecologic Endocrinology and Infertility. 7th edition. Philadelphia: Lippincott Williams and Wilkins; 2005. Endometriosis. [Google Scholar]
- 23.Lavelle KJ, Melman AW, Cleary RE. Ureteral obstruction owing to endometriosis: reversal with synthetic progestin. J Urol. 1976;116:665–666. doi: 10.1016/s0022-5347(17)58959-4. [DOI] [PubMed] [Google Scholar]
- 24.Gantt PA, Hunt JB, McDonough PG. Progestin reversal of ureteral endometriosis. Obstet Gynecol. 1981;57:665–667. [PubMed] [Google Scholar]
- 25.Umar SA, MacLennan GT, Cheng L. Endometriosis of the ureter. J Urol. 2008;179:2412. doi: 10.1016/j.juro.2008.03.083. [DOI] [PubMed] [Google Scholar]
- 26.Brough RJ, O’Flynn K. Recurrent pelvic endometriosis and bilateral ureteric obstruction associated with hormone replacement therapy. BMJ. 1996;312:1221–1222. doi: 10.1136/bmj.312.7040.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Antonelli A, Simeone C, Zani D, Sacconi T, Minini G, Canossi E, Cunico SC. Clinical aspects and surgical treatment of urinary tract endometriosis: our experience with 31 cases. Eur Urol. 2006;49:1093–1097. doi: 10.1016/j.eururo.2006.03.037. [DOI] [PubMed] [Google Scholar]


