Abstract
Objective: The effect of transcutaneous electrical nerve stimulation (TENS) on immuno-inflammatory response was tested and the differences between electroacupuncture (EA) and TENS in immuno-inflammatory response in patients undergoing supratentorial craniotomy were explored. Methods: 51 patients received craniotomy were randomly divided into 3 groups: control (group C, n=18), EA (group A, n=19) and TENS (group T, n=14) groups. Blood samples were collected before anesthesia (T0) and 30 min (T1), 2 h (T2) and 4 h (T3) after induction of anesthesia to measure the levels of tumor necrosis factor-α (TNF-α), interleukin (IL)-8, IL-10, IgM, IgA and IgG. Results: No significant difference existed between group A and group T during craniotomy. IgM and IgA decreased significantly in group C compared with groups A and T at T2 and T3 time points. Compared with group C, there were significant differences in TNF-α, IgM and IgA levels at T0 in groups A and T; no significant difference was found in the levels of IgG, IL-10 and IL-8. Conclusion: EA and TENS could reduce immunosuppression in patients undergoing supratentorial craniotomy and it has significance in choice of treatment in immunosuppressive therapy.
Keywords: Transcutaneous electrical nerve stimulation (TENS), electroacupuncture (EA), immuno-inflammatory, supratentorial craniotomy
Introduction
Postoperative infections are common and potential fatal complications in neurosurgical intensive care medicine. An impairment of immune function after central nervous system surgery is associated with higher risk of infection and postoperative complications [1]. A major component of the biological response to craniotomy is induction of inflammation. This type of immune response is driven by the release of intracellular antigens which are normally hidden from leukocytes [2]. Noxious stimulus during neurosurgical procedures such as insertion of cranial pins and scalp incision could result in sudden increases in blood pressure and heart rate [3]. In addition, many neuro-hormonal responses may lead to following tissue injuries and then release some cytokines.
As one of the therapeutic measure in traditional chinese medicine (TCM), acupuncture has been practiced in clinics for thousands of years, and it has been found to have a bidirectional neuron-endocrine-immune system regulating effect; acupuncture antagonizes systemic inflammatory response with no side effects. Early studies showed that pretreatment of electroacupuncture at the Zusanli (ST36) and Neiguan (PC6) acupoints attenuated the LPS-induced inflammatory response and mitigated acute kidney injury [4]. Our previous study showed that EA can attenuate immunosuppression in patients undergoing supratentorial craniotomy [5].
Transcutaneous electrical nerve stimulation (TENS), a non-pharmacological treatment for pain relief, has been used to alleviate a variety of painful conditions [6]. It was reported could increase local blood flow in skin, and alleviate mucous membrane damage by activating neural fibers and regulating vascular resistance [7]. Previous clinical studies have demonstrated a positive effect of TENS analgesia in patients with osteoarthritis pain, low back pain and postoperative pain [8-10]. TENS also demonstrated significant reduction of pain sensitivity in rat [11,12]. TENS was also useful to improve manipulation capability of stroke patients with sensory loss [13].
In this study, we tested the levels of TNF-α, IL-8, IL-10, IgM, IgA and IgG in patients received EA and TENS who underwent supratentorial craniotomy, and compared whether there is a difference between in immuno-inflammatory response.
Materials and methods
Patients and ethnic consideration
All the operations and related examinations were conducted in Beijing Tiantan Hospital (Beijing, China). This study was approved by an Institutional Review Board of Beijing Tiantan Hospital (TTH-2013-023), and was conducted in accordance with good clinical practice, all applicable regulatory requirements and the guiding principles of the Declaration of Helsinki. Written informed consents were obtained from all subjects prior to admission to the study.
This randomized controlled trial was performed in 51 patients who aged 18-60 years and received supratentorial craniotomy. Patients whose surgery was assessed to be grade 1-2 by the American Society of Anesthesiologists (ASA) were recruited. The patients were excluded if they were: patients with immune, having renal or central nervous system dysfunction, patients with congestive heart failure, receiving exogenous hormone therapy (including steroids), having prior experience of acupuncture, pregnancy, malnutrition, diabetes, malignancy, infection or inflammation.
Grouping patients
No analgesics or tranquilizers were administered before the operation. After operation, the induction an intravenous infusion was given to these patients, and non-invasive blood pressure (NIBP), heart rate (HR), oxygen pulse saturation (SPO2) and the bispectral index (BIS) were monitored. The operation information of characteristics and details in patients are listed in Table 1.
Table 1.
Patient characteristics and operative data
| Group C | Group A | Group T | |
|---|---|---|---|
| Gender (M/F) | 10/8 | 9/10 | 6/8 |
| Age (years) | 40±10 | 43±11 | 39±9 |
| Duration of surgery (min) | 247±42 | 251±45 | 241±39 |
| ASA physical status (I/II) | 13/5 | 14/5 | 8/6 |
| Tumor type (glioma/meningioma/other tumors) | 8/5/5 | 9/6/4 | 7/4/3 |
ASA = American Society of Anesthesiologists. There is no significant difference among Group C, A and T.
The 51 patients were randomly divided into 3 groups. Group C was a control group. In this group, patients didn’t receive EA or TENS during the operation. For group A patients, EA was applied to LI4 (Hegu), TE5 (Waiguan), BL63 (Jinme), LR3 (Taichong), ST36 (Zusanli), GB40 (Qiuxu), BL10 (Tianzhu), GB20 (Fengchi), BL2 (Cuanzhu) and EX-HN4 (Yuyao) on the same side during the craniotomy. For acupuncture points of LI 4, TE5, BL63, LR3, ST36 and GB40, each acupuncture point was applied with one needle; LI4 and TE5, BL63 and LR3, ST36 and GB40 were connected with the acupuncture point nerve stimulator in pairs. BL10 and GB20 were penetrated by a single needle; as for BL2 and EX-HN4, the pairs of needles were then connected with the acupuncture point nerve stimulator to start the EA. Group T was a TENS group, in this group, TENS electrodes were fixed at the same position as EA applied, but the electrodes didn’t penetrate the skin.
Surgery procedure
After intravenous infusion was performed, NIBP, HR, SPO2 and BIS were monitored, and then EA and TENS were started. EA stimulation was delivered via a LH202H HANS acupuncture point nerve stimulator (Beijing Huawei Co Ltd, China) using dense-dispersed wave, with frequency of 2 Hz/100 Hz and alternated every 3 s. The stimulation intensity was adjusted to the level of maximal tolerance of each patient and stimulation and lasted from the induction of anesthesia till the end of the operation.
During this period, target concentration infusion anesthesia was performed using propofol and sufentanil. The induction plasma concentration of propofol was 5 μg/mL and sufentanil was 0.5 ng/mL. When the patients were unconscious, the plasma concentration of propofol was reduced to 3.2 μg/mL and the concentration of sufentanyl was reduced to 0.3 ng/mL, vecuronium bromide was administered with dose of 0.1 mg/kg. After patients’ muscle relaxed, tracheal intubation was performed. The mechanical ventilation was applied with volume of 10 mL/kg tidal, 12 times/min respiratory frequency and 1 L/min oxygen flow. Intermittent administration of 0.05 mg/kg vecuronium bromide was given to maintain their muscle relaxation. The concentration of sufentanil was adjusted to maintain the mean arterial pressure (MAP) and HR in the basic range of +10% to -20%. In cases of hypotension (MAP<20% of baseline), bradycardia (HR<50 beats/min) or hypertension (MAP>10% of baseline values), 6 mg ephedrine, 0.5 mg atropine or 0.2-0.5 mg nicardipine was administered respectively.
Sampling collection
Blood samples were taken in SSTII advance tubes (Becton Dickinson, UK) before anesthesia (T0) and 30 min (T1), 2 h (T2) and 4 h (T3) after induction of anesthesia for measurement of cytokine and immunoglobulin concentrations.
Multiplex cytometric bead assay
A cytometric bead assay kit (Becton Dickinson) was employed to measure levels of TNF-α, IL-8, IL-10, IgM, IgA, and IgG in plasma according to the manufacturer’s instructions.
Statistical analysis
Data were recorded as means ± SD and statistical analysis were performed using SPSS 13.0 statistical software. Data were analyzed using repeated-measures analysis of variance and separate effect analysis. The Maunchly test was used to judge whether there were relations between the repeatedly measured data. When P<0.05, the Greenhouse-Geisser correction was used to correct the results. Differences were considered as significant at P<0.05. The baseline analysis showed significant differences between groups in TNF-α, IgA and IgM, so the differences value between T0 and other time points were used to compare.
Results
Basic information of the 51 patients was listed in Table 1. No subject withdrew from the trial. There was no difference in arterial blood pressure or HR among groups before, during or after EA or TENS stimulation performed. There was no operative morbidity or mortality occurred.
Comparison within the groups
As shown in Table 2, IL-10 in peripheral blood increased significantly 2 h (T2) and 4 h (T3) after induction of anesthesia in group A compared with T0 (increased 128% and 286% respectively). IL-8 in peripheral blood increased significantly at T3 (66.5%) in group A compared with T0. While the levels of TNF-α and IgG did not change.
Table 2.
Cytokine and immunoglobulin levels during surgery
| Group | T0 | T1 | T2 | T3 | |
|---|---|---|---|---|---|
| TNF-α (pg/ml) | C | 1.47±0.13 | 1.66±0.13 | 1.54±0.12 | 1.67±0.14 |
| A | 2.63±0.12# | 2.84±0.13 | 2.81±0.11 | 2.89±0.14 | |
| T | 2.75±0.14# | 2.66±0.15 | 2.61±0.13 | 2.76±0.16 | |
| IL-10 (pg/ml) | C | 1.04±0.31 | 0.88±0.35 | 2.01±2.10 | 5.40±3.90 |
| A | 3.58±0.30 | 3.75±0.34 | 8.16±2.05* | 13.8±3.80* | |
| T | 2.87±0.35 | 2.91±0.4 | 2.84±2.38 | 3.42±4.43 | |
| IL-8 (pg/ml) | C | 9.25±1.59 | 7.00±0.88 | 6.59±0.95 | 12.02±1.16 |
| A | 8.92±1.55 | 8.26±0.85 | 10.27±0.92 | 14.85±1.13* | |
| T | 10.55±1.80 | 7.97±1.00 | 7.76±1.07 | 9.69±1.32 | |
| IgG (g/l) | C | 0.50±0.05 | 0.56±0.06 | 0.52±0.06 | 0.51±0.05 |
| A | 0.48±0.05 | 0.49±0.06 | 0.51±0.06 | 0.48±0.05 | |
| T | 0.49±0.06 | 0.47±0.07 | 0.46±0.07 | 0.47±0.06 |
P<0.05 vs before anesthesia in same group;
P<0.05 vs group C.
IgM and IgA in peripheral blood decreased significantly in group C at T2 and T3 compared with T0 (decreased 28.5% and 20.2%); IgA decreased 28.1% at T2 and 23.7% at T3; Figures 1 and 2).
Figure 1.
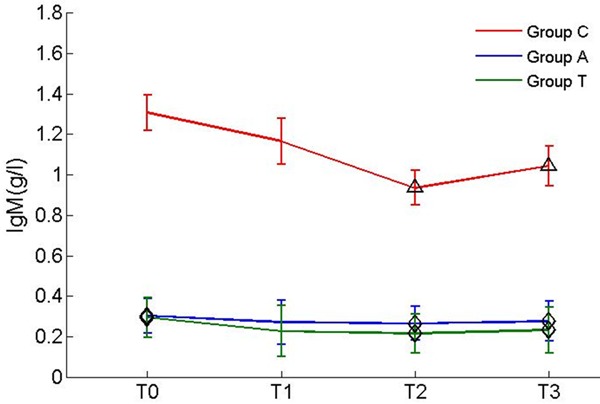
Levels of IgM during surgery. Δp<0.05 vs before anesthesia in same group; ◊P<0.05 vs group C.
Figure 2.
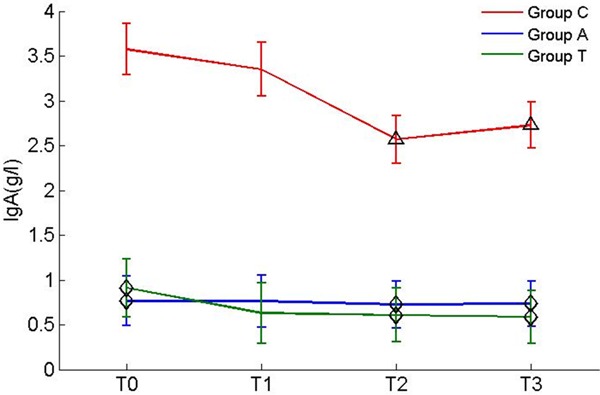
Levels of IgA during surgery. Δp<0.05 vs before anaesthesia in same group; ◊P<0.05 vs group C.
Comparisons among the three groups
There was no significant difference between the measurements in groups A and T during craniotomy. Comparison of the 3 groups showed that there were differences in the baseline levels of TNF-α, IgM and IgA, but no statistical differences were observed in the changing of TNF-α level. There was no significant difference in the levels of IL-10, IL-8 and IgG among the 3 groups. In the comparison of immunoglobulin levels during craniotomy, we found the peripheral blood IgM and IgA levels in group C were significantly decreased compared with those in groups A and group T at T2 and T3 (Figures 1 and 2).
Discussion
Surgery and the resultant stress response lead to a suppression of immune function [14]. Our study found that the peripheral blood IgA and IgM of patients in group C decreased significantly compared with those in groups A and T at 2 h and 4 h after the induction of anesthesia. This indicated that EA and TENS could improve the immune function suppressed by surgery. We also noted that the IL-10 levels in peripheral blood were increased significantly compared with those at T0, 2 h and 4 h after the induction in group A. The IL-8 levels in peripheral blood were increased significantly compared with those at T0 and 4 h after the induction in group A. The time at which IL-10 levels increased was earlier than that of IL-8 levels. Our study may be a demonstration that EA can modulate immune balance.
Acupuncture analgesia is the foundation of acupuncture anesthesia [15]. Previous studies showed that EA had analgesic action in neck pain, shoulder pain, elbow pain, back pain and knee pain [16]. The mechanism how acupuncture exerts treatment effect is very complex, which includes traditional chinese medicine theory and western anatomy physiology. Traditional chinese medicine theory assumes that there are two opposite forces in nature-Yin and Yang, whose origin is Qi. When the person is healthy, he is in the balance of Yin and Yang. In this state, Qi flows freely through the main and collateral channels, which put the Qi from the inside organ to the surface of the skin. There are a lot of acupoints along the meridians. Stimulating the acupoints could correct the imbalance of Yin and Yang can restore a person to health. The selection of acupoints is by theory of meridians and traditional chinese medicine differentiates diagnostic classification.
When nidus lies in upper of body, the lower acupoints become a therapeutic target. So we selected the acupoints under the wrist ankle especially at foot in our study. In our study, tumors were located at anterior cranial fossa; operation window is located in the temporal side, which in the field of Shaoyang, Yangming and Taiyang meridians. So we selected the following acupoints: LI4 (Hegu), TE5 (Waiguan), BL63 (Jinme), LR3 (Taichong), ST36 (Zusanli), GB40 (Qiuxu), BL10 (Tianzhu), GB20 (Fengchi), BL2 (Cuanzhu) and EX-HN4 (Yuyao).
In vitro studies had shown that electrical stimulation increase the migration and proliferation of fibroblasts, the movement of macrophages, and phagocyte functions in wound healing [17]. TENS also increased blood flow to wounds and fibroblasts, and induced by protein and DNA synthesis, reduced edema and prevented bacterial growth. Koca Kutlu et al [18] reported that wound closure was progress more rapidly in TENS group compared with Saline Solution, Povidone-Iodine, and Lavender Oil groups. Recent findings reveal that EA treatment relieved inflammatory pain; it is proposed that that EA and TENS share the similar therapeutic effect on alleviating pain hypersensitivity. We compared the differences between groups A and T during craniotomy. Our results indicated that there was no significant difference between the 2 groups during craniotomy, which is in consistent with report of Wang et al. [19], they indicated that there is no significant difference in producing antinociception between EA and TENS at 3 different frequencies (2, 15 and 100 Hz).
TENS also could provide sensory feedback in stroke patients with sensory loss [13], alleviate mechanical hyperalgesia caused by Complete Freund’s Adjuvant [13,20], improve pain relief in patients with diabetic neuropathy [21], reduce simulator sickness symptoms and alleviate cognitive impairment [22]. TENS also improve the Diabetic Cytopathy (DCP) via Up-Regulation of CGRP and cAMP [23], reduce the severity and incidence of hypotension after epidural anesthesia [24], and could reduce postoperative analgesic requirement in elderly patients after total hip arthroplasty [10].
Conclusion
The limitations of this study include the small sample size and the fewer indicators. In future studies, we aim to increase the number of participants, and collect more laboratory assessment measures to evaluate immuno-inflammatory response, at the same time, we will also focus on acupuncture and rehabilitation therapy of the follow-up effect. In conclusion, our study demonstrates EA and TENS could reduce immunosuppression in patients undergoing supratentorial craniotomy.
Acknowledgements
This work was supported by the multidisciplinary study on chronic diseases in electric power system employees and the subprogram “the effect of different anesthetic methods on immune-inflammatory response in patients undergoing supratentorial craniotomy” (The state grid program of science and technology, SGHBOOOAJJS1400182) and the National Key Basic Research Program on innovation and application study of combined acupuncture drug (2013CB531901).
Disclosure of conflict of interest
None.
References
- 1.Liu S, Wang B, Li S, Zhou Y, An L, Wang Y, Lv H, Zhang G, Fang F, Liu Z, Han R, Jiang T, Kang X. Immune cell populations decrease during craniotomy under general anesthesia. Anesth Analg. 2011;113:572–577. doi: 10.1213/ANE.0b013e3182278237. [DOI] [PubMed] [Google Scholar]
- 2.Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pinosky ML, Fishman RL, Reeves ST, Harvey SC, Patel S, Palesch Y, Dorman BH. The effect of bupivacaine skull block on the hemodynamic response to craniotomy. Anesth Analg. 1996;83:1256–1261. doi: 10.1097/00000539-199612000-00022. [DOI] [PubMed] [Google Scholar]
- 4.Gu G, Zhang Z, Wang G, Han F, Han L, Wang K, Liu J, Li W. Effects of electroacupuncture pretreatment on inflammatory response and acute kidney injury in endotoxaemic rats. J Int Med Res. 2011;39:1783–1797. doi: 10.1177/147323001103900521. [DOI] [PubMed] [Google Scholar]
- 5.Li G, Li S, An L, Wang B. Electroacupuncture alleviates intraoperative immunosuppression in patients undergoing supratentorial craniotomy. Acupunct Med. 2013;31:51–56. doi: 10.1136/acupmed-2012-010254. [DOI] [PubMed] [Google Scholar]
- 6.DeSantana JM, Walsh DM, Vance C, Rakel BA, Sluka KA. Effectiveness of transcutaneous electrical nerve stimulation for treatment of hyperalgesia and pain. Curr Rheumatol Rep. 2008;10:492–499. doi: 10.1007/s11926-008-0080-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khalil Z, Merhi M. Effects of aging on neurogenic vasodilator responses evoked by transcutaneous electrical nerve stimulation: relevance to wound healing. J Gerontol A Biol Sci Med Sci. 2000;55:B257–263. doi: 10.1093/gerona/55.6.b257. [DOI] [PubMed] [Google Scholar]
- 8.Keskin EA, Onur O, Keskin HL, Gumus II, Kafali H, Turhan N. Transcutaneous electrical nerve stimulation improves low back pain during pregnancy. Gynecol Obstet Invest. 2012;74:76–83. doi: 10.1159/000337720. [DOI] [PubMed] [Google Scholar]
- 9.Zaniewska R, Okurowska-Zawada B, Kulak W, Domian K. [Analysis of quality of life in patiens with low back pain after receiving transcutaneous electrical nerve stimulation (TENS)] . Med Pr. 2012;63:295–302. [PubMed] [Google Scholar]
- 10.Lan F, Ma YH, Xue JX, Wang TL, Ma DQ. Transcutaneous electrical nerve stimulation on acupoints reduces fentanyl requirement for postoperative pain relief after total hip arthroplasty in elderly patients. Minerva Anestesiol. 2012;78:887–895. [PubMed] [Google Scholar]
- 11.Sabino GS, Santos CM, Francischi JN, de Resende MA. Release of endogenous opioids following transcutaneous electric nerve stimulation in an experimental model of acute inflammatory pain. J Pain. 2008;9:157–163. doi: 10.1016/j.jpain.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Sluka KA, Lisi TL, Westlund KN. Increased release of serotonin in the spinal cord during low, but not high, frequency transcutaneous electric nerve stimulation in rats with joint inflammation. Arch Phys Med Rehabil. 2006;87:1137–1140. doi: 10.1016/j.apmr.2006.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kita K, Otaka Y, Takeda K, Sakata S, Ushiba J, Kondo K, Liu M, Osu R. A pilot study of sensory feedback by transcutaneous electrical nerve stimulation to improve manipulation deficit caused by severe sensory loss after stroke. J Neuroeng Rehabil. 2013;10:55. doi: 10.1186/1743-0003-10-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yokoyama M, Itano Y, Mizobuchi S, Nakatsuka H, Kaku R, Takashima T, Hirakawa M. The effects of epidural block on the distribution of lymphocyte subsets and natural-killer cell activity in patients with and without pain. Anesth Analg. 2001;92:463–469. doi: 10.1097/00000539-200102000-00035. [DOI] [PubMed] [Google Scholar]
- 15.Zhang WT, Jin Z, Huang J, Zhang L, Zeng YW, Luo F, Chen AC, Han JS. Modulation of cold pain in human brain by electric acupoint stimulation: evidence from fMRI. Neuroreport. 2003;14:1591–1596. doi: 10.1097/00001756-200308260-00010. [DOI] [PubMed] [Google Scholar]
- 16.Trinh K, Graham N, Gross A, Goldsmith C, Wang E, Cameron I, Kay T. Acupuncture for neck disorders. Spine (Phila Pa 1976) 2007;32:236–243. doi: 10.1097/01.brs.0000252100.61002.d4. [DOI] [PubMed] [Google Scholar]
- 17.Kloth LC, McCulloch JM. Promotion of wound healing with electrical stimulation. Adv Wound Care. 1996;9:42–45. [PubMed] [Google Scholar]
- 18.Koca Kutlu A, Cecen D, Gurgen SG, Sayin O, Cetin F. A Comparison Study of Growth Factor Expression following Treatment with Transcutaneous Electrical Nerve Stimulation, Saline Solution, Povidone-Iodine, and Lavender Oil in Wounds Healing. Evid Based Complement Alternat Med. 2013;2013:361832. doi: 10.1155/2013/361832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang JQ, Mao L, Han JS. Comparison of the antinociceptive effects induced by electroacupuncture and transcutaneous electrical nerve stimulation in the rat. Int J Neurosci. 1992;65:117–129. doi: 10.3109/00207459209003283. [DOI] [PubMed] [Google Scholar]
- 20.Fang JF, Liang Y, Du JY, Fang JQ. Transcutaneous electrical nerve stimulation attenuates CFA-induced hyperalgesia and inhibits spinal ERK1/2-COX-2 pathway activation in rats. BMC Complement Altern Med. 2013;13:134. doi: 10.1186/1472-6882-13-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stein C, Eibel B, Sbruzzi G, Lago PD, Plentz RD. Electrical stimulation and electromagnetic field use in patients with diabetic neuropathy: systematic review and meta-analysis. Braz J Phys Ther. 2013;17:93–104. doi: 10.1590/S1413-35552012005000083. [DOI] [PubMed] [Google Scholar]
- 22.Chu H, Li MH, Huang YC, Lee SY. Simultaneous transcutaneous electrical nerve stimulation mitigates simulator sickness symptoms in healthy adults: a crossover study. BMC Complement Altern Med. 2013;13:84. doi: 10.1186/1472-6882-13-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ding L, Song T, Yi C, Huang Y, Yu W, Ling L, Dai Y, Wei Z. Transcutaneous electrical nerve stimulation (TENS) improves the diabetic cytopathy (DCP) via up-regulation of CGRP and cAMP. PLoS One. 2013;8:e57477. doi: 10.1371/journal.pone.0057477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arai YC, Ito A, Ohshima K, Hibino S, Niwa S, Kawanishi J, Numanami H, Sakakima Y, Mizuno S, Tawada Y, Maruyama Y, Sato J, Nishihara M, Inoue S, Ushida T. Transcutaneous Electrical Nerve Stimulation on the PC-5 and PC-6 Points Alleviated Hypotension after Epidural Anaesthesia, Depending on the Stimulus Frequency. Evid Based Complement Alternat Med. 2012;2012:727121. doi: 10.1155/2012/727121. [DOI] [PMC free article] [PubMed] [Google Scholar]


