Abstract
Objective: The aim of this study was to identify the influencing factors related to outcome of patients with hilar cholangiocarcinoma. Methods: From January 1999 to January 2009, 204 cases of hilar cholangiocarcinoma undergoing surgery were analyzed retrospectively. Bismuth-Corlette classification showed type I in 18 patients, type II in 40, type IIIa in 65, type IIIb in 54, type IV in 27. Survival analysis was performed by the Kaplan-Meier method and the relationship between each of the clinicopathologic variables and survival was assessed by the log-rank test. Multivatiate results were confirmed using Cox regression. Results: Radical resection was accomplished in 161 of 204 patients (78.9%). Radical resection offered the best chance of long-term survival, with the 1-, 3-, and 5-year survival rate were 62.6%, 42.4%, 23.7%, respectively. Univariate analysis showed that lymph node metastasis, surgical margin, operative procedure and tumor differentiation were prognostic impacts. The difference was statistically significant (P < 0.05). Cox multivariate analysis showed that lymph node metastasis and surgical margin are two separate prognostic factors. Conclusion: Racical resection is the key to improve the long-term survival rate of hilar cholangiocarcinoma and a favorable outcome after resection is mainly determined by curative resection and the absence of lymph node metastasis.
Keywords: Cholangiocarcinoma, survival, prognosis
Introduction
Klatskin tumor (or hilar cholangiocarcinoma) is a cholangiocarcinoma which occurs at the confluence of the right and left hepatic bile ducts. It was named after Dr. Gerald Klatskin. Because of their location, these tumors tend to become symptomatic late in their development and therefore are not usually resectable at the time of presentation. This is variable as, due to obstruction, jaundice may present early and compel the patient to seek help. Complete resection of the tumor offers hope of long-term survival, and of late there has been renewed interest in liver transplantation from deceased donors along with adjuvant therapy. Prognosis has been improved with advancement of preoperative diagnostic techniques and surgical techniques. The aim of this retrospective study was to indentify useful prognostic factors for patients with hilar cholangiocarcinnoma.
Patients and methods
Clinical data of patients
From January 1999 to January 2009 in the Liaoning Tumor Hospital, Shen Zhou Hospital, Feng Tian Hospital and the First Hospital of China Medical University, a total of 204 patients were admitted to our study.
They were 122 men and 82 women, aged from 25 to 76 years (mean 55.6 years). No severe diseases were encountered in their circulatory, respiratory and urinary systems. The clinical symptoms were jaundice (89.3%), pruritus (53.8%) and abdominal pain (36.1%). The diagnosis was established by ultrasonography, computed tomography, magnetic resonance cholangiopancreatography, and endoscopic pathologic appearances at surgery.
Tumor localization was classified according to Bismuth and Corlette. The tumor was localized above the cystic duct and below the hepatic bifurcation (type I) in 18 (8.8%) and at the hepatic junction (type II) in 40 (19.6%) cases. 119 cases (58.3%) were type III. In 65 of these, tumor extension involved the right hepatic duct (type IIIa) and in 54 it involved the left hepatic duct (type IIIb). There were 27 (13.2%) type IV lesions with infiltration into both the left and right hepatic ducts.
Data for these patients were extracted from the hospital database and interviews, including gender, age, surgical margin, lymph node metastasis, operative procedure, CA 19-9 level, CEA (carcinoembryonic antigen) level, tumor differentiation, total bilirubin, adjuvant chemotherapy, tumor diameter, combine bile duct stone or not and Bismuth-Corlette classification. These data were obtained form a retrospective review of medical records. Complete information about the survival status could be obtained for all patients.
Statistical analysis
Survival time was calculated from the date of surgery to death or censored date. Patients who died of hilar cholangiocarcinoma were treated as event observations, and patients who died of unrelated causes and were alive at the last follow-up were treated as censored observations. Survival curves were constructed using the Kaplan-Meier method and compared using the log-rank test. Only the variables that were statistically significant by univariate analysis were included in a multivariate analysis to establish a hierarchy among the various prognostic factors. Multivariate results were confirmed using Cox proportional hazards regression. The stability of the model was certified by using a likelihood ratio step-forward and step-backward fitting procedure. The level of significance was taken from the last step of the regression analysis. A P value ≤ 0.05 was considered to be statistically significant.
Results
No patient died in the hospital. Radical resection offered the best chance of long-term survival, with the 1-, 3-, and 5-year survival rate were 62.6%, 42.4%, 23.7%, respectively. This contrasts to 1-, 3-, and 5-year survival rate were 51.3%, 22.4%, 4.5%, respectively, in patients with palliative resection.
Tumor removal was accomplished by resection of the bile duct bifurcation (group I) in 53 patients. A combination of both excision of the bile duct bifurcation and hepatic resection (group II) was performed in 87 patients. Combined bile duct, hepatic and vascular resection (group III) was performed in 64 patients.
Two of these patients underwent resection and reconstruction of the portal vein n combination with bile duct excision without liver resection. Hepatic resection in groups II and III consisted of anatomic (n = 50) and extended (n = 22) left hemihepatectomy, as well as anatomic (n = 31) and extended (n = 48) right hemihepatectomy. In five patients, resection of the liver was restricted to the central segment IVb and V. The caudate lobe was removed in79 of 204 (38.7%) cases of lover resection. Vascular extensions consisted of solitary resection and reconstruction of the portal vein bifurcation in 52 cases, combined resection of portal vein and right hepatic artery in two, combined resection of portal vein and retrohepatic caval vein in one, and isolated resection of the caval vein in two. In all cases, an extensive lymphadenectomy was performed in the hepatoduodenal ligament and along the common hepatic artery. The bilioenteric continuity was reestablished using a Rous-en-Y loop of jejunum.
R0 resection was accomplished in 161 of 204 patients (78.9%). The rate of curative resections did not differ after solitary resection of the bile duct bifurcation (group I, 76.4%), combined excision of bile duct bifurcation and hepatic resection (group II, 80.2%), or combined bile duct, hepatic, and vascular resection (group III, 75.6%). In 18.6% of patients (group I, n = 7; group II, n = 21; group III, n = 10), there was microscopic tumor infiltration at the resection margins (R1). Tumor infiltration affected the proximal resection line of the bile ducts in 29 patients, while the distal resection line was infiltrated in only 6 patients. Other 3 patients, residual tumor was found histologically at the hepatic resection margin. Macroscopic residual tumor (R2) was left behind in 2.5% of patients (group I, n = 2; group II, n = 0; group III, n = 3).
Univariate analysis revealed that lymph node metastasis, surgical margin, operative procedure and tumor differentiation showed significant prognostic value for survival (Table 1).
Table 1.
Univariate analysis of clinicopathologic variables
| Variable | No. of patients | Median survival (month) | X2 | P value |
|---|---|---|---|---|
| Gender | ||||
| Male | 122 | 23.5 | ||
| Female | 82 | 24.0 | 0.67 | 0.77 |
| Age | ||||
| < 60 | 52 | 26.1 | ||
| ≥ 60 | 152 | 22.4 | 1.14 | 0.19 |
| Surgical margin | ||||
| Negative | 153 | 26.0 | ||
| Positive | 51 | 15.1 | 4.31 | 0.03 |
| lymph node metastasis | ||||
| Negative | 75 | 24.2 | ||
| Positive | 129 | 21.2 | 5.44 | 0.02 |
| Operative procedure | ||||
| Radical | 55 | 24.7 | ||
| Palliative | 149 | 10.5 | 5.57 | 0.02 |
| CA 19-9 level | ||||
| < 37 ku/ml | 123 | 24.1 | ||
| ≥ 37 ku/m l | 81 | 23.8 | 1.634 | 0.39 |
| CEA level | ||||
| < 15 ng/ml | 68 | 22.2 | ||
| ≥ 15 ng/ml | 136 | 24.7 | 2.24 | 0.11 |
| Tumor differentiation | ||||
| Well | 112 | 26.1 | ||
| Moderate | 23 | 22.1 | ||
| Poorly | 69 | 15.6 | 13.74 | < 0.01 |
| Total bilirubin | ||||
| < 342 µmol/L | 89 | 31.2 | ||
| ≥ 342 µmol/L | 115 | 24.6 | 3.48 | 0.06 |
| Adjuvant chemotherapy | ||||
| Yes | 26 | 24.4 | ||
| No | 178 | 22.0 | 2.97 | 0.07 |
| Tumor diameter | ||||
| < 3 cm | 74 | 26.1 | ||
| 3 cm-5 cm | 77 | 23.6 | ||
| ≥ 5 cm | 53 | 19.1 | 2.54 | 0.06 |
| Combined bile duct stone | ||||
| Yes | 87 | 23.5 | ||
| No | 117 | 24.1 | 1.23 | 0.01 |
| Bismuth-Corlette classification | ||||
| I | 18 | 27.1 | ||
| II | 40 | 22.5 | ||
| IIIa | 65 | 23.1 | ||
| IIIb | 54 | 25.0 | ||
| IV | 27 | 12.0 | 2.50 | 0.06 |
The prognostic factors in the univariate analysis were entered into a multivariate model to identify independent predictors of long-term survival. Among the four significant variables, surgical margin and lymph node metastasis were identified as independent prognostic factors. Of the two, lymph node metastasis denoted an increase in the likelihood of death of 1.377 times if the patient had lymph node metastasis. And surgical margin as a favorable factor (relative risk, 0.543) (Table 2; Figures 1, 2).
Table 2.
Multivariate analysis of clinicopathologic variables
| Variable | β | Wald X2 | P value | Relative risk (RR) | RR (95% CI) | |
|---|---|---|---|---|---|---|
|
| ||||||
| Lower | Upper | |||||
| Surgical margin | -0.713 | 7.261 | 0.008 | 0.543 | 0.348 | 0.847 |
| Lymph node metastasis | 0.320 | 7.123 | 0.008 | 1.377 | 1.089 | 1.741 |
Figure 1.
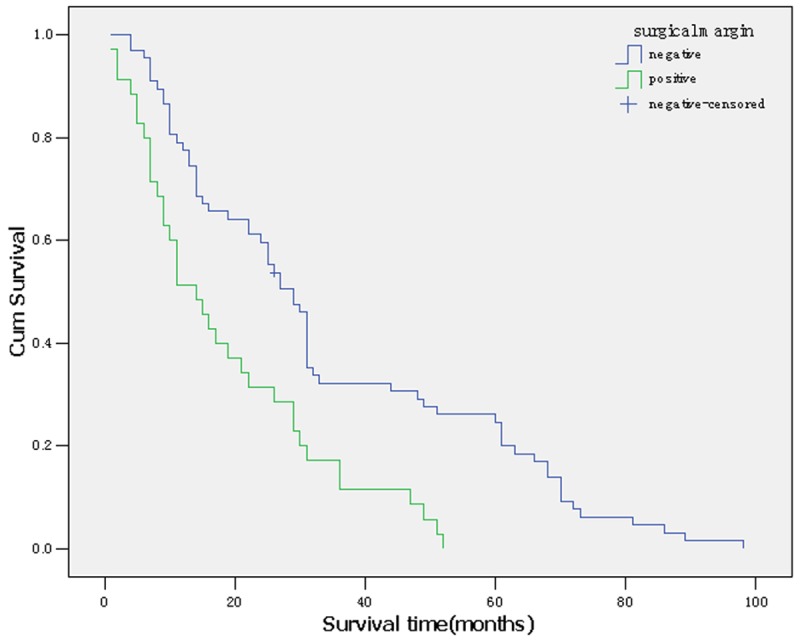
Survival curve according to surgical margin.
Figure 2.
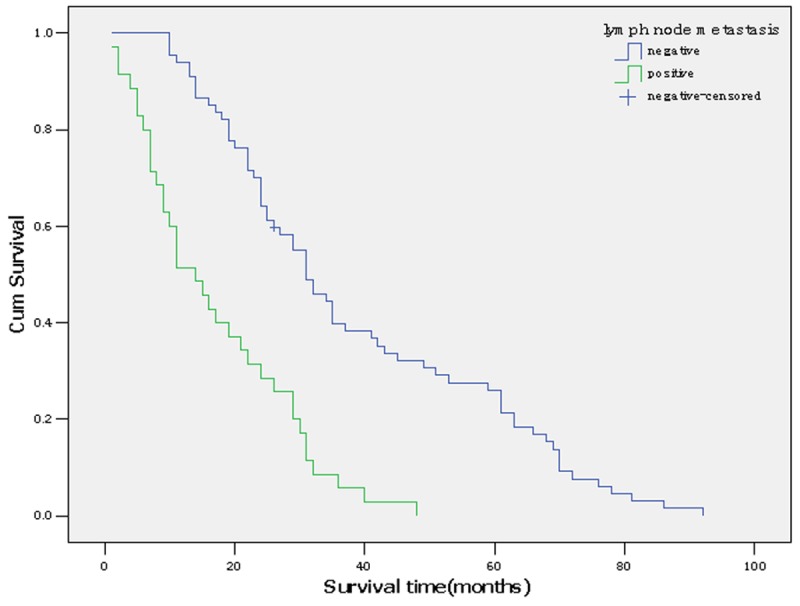
Survival curve according to lymph node metastasis.
Discussion
Cholangiocarcinoma is a rare malignant tumor of the biliary system with a poor prognosis. It is a second most common malignancy of primary liver tumors worldwide [1]. Cholangiocarcinoma is commonly classified into 3 groups based on the location of the tumor: intrahepatic, hilar and distal types. Hilar cholangiocarcinoma or Klatskin tumor arising from the upper one third of the main bile duct to transverse hilar fissure has low rates of radical resection and poor long-term survival. In recent years, its prognosis has been improved with advancement of preoperative diagnostic techniques and surgical techniques.
Radical resection was found to be the most important measure for a cure and long-term survival. In contrast to palliative bypass procedures, radical resection does not only restore bile flow, but removes the tumor. To achieve R0 resection, we removed entire tumor including the suprapancreatic extrahepatic biliary tract, gallbladder, and cystic duct, together with clearance of the suprapancreatic tissues and related lymph nodes. We proposed that the bile duct be transected 5 mm above the tumor, or 10 mm above if possible. Skeletonization of the hepatoduodenal ligament should be done. Partial hepatectomy is dependent on Bismuth-Corlette type of the tumor. Without doubt, the more radical operations are associated with a higher operative mortality rate. Surgical morbidity and mortality after resection have to be carefully balanced against the risks and results of primary palliative biliary drainage. The feasibility of extensive liver resection depends on the volume and the regenerative potential of the remaining liver. Especially in the group of patients with combined hepatic and vascular resection, it is due to surgical complications, such as portal vein thrombosis.
The role of liver transplantation in the treatment of hilar cholangiocarcinoma is highly controversial. Most investigators do not envisage an indication for liver transplantation in view of the high rate of tumor recurrence and the shortage of donor organs. It requires necessary skills and techniques, clearance of lymph nodes perioperative management. The high risks of postoperative lymph node metastasis and tumor recurrence must be taken into consideration [2,3].
Surgical margin status is a prognostic factor in several cancers. In this retrospective cohort study, the results of multiple factors analysis showed that surgical margin status was an independent prognostic factor by multivariate analysis. Patients with negative surgical margin had long term survival than those with positive margin. Median survivals of patients who had negative resection margin (R0) were markedly longer than those who had macroscopic positive margin (R2) and microscopic positive margin (R1). Accurate evaluation of intraductal longitudinal spread by precise cholangiography, choice of appropriate hepatectomy, and hepatic ductal division at the separating limits from the vasculature are mandatory to ensure negative ductal margins [4]. In cases of portal vein resection, the preceding portal reconstruction enabled ductal division at the separating limits from the vasculature by full mobilization of the remnant portal branch, as in a conventional hepatectomy without portal vein resection [5,6]. We do not routinely use frozen sections to assess the remnant ductal stumps because the determination, whether positive or negative, is sometimes difficult even in permanent section. Furthermore, even if the remnant is positive, additional ductal resection at the separating limits is not feasible.
Recent researches have reported rates for lymph node metastasis of 24-47% for hilar cholangiocarcinoma [7,8]. In our study, survival was compromised by the presence of lymph node metastasis as demonstrated by both univariate and multivariate analysis, with an increase in the likelihood of mortality risk of 1.377 times. Other previous studies also got the lymph node metastasis was an independent prognostic factor for hilar cholangiocarcinoma [8]. In our study, three patients with nodal involvement have survived for more than 5 years. Maybe the performance of lymph node dissection during our resections contributed to locoregional control.
Adjuvant chemotherapy and radiation is a controversial issue in cholangiocarcinoma. Our study did not show any impact of adjuvant chemotherapy, maybe because of the small number of treated patients. Takada et al. [9] compared therapy with mitomycin C and 5-FU to surgery alone in a randomized controlled trial of patients who underwent radical resection of cholangiocarcinoma. They reported that the 5-year survival rates for patients with hilar or distal cholangiocarcinoma did not differ based on postoperative chemotherapy or surgery alone. But many previous retrospective studies showed benefits of adjuvant chemotherapy [10,11]. Gerhards et al. [12] reported that in 91 patients who underwent surgical resection of hilar cholangiocarcinoma, overall median survival was significantly longer in patients treated with adjuvant radiotherapy than in those who underwent resection alone. Hughes et al. [13] reported that 68 patients with distal cholangiocarcinoma found that patients who underwent surgery and received chemoradiation had significantly longer actuarial mean survival compared with those who underwent surgery alone. Furthermore, a meta-analysis showed that chemotherapy as a part of adjuvant therapy which included radiotherapy and concurrent chemoradiotherapy may be beneficial in resectable cholangiocarcinoma patients with high risk features, such as lymph node metastases and positive surgical margins [14]. Some new anticancer drugs including gemcitabine, oxaliplatin, capecitabine, and S-1 have been reported recently to have favorable anticancer effects on patients with unresectable biliary tract carcinoma [15-17]. So randomized controlled trials should be conducted to define the role of postoperative adjuvant chemotherapy and radiotherapy.
In contrast to previous researches, our study did not find a survival disadvantage in case of perineural tumor infiltration. The finding that the long-term prognosis after resection was independent of histologically proven vascular tumor infiltration has an important impact on the surgeon’s decision as to whether a locally invasive hilar cholangiocarcinoma should be resected. Furthermore, it is our experience that whenever tumor-infiltrated vascular segments in the hepatoduodenal ligament can be resected without complications, the patients had no impaired long-term survival. This is an important argument to continue our surgical approach using combined vascular and hepatic resection.
In conclusion, factors such were statistical significantly associated with the survival time of hilar cholangiocarcinoma patients. However, the limitations of this study are retrospective design ad the relatively small number of patients studied. Prospective studies enrolling a larger number of patients are required to confirm the results of this study.
Disclosure of conflict of interest
None.
References
- 1.Olnes MJ, Erlich R. A review and update on cholangiocarcinoma. Oncology. 2004;66:167–179. doi: 10.1159/000077991. [DOI] [PubMed] [Google Scholar]
- 2.Pichlmayr R, Weimann A, Oldhafer KJ, Schlitt HJ, Klempnauer J, Bornscheuer A, Chavan A, Schmoll E, Lang H, Tusch G, et al. Role of lover transplantation in the treatment of unresectable lover cancer. World J Surg. 1995;19:807–813. doi: 10.1007/BF00299775. [DOI] [PubMed] [Google Scholar]
- 3.Partesky C. Treatment of hilar cancers. J Chir (Paris) 1998;135:162–167. [PubMed] [Google Scholar]
- 4.Nimura Y, Kamiya J, kondo S, Nagino M, Uesaka K, Oda K, Sano T, Yamamoto H, Hayakawa N. Aggressive preoperative management and extended surgery for hilar cholangiocarcinoma: Nagoya experience. J Hepatobiliary Pancreat Surg. 2000;7:155–162. doi: 10.1007/s005340050170. [DOI] [PubMed] [Google Scholar]
- 5.Kondo S, Katoh H, Hirano S, Ambo Y, Tanaka E, Okushiba S. Portal vein resection and reconstruction prior to hepatic dissection during right hepatectomy and caudate lobectomy for hepatobiliary cancer. Br J Surg. 2003;90:694–697. doi: 10.1002/bjs.4084. [DOI] [PubMed] [Google Scholar]
- 6.Kondo S, Katoh H, Hirano S, Ambo Y, Tanaka E, Kawarada Y, Kaji M, Ohtake S, Okushiba S, Morikawa T. Wedge resection of the portal bifurcation concomitant with left hepatectomy plus biliary reconstruction for hepatobiliary cancer. J Hepatobiliary Pancreat Surg. 2002;9:603–606. doi: 10.1007/s005340200081. [DOI] [PubMed] [Google Scholar]
- 7.Hirano S, Kondo S, Tanaka E, Shichinohe T, Tsuchikawa T, Kato K, Matsumoto J, Kawasaki R. Outcome of surgical treatment of hilar cholangiocarcinoma: a special reference to postoperative morbidity and mortality. J Hepatobiliary Pancreat Sci. 2010;17:455–62. doi: 10.1007/s00534-009-0208-1. [DOI] [PubMed] [Google Scholar]
- 8.Lee SG, Song GW, Hwang S, Ha TY, Moon DB, Jung DH, Kim KH, Ahn CS, Kim MH, Lee SK, Sung KB, Ko GY. Surgical treatment of hilar cholangiocarcinoma in the new era: the Asian experience. J Hepatobiliary Pancreat Sci. 2010;17:476–89. doi: 10.1007/s00534-009-0204-5. [DOI] [PubMed] [Google Scholar]
- 9.Takada T, Amano H, Yasuda H, Nimura Y, Matsushiro T, Kato H, Nagakawa T, Nakayama T Study Group of Surgical Adjuvant Therapy for Carcinomas of the Pancreas and Biliary Tract. Is postoperative adjuvant chemotherapy useful for gallbladder carcinoma? A phase III multicenter prospective randomized controlled trial in patients with resected pancreaticobiliary carcinoma. Cancer. 2002;95:1685–95. doi: 10.1002/cncr.10831. [DOI] [PubMed] [Google Scholar]
- 10.Murakami Y, Uemura K, Sudo T, Hashimoto Y, Nakashima A, Kondo N, Sakabe R, Ohge H, Sueda T. Prognostic factors after surgical resection for intrahepatic, hilar, and distal cholangiocarcinoma. Ann Surg Oncol. 2011;18:651–658. doi: 10.1245/s10434-010-1325-4. [DOI] [PubMed] [Google Scholar]
- 11.Murakami Y, Uemura K, Sudo T, Hayashidani Y, Hashimoto Y, Nakamura H, Nakashima A, Sueda T. Adjuvant gemcitabine plus S-1 chemotherapy improves survival after aggressive surgical resection for advanced biliary carcinoma. Ann Surg. 2009;250:950–956. doi: 10.1097/sla.0b013e3181b0fc8b. [DOI] [PubMed] [Google Scholar]
- 12.Gerhards MF, van Gulik TM, González González D, Rauws EA, Gouma DJ. Results of postoperative radiotherapy for resectable hilar cholangiocarcinoma. World J Surg. 2003;27:173–9. doi: 10.1007/s00268-002-6434-1. [DOI] [PubMed] [Google Scholar]
- 13.Hughes MA, Frassica DA, Yeo CJ, Riall TS, Lillemoe KD, Cameron JL, Donehower RC, Laheru DA, Hruban RH, Abrams RA. Adjuvant concurrent chemoradiation for adenocarcinoma of the distal common bile duct. Int J Radiat Oncol Biol Phys. 2007;68:178–82. doi: 10.1016/j.ijrobp.2006.11.048. [DOI] [PubMed] [Google Scholar]
- 14.Horgan AM, Amir E, Walter T, Knox JJ. Adjuvant therapy in the treatment of biliary tract cancer: a systematic review nd meta-analysis. J. Clin. Oncol. 2012;30:1934–1940. doi: 10.1200/JCO.2011.40.5381. [DOI] [PubMed] [Google Scholar]
- 15.André T, Tournigand C, Rosmorduc O, Provent S, Maindrault-Goebel F, Avenin D, Selle F, Paye F, Hannoun L, Houry S, Gayet B, Lotz JP, de Gramont A, Louvet C GERCOR Group. Gemcitabine combined with oxaliplatin (GEMOX) in advanced biliary tract adenocarcinoma: a GERCOR study. Ann Oncol. 2004;15:1339–43. doi: 10.1093/annonc/mdh351. [DOI] [PubMed] [Google Scholar]
- 16.Patt YZ, Hassan MM, Aguayo A, Nooka AK, Lozano RD, Curley SA, Vauthey JN, Ellis LM, Schnirer II, Wolff RA, Charnsangavej C, Brown TD. Oral capecitabine for the treatment of hepatocellular carcinoma, cholangiocarcinoma, and gallbladder carcinoma. Cancer. 2004;101:578–86. doi: 10.1002/cncr.20368. [DOI] [PubMed] [Google Scholar]
- 17.Ueno H, Okusaka T, Ikeda M, Takezako Y, Morizane C. Phase II study of S-1 in patients with advanced biliary tract cancer. Br J Cancer. 2004;91:1769–74. doi: 10.1038/sj.bjc.6602208. [DOI] [PMC free article] [PubMed] [Google Scholar]


