Abstract
Objective: Metastasis-associated in colon cancer-1 (MACC1), a new gene associated with primary and metastatic colon cancer, promotes tumor cell growth as well as the development of distant metastasis. The aim of this study is to investigate the expression of MACC1 protein in colon cancer and its association with clinicopathological parameters and postoperative liver metastasis. Materials and Methods: Expression of MACC1 protein was detected immunohistochemically in paraffin-embedded specimens of 96 cases of colon cancer. Relationship between MACC1 protein expression and clinicopathological parameters, postoperative liver metastasis were analyzed. Results: Immunohistochemistry examination showed that MACC1 protein expression was significantly more abundant in colon cancer tissues than in normal colon tissues (P = 0.038), Positive rate of MACC1 expression in colon cancer tissues was increased significantly in patients with lymph node metastases (P = 0.001) and higher T stages (P = 0.006). Postoperative live metastasis-free survival period was significantly longer in negative MACC1 expression group than that of positive MACC1 expression group (36.4 ± 2.85 vs. 28.6 ± 2.02 months, P = 0.014). Multivariate analysis showed that MACC1 expression level is an independent prognostic factor for postoperative live metastasis-free survival (95% confidence interval [CI] =1.32-3.38, P = 0.006). Conclusions: Our results suggest that MACC1 expression level might play an important role in colon cancer invasion and MACC1 expression level is an independent biomarker for postoperative liver metastasis in patients with colon cancer.
Keywords: Colon neoplasm, metastasis-associated in colon cancer 1, liver metastasis
Introduction
Around the world, colorectal cancer is one of the most frequent malignancies with over 1.2 million new cancer cases and 608,700 deaths estimated to have occurred [1]. Among all colorectal cancer cases, nearly 75% of patients are diagnosed with colon cancer which has a high propensity for liver metastasis [2]. The major cause of death from colon cancer is liver metastasis, which is usually resistant to conventional therapies [3]. 35%-55% of patients with colon cancer will develop liver metastases during the course of illness. Survival following hepatic resection of colorectal metastasis now approaches 35%-50%. However, the recurrence rate in 5 years is as high as approximately 65% [4]. Early treatment targeting colon cancer liver metastatic foci might be important for improving patient survival. To date, the precise mechanisms leading to liver metastasis in colon cancer remains unclear, and biomarkers for liver metastasis are still lacking.
Metastasis-associated in colon cancer 1 (MACC1), a novel regulator of tumor growth and metastasis, has recently been indicated as a potential prognostic factor for metastatic disease in primary hepatocellular carcinoma [5,6], lung adenocarcinoma [7,8], non-small cell lung cancer [9], gastric carcinoma [10,11], breast cancer [12], esophageal cancer [13] and colorectal cancer [14,15]. Expression of MACC1 was found to be significantly upregulated in malignant tissues compared to normal tissues or adenomas, and induction of MACC1 might occur at the crucial step of transition from the benign to the malignant phenotype and can be allocated to the adenoma-carcinoma sequence for colon cancer [14]. MACC1 has been identified as a prognosis biomarker for colon cancer, which promotes proliferation, invasion and hepatocyte growth factor (HGF)-induced scattering of colon cancer cells in vitro and in vivo [16]. MET, which encodes Met protein, has been proven to be a transcriptional target of MACC1. MACC1 controls the activity and expression of MET, and regulates HGF/Met signal pathway. Transduction of MACC1 mutants with the SH3 domain or the proline-rich motif deleted in colon cancer cells abrogated the above function of MACC1 [17].
We demonstrated previously that the hedgehog (Hh) signaling pathway plays a key role in the occurrence and development of colon cancer, and high glioma-associated oncogene homolog 1 (Gli-1) and smoothened (Smo) protein expression may promote postoperative liver metastasis of colon cancer [18,19]. The aim of the present study is to explore the potential role of MACC1 protein in colon cancer development and liver metastasis. For this purpose, MACC1 protein expression was detected in colon cancer and normal colon tissues by immunohistochemistry using tissue microarray. Additionally, the correlation of MACC1 protein expression with the clinicopathological data and prognostic variables was analyzed.
Materials and methods
Patients and tissue samples
Tissue samples of colon cancer were obtained from 96 patients who underwent surgical operation in the Second Hospital of Shandong University from July 2002 to December 2007. Samples were provided as Formalin-fixed and paraffin-embedded tissue specimens. All patients received no chemotherapy and/or other therapies before surgical operation. Table 1 presents the clinicopathological data of the 96 patients. All hematoxylin and eosin (H & E)-stained slides for each patient were reviewed by two pathologists. All patients were staged according to the American Joint Committee on Cancer (AJCC) TNM staging system for colon cancer (seventh edition). The study protocol was approved by the ethnics committee of Shandong University. All 96 patients provided written informed consent for their tissues to be used in this study.
Table 1.
Clinicopathological parameters in relation to MACC1 immunoreactivity
| Variable | Categorization | Patients (n) | MACC1 expressiona | P valueb | |
|---|---|---|---|---|---|
|
| |||||
| positive | negative | ||||
| Gender | |||||
| male | 60 | 31 | 29 | ||
| female | 36 | 20 | 16 | 0.230 | |
| Age | |||||
| ≥ 60 | 50 | 28 | 22 | ||
| < 60 | 46 | 23 | 23 | 0.638 | |
| Tumor size | |||||
| ≥ 5 cm | 42 | 21 | 21 | ||
| < 5 cm | 54 | 30 | 24 | 0.680 | |
| Tumor grade (WHO) | |||||
| G1 + G2 | 64 | 33 | 31 | ||
| G3 + G4 | 32 | 18 | 14 | 0.702 | |
| Lymph node status | |||||
| N+ | 51 | 39 | 12 | ||
| N0 | 45 | 12 | 33 | 0.001 | |
| T stages | |||||
| T2 | 30 | 8 | 22 | ||
| T3 | 45 | 28 | 17 | ||
| T4 | 21 | 15 | 6 | 0.006 | |
MACC1 protein expression, positive means TS 4-6, and negative means TS 0-3;
P values were evaluated by chi-square test or the Fisher’s exact test
Tissue microarray (TMA)
For the fabrication of the TMAs, we reviewed the patients’ tissue slides, collected two 2-mm cores from each paraffin block, and embedded them in a recipient block to plant 30 tissues per block. Each tissue core was assigned with a unique tissue microarray location number that was linked to a database containing other clinicopathological data. TMA sections were then cut from the block in preparation for immunohistochemistry experiments.
Immunohistochemistry (IHC)
The expression of MACC1 was detected by streptavidin-peroxidase-biotin (SP) immunohistochemical method according to the manufacturer’s instructions [18,20]. In brief, paraffin-embedded specimens were cut into 4 μm sections and baked at 60°C for 60 min. The sections were deparaffinized with xylenes and rehydrated. Then sections were submerged into EDTA antigenic retrieval buffer in a pressure cooker for 10 minutes and then cooled at room temperature for 20 minutes. The sections were treated with 3% hydrogen peroxide in methanol to quench the endogenous peroxidase activity, followed by incubation with normal serum to block nonspecific binding. The sections were incubated with MACC1 monoclonal antibody (1:100; HPA020103; SIGMA, St, Louis, USA) overnight at 4°C. After washing, the tissue sections were incubated with biotinylated secondary antibody (Maixin Biotechnology Company, Fuzhou, China) for 1 h at room temperature, followed by incubation with streptavidin-horseradish peroxidase for 20 minutes. After washing with PBS, diaminobenzidine (DAB) was added for visualization. The sections were counterstained with haematoxylin. For negative controls, the anti-MACC1 antibody was replaced with PBS.
Evaluation of immunohistochemical staining
Evaluation of MACC1 staining was conducted according to the previously described methods as suggested by Chundong G et al [7,21]. Positive staining of MACC1 protein presents brown in cytoplasm, partly in nucleus. An average of 1,500 cells was evaluated per section. The stained specimens were then categorized into 6 degree classes according to the quantitative score. Initially, 4 degrees of the proportional score (PS) for the positive staining cells were assigned according to the frequency of positive tumor cells (0, none; 1, 1/100 to 1/4; 2, 1/4 to 1/2; and 3, > 1/2). Thereafter, 4 degrees of the intensity score (IS) were assigned according to the intensity of the staining (0, none; 1, weak; 2, intermediate; and 3, strong). The proportional score and the intensity score were then added to obtain a total score (TS), which ranged from 0 to 6. According to the TS, the MACC1 expression of the tumor was categorized as negative when the score was 0-3 and as positive when the score was 4-6. The stained slides were reviewed and scored independently by two observers blinded to the patients’ information. Cases with discrepancies in TS were discussed together with other pathologists until consensus was reached.
Statistical analysis
The data were analyzed using using the statistical software package SPSS 13.0 (SPSS, Chicago, IL, USA). A two-sided Fisher’s exact test or Χ2 test was performed to analyze possible associations between MACC1 expression and clinical parameters. The cumulative survival time was computed using the Kaplan-Meier method and compared by the log-rank test. Multivariate analysis was performed according to Cox proportional hazards model. Differences were considered to be statistically significant for p values of less than 0.05.
Results
MACC1 protein expression and localization was studied on a large TMA including normal (n = 26) and timorous colon tissues (n = 96). MACC1 staining was detectable in the cytoplasm of benign and malignant colon epithelial cells. In the normal colon tissues, MACC1 protein expression was less abundant (median TS = 1) than in the colon cancer cells (median TS = 4) (P = 0.038). In the cells of the colon cancer, MACC1 expression was present in different intensities and different cell distributions. Following the staging criteria of stain intensity, 5 cases were identified as completely score “0” (Figure 1A), 18 cases (18.8%) were identified as score “1” (Figure 1B), 34 cases (35.4%) were identified as score “2” (Figure 1C), and 39 cases (57.3%) were identified as score “3” (Figure 1D). We evaluated MACC1 protein expression in colon cancer cells by determining the TS, with scores of 1, 2, 3, 4, 5, 6. Among the 96 cases with colon cancer, 51 cases (53.1%) were identified as “positive” for IHC staining of MACC1 protein with TS 4-6 and 45 cases (46.9%) were negative with TS 0-3, respectively.
Figure 1.
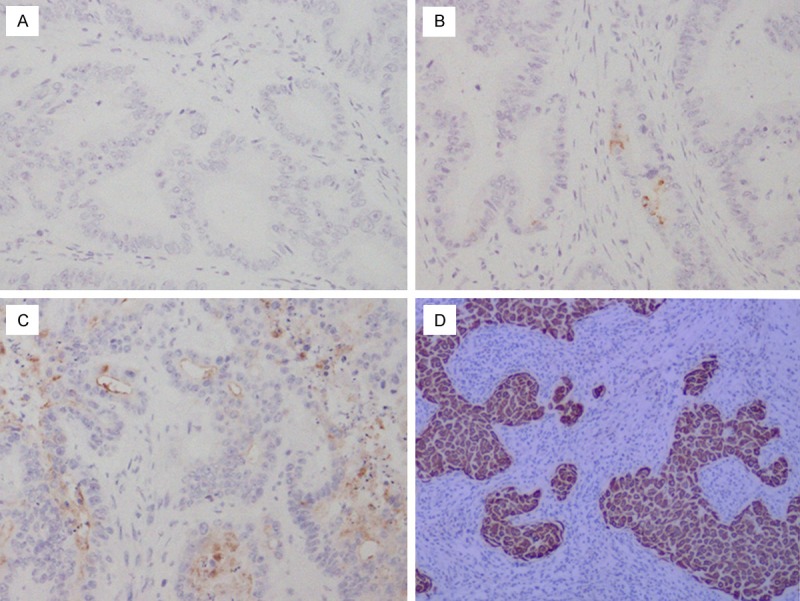
Immunohistochemical expression analysis of MACC1 protein in colon cancer tissues using a TMA (SP, 200 ×). A. Colon cancer tissue, MACC1 negative staining. B. Colon cancer tissue, MACC1 negative staining, the staining is weak. C. colon cancer tissue, MACC1 positive staning, the staining is intermediate the staining is moderate. D. colon cancer tissue, MACC1 positive, the staining is strong.
No significant correlation was found between the expression level of MACC1 and biological factors such as patients’ age (P = 0.638), gender (P = 0.230), tumor size (P = 0.680), tumor grade (P = 0.702). In contrast, statistical analyses indicated that MACC1 expression was positively related to lymph node status, T stages and the correlation was statistically significant (P = 0.001/0.006, respectively). The results of these analyses are summarized in Table 1.
Of the 96 patients with colon cancer, none was lost to follow-up. The median observation period was 38.4 months (ranged, 5.6-60.0 months), with 62 postoperative live metastases. The median postoperative live metastasis-free survival was 30.27 ± 8.99 months. To answer the question whether MACC1 overexpression might have an impact on patients’ clinical outcome, univariate survival probability curves were calculated based on the immunohistochemical results. Using Kaplan-Meier analysis we found that postoperative live metastasis-free survival in the group of negative MACC1 protein expression was significantly longer than that of positive expression (36.4 ± 2.85 vs. 28.6 ± 2.02 months, P = 0.014) (Figure 2). Multivariate analysis showed MACC1 protein expression status was evaluated as an independent prognostic factor for postoperative live metastasis-free survival (95% confidence interval [CI] = 1.32-3.38, P = 0.006, Table 2).
Figure 2.
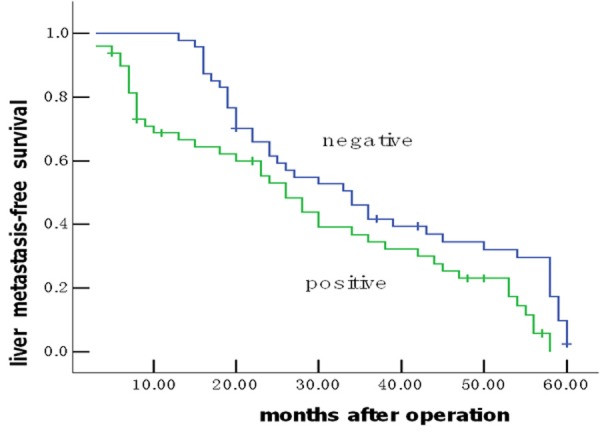
Colon cancer patients expressing positive MACC1 protein show unfavourable prognosis. Univariate Kaplan-Meier analysis was performed on the basis of MACC1 expression results derived from the TMA. Patients with negative MACC1 expression displayed longer liver metastasis-free survival estimation (blue graph) compared to patients with positive MACC1 expression (green graph) (P = 0.014).
Table 2.
Contributing factors for postoperative liver metastasis
| Variable | B | SE | Wald | P value |
|---|---|---|---|---|
| MACC1 expression | 1.583 | 0.480 | 0.242 | 0.006 |
| T stages | 1.550 | 0.436 | 0.208 | 0.010 |
| Lymph node status | 1.364 | 0.384 | 0.186 | 0.014 |
Discussion
MACC1 gene, which located on chromosome 7p21.1, was identified by a genome-wide screen of human colon cancer samples, and its expression was closely related to the metastasis of colon cancers [16]. There has been accumulating evidence showing that aberrant expression of MACC1 protein is associated with the development of some malignant tumors [22,23]. For example, overexpression of MACC1 were detected in ovarian cancer tissues and MACC1 knockdown by specific small hairpin RNA in OVCAR-3 cells resulted in significant inhibition of cell proliferation, migration and invasion, meanwhile obvious enhancement of apoptosis [24]. MACC1 expression in lung adenocarcinoma was significantly higher in patients with recurrence than those without recurrence [5]. MACC1 was more highly expressed in hepatocellular carcinoma (HCC) than in non-HCC tissues, its expression was associated with overall survival (OS) and disease-free survival (DFS). Moreover, stratified analysis showed that tumor-node-metastasis (TNM) stage I patients with high MACC1 levels had shorter OS and DFS than those with low MACC1 [6]. Expression of MACC1 in gastric carcinoma was significantly associated with larger tumor size, deeper tumor invasion, presence of lymph node metastasis, lymphatic involvement, venous invasion, distant metastasis and advanced clinical stage [25]. MACC1 was more frequently expressed in peritoneal-disseminated gastric carcinomas and might serve as a new parameter for the prognostic prediction of gastric cancer [10].
In the present study, we examined the MACC1 expression in paraffin-embedded tumor samples using the IHC method with the monoclonal antibody. Our results showed that MACC1 is in the cytoplasm of cells. Positive MACC1 expression in the colon cancer tissues was detected in 51 cases (53.1%) of the patients. Furthermore, MACC1 expression level is correlated with lymph node metastasis and T status, which suggests that MACC1 may have some correlation with worse biological behavior and clinical aggressiveness of colon cancer.
Cancer metastasis is a complex process involving many genes that function in the tumor cell and at the target organ. The molecular mechanism underlying liver metastasis in colon cancer is unclear. Traditional clinicopathological parameters including the depth of invasion, the presence of venous invasion, and lymph node metastasis, have limited prognostic values [26]. Other variables, such as CD10, CD44, VEGF, TGF-a, P-cadherin and the density of macrophages in the invasive front have been shown to be correlated with liver metastasis, however the predictive efficacy of these factors remains uncertain [18,27,28,29]. In the present study, postoperative live metastasis-free survival in the group of negative MACC1 expression was significantly longer than that of positive expression. Furthermore, result of multivariate analysis demonstrated that MACC1 expression was an independent prognostic factor for postoperative live metastasis-free survival. In previous studies, Shirahata A et al reported MACC1mRNA expression in advanced colorectal carcinoma showed significant correlation with peritoneal dissemination and higher stage of TNM classification [14]. Kawamura M et al demonstrated high expression of MACC1 in rectal cancer patients treated with chemoradiotherapy followed by surgery was associated with reduced relapse-free survival and the prognosis was worse when MACC1 was highly expressed [2]. Isella C et al found MACC1 mRNA levels were an independent prognostic factor of recurrence after liver resection of colorectal cancer metastasis [30]. To our knowledge, this is the first report of the relationship between expression of MACC1 protein and postoperative live metastasis of colon cancer. These findings suggested that the aberrant expression of MACC1 might contribute to the development of colon cancer and postoperative liver metastasis.
In summary, MACC1 expression in colon cancer was significantly higher in tumor cells than in normal ones. Positive MACC1 protein expression in colon cancer is significantly correlated with lymph node metastasis, T stage, and shorter postoperative liver metastasis-free survival. The results provide evidence that positive MACC1 protein expression may be important in the acquisition of a more invasive phenotype. Furthermore, the expression of MACC1 protein, as detected by IHC, may be useful as a prognostic biomarker for the poor liver metastasis-free survival of colon cancer patients.
Acknowledgements
This study was supported by (a) Shandong Province Natural Science Foundation (Grant NO. ZR2012HM044) and (b) Xinjiang Uygur Autonomous Region Natural Science Foundation (Grant NO. 201233146-13).
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Kawamura M, Saigusa S, Toiyama Y, Tanaka K, Okugawa Y, Hiro J, Uchida K, Mohri Y, Inoue Y, Kusunoki M. Correlation of MACC1 and MET expression in rectal cancer after neoadjuvant chemoradiotherapy. Anticancer Res. 2012;32:1527–31. [PubMed] [Google Scholar]
- 3.Fidler IJ. Critical factors in the biology of human cancer metastasis: twenty-eight GHA Clowes Memorial Award Lecture. Cancer Res. 1990;50:6130–8. [PubMed] [Google Scholar]
- 4.Mayo SC, Pawlik TM. Current management of colorectal hepatic metastasis. Expert Rev Gastroenterol Hepatol. 2009;3:131–44. doi: 10.1586/egh.09.8. [DOI] [PubMed] [Google Scholar]
- 5.Shirahata A, Fan W, Sakuraba K, Yokomizo K, Goto T, Mizukami H, Saito M, Ishibashi K, Kigawa G, Nemoto H, Sanada Y, Hibi K. MACC 1 as a marker for vascular invasive hepatocellular carcinoma. Anticancer Res. 2011;31:777–80. [PubMed] [Google Scholar]
- 6.Qiu J, Huang P, Liu Q, Hong J, Li B, Lu C, Wang L, Wang J, Yuan Y. Identification of MACC1 as a novel prognostic marker in hepatocellular carcinoma. J Transl Med. 2011;29:166–76. doi: 10.1186/1479-5876-9-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chundong G, Uramoto H, Onitsuka T, Shimokawa H, Iwanami T, Nakagawa M, Oyama T, Tanaka F. Molecular diagnosis of MACC1 status in lung adenocarcinoma by immunohistochemical analysis. Anticancer Res. 2011;31:1141–5. [PubMed] [Google Scholar]
- 8.Shimokawa H, Uramoto H, Onitsuka T, Chundong G, Hanagiri T, Oyama T, Yasumoto K. Overexpression of MACC1-mRNA in lung adenocarcinoma is associated with postoperative recurrence. J Thorac Cardiovasc Surg. 2011;141:895–8. doi: 10.1016/j.jtcvs.2010.09.044. [DOI] [PubMed] [Google Scholar]
- 9.Hu X, Fu X, Wen S, Zou X, Liu Y. Prognostic value of MACC1 and c-met expressions in non-small cell lung cancer. Zhongguo Fei Ai Za Zhi. 2012;15:399–403. doi: 10.3779/j.issn.1009-3419.2012.07.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shirahata A, Sakata M, Kitamura Y, Sakuraba K, Yokomizo K, Goto T, Mizukami H, Saito M, Ishibashi K, Kigawa G, Nemoto H, Hibi K. MACC 1 as a marker for peritoneal-disseminated gastric carcinoma. Anticancer Res. 2010;30:3441–4. [PubMed] [Google Scholar]
- 11.Wang L, Wu Y, Lin L, Liu P, Huang H, Liao W, Zheng D, Zuo Q, Sun L, Huang N, Shi M, Liao Y, Liao W. Metastasis-associated in colon cancer-1 upregulation predicts a poor prognosis of gastric cancer, and promotes tumor cell proliferation and invasion. Int J Cancer. 2013;133:1419–30. doi: 10.1002/ijc.28140. [DOI] [PubMed] [Google Scholar]
- 12.Huang Y, Zhang H, Cai J, Fang L, Wu J, Ye C, Zhu X, Li M. Overexpression of MACC1 and its significance in human breast cancer progression. Cell Biosci. 2013;3:16. doi: 10.1186/2045-3701-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu M, Xu Y, Mao X, Gao Y, Shao L, Yan F. Overexpression of metastasis-associated in colon cancer-1 associated with poor prognosis in patients with esophageal cancer. Pathol Oncol Res. 2013;19:749–53. doi: 10.1007/s12253-013-9638-9. [DOI] [PubMed] [Google Scholar]
- 14.Shirahata A, Shinmura K, Kitamura Y, Sakuraba K, Yokomizo K, Goto T, Mizukami H, Saito M, Ishibashi K, Kigawa G, Nemoto H, Hibi K. MACC1 as a marker for advanced colorectal carcinoma. Anticancer Res. 2010;30:2689–92. [PubMed] [Google Scholar]
- 15.Arlta F, Stein U. Colon cancer metastasis: MACC1 and Met as metastatic pacemakers. Int J Biochem Cell Biol. 2009;41:2356–59. doi: 10.1016/j.biocel.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Stein U, Walther W, Arlt F, Schwabe H, Smith J, Fichtner I, Birchmeier W, Schlag PM. MACC1, a newly identified key regulator of HGF-MET signaling, predicts colon cancer metastasis. Nat Med. 2009;15:59–67. doi: 10.1038/nm.1889. [DOI] [PubMed] [Google Scholar]
- 17.Kokoszyńska K, Kryński J, Rychlewski L, Wyrwicz LS. Unexpected domain composition of MACC1 links MET signaling and apoptosis. Acta Biochim Pol. 2009;56:317–23. [PubMed] [Google Scholar]
- 18.Ding YL, Zhou Y, Xiang L, Ji ZP, Luo ZH. Expression of glioma-associated oncogene homolog 1 is associated with invasion and postoperative liver metastasis in colon cancer. Int J Med Sci. 2012;9:334–8. doi: 10.7150/ijms.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ding YL, Wang QS, Zhao WM, Xiang L. Expression of smoothened protein in colon cancer and its prognostic value for postoperative liver metastasis. Asian Pac J Cancer Prev. 2012;13:4001–5. doi: 10.7314/apjcp.2012.13.8.4001. [DOI] [PubMed] [Google Scholar]
- 20.Tan S, Zhang L, Zhong XM, Yang ZL, Zhao LY, Gao YJ, Shao HY, Qin FX, Chen XC, Zhang HJ, Chen H, Wang L. Monoclonal Antibodies against Nucleophosmin Mutants: Potentials for the Detection of Acute Myeloid Leukemia. Int J Med Sci. 2011;8:309–14. doi: 10.7150/ijms.8.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Onitsuka T, Uramoto H, Ono K, Takenoyama M, Hanagiri T, Oyama T, Izumi H, Kohno K, Yasumoto K. Comprehensive molecular analyses of lung adenocarcinoma with regard to the epidermal growth factor receptor, K-ras, MET, and hepatocyte growth factor status. J Thorac Oncol. 2010;5:591–96. doi: 10.1097/JTO.0b013e3181d0a4db. [DOI] [PubMed] [Google Scholar]
- 22.Stein U. MACC1-a novel target for solid cancers. Expert Opin Ther Targets. 2013;17:1039–52. doi: 10.1517/14728222.2013.815727. [DOI] [PubMed] [Google Scholar]
- 23.Juneja M, Ilm K, Schlag PM, Stein U. Promoter identification and transcriptional regulation of the metastasis gene MACC1 in colorectal cancer. Mol Oncol. 2013;7:929–43. doi: 10.1016/j.molonc.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang R, Shi H, Chen Z. Effects of metastasis-associated in colon cancer 1 inhibition by small hairpin RNA on ovarian carcinoma OVCAR-3 cells. J Exp Clin Cancer Res. 2011;30:83. doi: 10.1186/1756-9966-30-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma J, Ma J, Meng Q, Zhao ZS, Xu WJ. Prognostic value and clinical pathology of mACC-1 and c-MET expression in gastric carcinoma. Pathol Oncol Res. 2013;19:821–32. doi: 10.1007/s12253-013-9650-0. [DOI] [PubMed] [Google Scholar]
- 26.Bird NC, Mangnall D, Majeed AW. Biology of colorectal liver metastases: A review. J Surg Oncol. 2006;94:68–80. doi: 10.1002/jso.20558. [DOI] [PubMed] [Google Scholar]
- 27.Zhou Q, Peng RQ, Wu XJ, Xia Q, Hou JH, Ding Y, Zhou QM, Zhang X, Pang ZZ, Wan DS, Zeng YX, Zhang XS. The density of macrophages in the invasive front is inversely correlated to liver metastasis in colon cancer. J Transl Med. 2010;8:13. doi: 10.1186/1479-5876-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun L, Hu H, Peng L, Zhou Z, Zhao X, Pan J, Sun L, Yang Z, Ran Y. P-cadherin promotes liver metastasis and is associated with poor prognosis in colon cancer. Am J Pathol. 2011;179:380–90. doi: 10.1016/j.ajpath.2011.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zlobec I. Novel biomarkers for the prediction of metastasis in colorectal cancer. Expert Opin Med Diagn. 2013;7:137–46. doi: 10.1517/17530059.2013.753054. [DOI] [PubMed] [Google Scholar]
- 30.Isella C, Mellano A, Galimi F, Petti C, Capussotti L, De Simone M, Bertotti A, Medico E, Muratore A. MACC1 mRNA levels predict cancer recurrence after resection ofcolorectal cancer liver metastases. Ann Surg. 2013;257:1089–95. doi: 10.1097/SLA.0b013e31828f96bc. [DOI] [PubMed] [Google Scholar]


