Abstract
Aims: This study is to assess the value of bulbocavernosus reflex (BCR) and pudendal nerve somatosensory evoked potential (SSEP) in the topical diagnosis of cauda equina syndrome (CES) with or without sphincter dysfunction in male patients. Methods: In this study, 40 healthy male adults (control group) and 53 male adult patients (experimental group) were included. The experimental group was subdivided into sphincter subgroup (24 patients with sphincter dysfunction) and non-sphincter subgroup (29 patients without sphincter dysfunction). All subjects underwent BCR and SSEP examinations. The mean latencies of BCR and SSEP P41 were calculated and compared between the control group and the experimental group. Latencies above the average value of +2.58S were considered abnormal. The abnormality rates of BCRs and SSEPs in sphincter and non-sphincter subgroups were calculated, respectively. Results: BCR and SSEP latencies in the experimental group were remarkably prolonged than those in the control group. BCR and SSEP latencies in sphincter subgroup were remarkably prolonged than those in non-sphincter subgroup. Among the 106 nerves in the experimental group, 87 nerves had prolonged BCR latencies and 3 nerves had no wave elicited, with an abnormality rate of 84.9%. The abnormality rates of BCR were 95.8% and 74.1% in sphincter subgroup and non-sphincter subgroup, respectively. Among the 53 nerves in the experimental group, 39 nerves had prolonged SSEP P41 latencies and 2 nerves had no wave elicited, with an abnormality rate of 77.4%. The abnormality rates of SSEP P41 were 91.7% and 65.5% in sphincter subgroup and non-sphincter subgroup, respectively. Conclusions: Both BCR and SSEP were changed in CES patients with or without sphincter dysfunction, and they were especially changed in patients with sphincter dysfunction. BCR and SSEP are valuable in the diagnosis of cauda equina lesions and their severity in males.
Keywords: Cauda equina syndrome, bulbocavernosus reflex, somatosensory evoked potential, sphincter dysfunction
Introduction
Cauda equina syndrome (CES) is resulted in from an insult to the collection site of lumbosacral nerve roots that arise from the caudal spinal cord. Injuries to cauda equina and root of spinal nerves and anterior horn of the sacral spinal cord lead to CES. CES is characterized by i) onset after acute factors; ii) obstacles in saddle area sensation that emerge first as an indicator of early diagnosis, and sphincter and sexual dysfunction that indicate more serious damages; and iii) symmetrical or nearly symmetrical symptoms [1,2]. CES usually presents with symptoms of lower back and leg pain, lower extremity weakness and sensory loss, perineal anesthesia, sphincter dysfunction and erectile dysfunction [3-5]. CES was divided into four stages: pre-phase, early-phase, mid-phase and late-phase. In pre-phase, the manifestations may be limited to waist symptoms and/or limbs radiating pain; saddle area numbness may emerge in early-phase; in mid-phase, the numbness may aggravate and constipation, bowel and bladder weakness, sexual hypofunction may emerge; and in late-phase, saddle area sensation is completely lost, accompanied by feces and urine retention or incontinence, sexual dysfunction [6]. Of note, loss of sphincter dysfunction can reflect the severity of cauda equine lesion. Because sphincter dysfunction only occurs in mid-phase and late-phase, it is often considered essential to the diagnosis of CES [7-10]. However, many cases of CES with complete sparing of sacral dysfunction have been described [1,11]. Of note, there are few effective and objective detection tools for the early clinical diagnosis and treatment of CES. In addition, effective and objective detection tools for the judgment of the severity of CES in males are rare.
Bulbocavernosus reflex (BCR) and pudendal nerve somatosensory evoked potential (SSEP) are determined using electromyogram technique and evoked potential technique in combination with nerve conduction determination based on neuroelectrophysiologic principle, which can objectively record the conduction time of pudendal nerve in both peripheral and central parts, as well as the conduction of the sacral reflex arc. BCR and SSEP of the dorsal nerve of penis have been reported to be used in the diagnosis of neurogenic impotence in men [12-16]. In addition, many studies proved BCR and SSEP to be the only objective method for the diagnosis of neurogenic impotence [17]. However, the use of BCR and SSEP examination in the topical diagnosis of nervous system diseases such as CES in males is rarely reported [18-21]. In this study, we try to explore the values of BCR and SSEP in the topical diagnosis of CES by comparing BCRs and SEEPs between 40 healthy male adults (control group) and 53 male patients with CES (experimental group).
Materials and methods
Subjects
In this study, 53 patients, clinically diagnosed as CES, were included into the experimental group (age range of 31-63 years and average age of 45.1 ± 6.83 years; height range of 168-179 cm and average height of 171 ± 5.4 cm). All cases were resulted in by trauma of disc by inappropriate treatment such as waist massage violence that led to cauda equina damage. All patients suffered from perineal anesthesia, 31 patients had lower-extremity weakness and sensory loss, and 24 patients had sphincter dysfunction. The patients were divided into sphincter subgroup (24 patients with sphincter dysfunction) and non-sphincter subgroup (29 patients without sphincter dysfunction). In the control group, 40 healthy male adults were included (age range of 29-64 years and average age of 43.7 ± 6.25 years; height range of 168-178 cm and average height of 170 ± 5.2 cm). No significant differences in ages and heights existed between the experimental group and the control group (P > 0.05). All patients with sphincter dysfunction in the experimental group underwent B-ultrasonic examinations to rule out bladder outlet obstruction. Written informed consent was obtained from all participants. All studies involving patients were conducted under the protocol approved by the Institutional Research Review Board at Wenzhou Medical University, China.
BCR
Keypoint EMG/EP system (Dantec Company, Skovlunde, Demark) was used in the examination of BCR. Skin temperature was kept above 32°C. Subjects took lithotomy position with an earth electrode attached to the thigh. Saddle-shaped surface electrode was used as stimulating electrode, where penises were laid on. Concentric needle electrodes were used for BCR record and were inserted into right and left bulbocavernosus muscles in turn. The stimulating intensity was 7 times of sensory threshold. Electrode impedance was < 5 KΩ, and the frequency of the square wave was 1.9 pulses per second. The average value of 20 reflection waves was recorded, and the latency was calculated based on the distance between the wave and the base line. Scanning time was 5 ms/div, persistence time was 100 ms and bandwidth was 10 Hz-2 KHz. BCR reflects the conduction function of pudendal afferent nerve, pudendal efferent nerve and S2-4 reflex arc. The mean latencies of BCR were calculated and compared between the control group and the experimental group. Latencies above the average value of +2.58S were considered abnormal. The abnormality rates of BCRs in sphincter and non-sphincter subgroups were calculated, respectively.
SSEP
Keypoint EMG/EP system (Dantec Company, Skovlunde, Demark) was used in the examination of SSEP. Skin temperature was kept above 32°C. Subjects took lithotomy position with an earth electrode attached to the thigh. Saddle-shaped surface electrode was used as stimulating electrode, where penises were laid on. The recording electrode was put on Cz-2 and the reference electrode was on Fz. Stimulating intensity was 3 times of sensory threshold. Electrode impedance was < 5 KΩ, and the frequency of the square wave was 5 pulses per second, superimposed by 200 times. Scanning time was 0.2 ms/div, persistence time was 100 ms and bandwidth was 10 Hz-5 KHz. SSEP P41 wave was detected to assess the sensory central conduction of the pudendal nerve. The mean latencies of SSEP P41 were calculated and compared between the control group and the experimental group. Latencies above the average value of +2.58S were considered abnormal. The abnormality rates of SSEPs in sphincter and non-sphincter subgroups were calculated, respectively.
Statistical analysis
All data were analyzed with SPSS 13.0 Statistical Package (IBM, USA). t-test was used to compare the mean latencies of BCR and SSEP P41 between the control group and the experimental group. Analysis of variance was used to compare the mean latencies of BCR and SSEP P41 among sphincter subgroup, non-sphincter subgroup and the control group. Chi-square test was used to compare the abnormality rates of BCRs and SSEPs between sphincter subgroup and non-sphincter subgroup.
Results
CES patients have abnormal BRR latencies
To compare BCR latencies between the experimental group and the control group and those among sphincter subgroup, non-sphincter subgroup and the control group, t-test and Analysis of variance were performed, respectively. The results showed that BCR latencies in the experimental group were remarkably prolonged compared with those in the control group (P < 0.05; Table 1). However, there was no statistical difference in the mean BCR latencies on two sides among sphincter subgroup, non-sphincter subgroup and the control group (P > 0.05; Table 1). These data suggested that CES patients had pudendal nerve or sacral reflex arc diseases.
Table 1.
Comparison of BCR latencies between the experimental group and the control group (means ± SD)
| Group | N | BCR latencies (ms) | ||
|---|---|---|---|---|
|
| ||||
| Left | Right | Mean | ||
| Experimental group | 53 | 63.8 ± 5.1 | 64.2 ± 5.4 | 64.0 ± 5.3 |
| Control group | 40 | 32.7 ± 5.3 | 33.6 ± 5.8 | 33.2 ± 5.4 |
| P | 0.001 | 0.000 | 0.001 | |
Patients with or without sphincter dysfunction have abnormal BCR and SSEP latencies
The mean SSEP P41 latencies in the experimental group and the control group were 42.1 ± 1.4 ms and 38.1 ± 1.1 ms, respectively. SSEP latencies in the experimental group were remarkably prolonged compared with those in the control group (P < 0.05) (Figures 1, 2). In addition, BCR and SSEP latencies in sphincter subgroup and non-sphincter subgroup were remarkably prolonged compared with those in the control group (P < 0.05; Table 2). BCR latencies in sphincter subgroup were remarkably prolonged than those in non-sphincter subgroup (P < 0.05; Table 2). There was no statistical difference in the mean SSEP latencies between sphincter subgroup and non-sphincter subgroup (P > 0.05; Table 2). These data indicated that patients with or without sphincter dysfunction had abnormal BCR and SSEP latencies.
Figure 1.
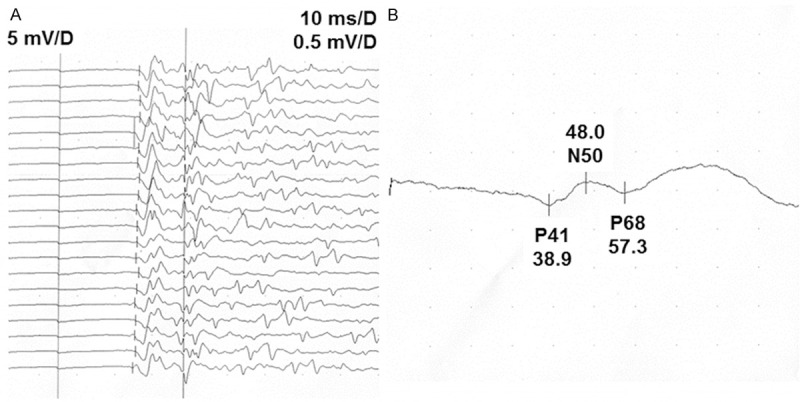
BCR and SSEP latencies in a 32-year-old healthy man. A. Latency curves. B. Enlarged latency curves. Stimulating electrode was fixed on the pudendal nerve and recording electrode was attached in the left bulbocavernous muscle. Average BCR latency was 35.4 ms; SSEP latency of P41 wave was 38.9 ms.
Figure 2.
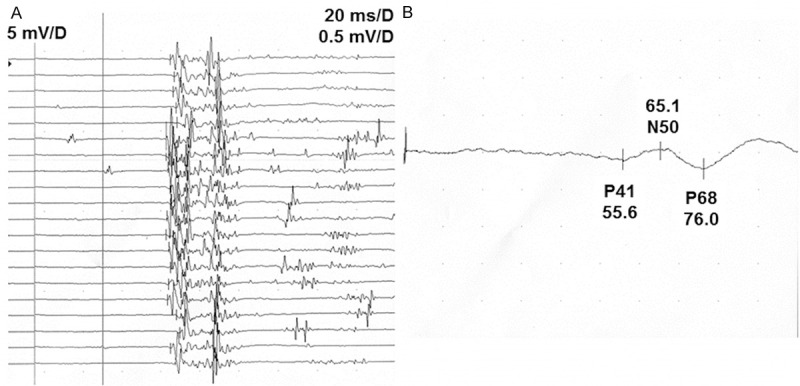
BCR and SSEP latencies in a 26-year-old man with CES. A. Latency curves. B. Enlarged latency curves. Stimulating electrode was fixed on the pudendal nerve and recording electrode was attached in the left bulbocavernous muscle. Average BCR latency was 84.5 ms; SSEP P41 latency was 55.6 ms.
Table 2.
Comparison of BCR and SSEP latencies among sphincter subgroup, non-sphincter subgroup and the control group (means ± SD)
| Group | N | BCR latencies (ms) | SSEP latencies (ms) | |
|---|---|---|---|---|
|
|
||||
| Left | Right | |||
| Sphincter subgroup | 24 | 68.7 ± 4.8a,b | 69.3 ± 5.0a,b | 42.9 ± 1.8a |
| Non-sphincter subgroup | 29 | 59.2 ± 5.5a | 59.8 ± 5.6a | 41.3 ± 1.2a |
| Control group | 40 | 32.7 ± 5.3 | 33.6 ± 5.8 | 38.1 ± 1.1 |
P < 0.05 compared with control group;
P < 0.05 compared with non-sphincter subgroup.
Patients with sphincter dysfunction have higher abnormality rates in BCR
To further investigate the effectiveness of BCR and SSEP in the diagnosis of CES, a comparison of latency abnormality rates of BCRs and SSEPs of P41 wave between sphincter subgroup and non-sphincter subgroup was performed. The BCR latency of the control group was 33.2 ± 5.4 ms. BCR latency > 47.1 ms was considered abnormal. Among the 80 nerves from the control group, 3 nerves had prolonged BCR latencies with an abnormality rate of 3.75%. Among the 48 nerves from sphincter subgroup, 46 nerves had abnormal BCR latencies, with an abnormality rate of 95.8%. Among the 58 nerves from non-sphincter subgroup, 43 nerves had abnormal BCR latencies, with an abnormality rate of 74.1%. Therefore, there was statistical difference in BCR latency abnormality rates among control group, sphincter subgroup and non-sphincter subgroup (P < 0.05; Table 3). Moreover, the SSEP P41 latency of the control group was 38.1 ± 1.1 ms. SSEP P41 latency > 40.9 ms was considered abnormal. Among the 40 nerves from the control group, 2 nerves had prolonged SSEP P41 latencies with an abnormality rate of 5%. Among the 24 nerves from sphincter subgroup, 16 nerves had abnormal SSEP P41 latencies, with an abnormality rate of 66.7%. Among the 29 nerves from non-sphincter subgroup, 19 nerves had abnormal SSEP P41 latencies, with an abnormality rate of 65.5%. Therefore, there is no statistical difference about latency abnormality rates of SSEPs between sphincter subgroup and non-sphincter subgroup (P > 0.05; Table 3). These data suggested that patients with sphincter dysfunction had higher abnormality rates in BCR.
Table 3.
Comparison of BCR and SSEP abnormality rates between sphincter subgroup and non-sphincter subgroup
| Group | BCR (%) | SSEP (%) |
|---|---|---|
| Sphincter subgroup | 46/48 (95.8%)a,b | 16/24 (66.7%)a |
| Non-sphincter subgroup | 43/58 (74.1%)a | 19/29 (65.5%)a |
| Control group | 3/80 (3.75%) | 2/40 (5%) |
P < 0.05 compared with control group;
P < 0.05 compared with non-sphincter subgroup.
Discussion
By stimulating pudendal nerves through sacral reflex arc, BCR causes bulbocavernosal muscle contraction. The conduction time in pudendal nerve and the sacral reflex arc can be objectively recorded. The latency can reflect the function of the peripheral pudendal nerve and the sacral reflex arc. In our study, BCR latencies in experimental group with or without sphincter dysfunction were remarkably prolonged than those in control group (P < 0.05), indicating that patients in the experimental group had pudendal nerve or sacral reflex arc diseases. The study suggested that BCR could reflect the function of sacral plexus sensitively and objectively, having diagnostic values for CES. The abnormality rates of BCR were 95.8% and 74.1% in sphincter subgroup and non-sphincter subgroup, indicating that patients with or without sphincter dysfunction had abnormal BCR. Therefore, it can be concluded that BCR has early diagnosis value for males with sacrococcygeal neuropathy especially CES in pre-phase and early phase without sphincter dysfunction. BCR latencies in sphincter subgroup were remarkably prolonged than those in non-sphincter subgroup (P < 0.05). There were statistical differences in BCR abnormality rates between sphincter subgroup and non-sphincter subgroup (P < 0.05). These results demonstrated the value of BCR in judging the disease severity in CES patients. Because sphincter dysfunction appears to aggravate damages to cauda equina, the detection of BCR shows more values for the objective determination of cauda equina lesion severity and the diagnosis of cauda equina lesions caused by sphincter dysfunction. The conclusion of our study concurred with the research by Li et al. [6].
SSEP is obtained by recording the electric potentials in the brain cortex after stimulating the pudendal nerve to generate signals that pass through the sacral spinal cord and then spread to the brain cortex. SSEP latency reflects the function of the spinal cord and the pudendal afferent nerve. SSEP recording methods in our study was the same with the research by Vodusek et al. [22]. In our study, SSEP latencies in the experimental group were remarkably prolonged than those in the control group (P < 0.05), indicating that patients in the experimental group had sacral pulp spinal cord or pudendal afferent nerve diseases. There was no statistical difference in SSEP latencies and abnormality rates between sphincter subgroup and non-sphincter subgroup (P > 0.05), indicating that patients with CES mainly had peripheral neuropathy. Furthermore, the combination of SSEP with BCR had values for localization diagnosis of females with sphincter dysfunction. As an electrophysiological diagnostic method, SSEP and BCR can be used in the clinical and sub-clinical diagnosis of sacral plexus neuropathy. There was no statistical difference in the mean latencies of BCR in two sides between the experimental group and the control group (P > 0.05), suggesting that both sides of the detection had similar clinical significance. Therefore, we can choose only one side for BCR in order to reduce the pain and the potential risk of complications. Only when this side leads to no waveform, we can choose the other side for inspection instead. In conclusion, the examination of BCR and SSEP is valuable in the topical and qualitative diagnosis of CES with or without sphincter dysfunction. It is an assistant diagnostic method and an early diagnostic method for CES in males. BCR and SSEP also have important values for determining the severity of CES.
Acknowledgements
This work was supported by Wenzhou Science and Technology Bureau (No. 2013S0461).
Disclosure of conflict of interest
All authors declare no financial competing interests. All authors declare no non-financial competing interests.
References
- 1.Lafuente DJ, Andrew J, Joy A. Sacral sparing with cauda equina compression from central lumbar intervertebral disc prolapse. J Neurol Neurosurg Psychiatry. 1985;48:579–81. doi: 10.1136/jnnp.48.6.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi JG, Jia LS, Li JS, Yuan W, Ni B, Chen DY, Xiao JJ, Ye XJ, Tan J. Clinical Study of the Mechanism of Cauda Equina Syndrome. Orthop J China. 2002;10:1283–1285. [Google Scholar]
- 3.Orendácová J, Cízková D, Kafka J, Lukácová N, Marsala M, Sulla I, Marsala J, Katsube N. Cauda equina syndrome. Prog Neurobiol. 2001;64:613–37. doi: 10.1016/s0301-0082(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 4.Kawasaki T, Hukuda S, Katsuura A, Inoue K, Chano T. Lumboperitoneal shunt for cauda equina syndrome in ankylosing spondylitis. J Spinal Disord. 1996;9:72–5. [PubMed] [Google Scholar]
- 5.Larner AJ, Pall HS, Hockley AD. Arrested progression of the cauda equina syndrome of ankylosing spondylitis after lumboperitoneal shunting. J Neurol Neurosurg Psychiatry. 1996;61:115–6. doi: 10.1136/jnnp.61.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li PH, He ZJ. Clinical Significance of the Electrophysiological Tests of BCR, ICR and PEP in Diagnosis and Prognosis of Cauda Equina Syndrome. Chinese J Orthop. 1999;19:99–102. [Google Scholar]
- 7.Balasubramanian K, Kalsi P, Greenough CG, Kuskoor Seetharam MP. Reliability of clinical assessment in diagnosing cauda equina syndrome. Br J Neurosurg. 2010;24:383–6. doi: 10.3109/02688697.2010.505987. [DOI] [PubMed] [Google Scholar]
- 8.McCarthy MJH, Aylott CEW, Grevitt MP, Hegarty J. Cauda equina syndrome: factors affecting long-term functional and sphincteric outcome. Spine (Phila Pa 1976) 2007;32:207–16. doi: 10.1097/01.brs.0000251750.20508.84. [DOI] [PubMed] [Google Scholar]
- 9.Qureshi A, Sell P. Cauda equina syndrome treated by surgical decompression: the influence of timing on surgical outcome. Eur Spine J. 2007;16:2143–51. doi: 10.1007/s00586-007-0491-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gleave JR, Macfarlane R. Cauda equina syndrome: what is the relationship between timing of surgery and outcome? Br J Neurosurg. 2002;16:325–8. doi: 10.1080/0268869021000032887. [DOI] [PubMed] [Google Scholar]
- 11.Xu SJ. Surgical Treatment of Lumbar Disc Herniation Combined withCauda Equina Syndrome. Chinese J Mod Oper Surg. 2011;15:123–125. [Google Scholar]
- 12.Arauz Bravo P, Montoya Pedron A, Acosta Lee T, Soler Vives A, Traba Sorribes L, Matos Gorgas Y, Gomez Martinez J, Mosqueda C, Palancar Heredia A. 166. Neurophysiological evaluation of erectile dysfunction. Clin Neurophysiol. 2008;119:e140. [Google Scholar]
- 13.Shafik A, Shafik IA, el-Sibai O, Shafik AA. Physioanatomical relationship of the external anal sphincter to the bulbocavernosus muscle in the female. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18:851–6. doi: 10.1007/s00192-006-0246-z. [DOI] [PubMed] [Google Scholar]
- 14.Shao B, Wang X, Li CL, Huang XF, Zheng RY, Ni PQ. The application of BCR and SSEP in diseases of the nervous system. Chinese J Neurol. 2002;35:388. [Google Scholar]
- 15.Shao B, Wang X, Li CL, Huang XF, Huang HB, Zheng RY, Ni PQ. The cavernosus reflex assessment in the diagnosis of nervous system diseases. Neurosci Bull. 2003;19:102–104. [Google Scholar]
- 16.Tas I, Yagiz On A, Altay B, Ozdedeli K. Electrophysiological assessment of sexual dysfunction in spinal cord injured patients. Spinal Cord. 2007;45:298–303. doi: 10.1038/sj.sc.3101949. [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Shao B, Ni PQ, Li CL, Huang XF. The normal analysis of BCR and SSEP. Zhejiang Med J. 2002;24:507–508. [Google Scholar]
- 18.Shao B, Ni PQ, Wang X, Zhu BL, Wang Z. Primary study of the bulbocavernosus reflex in female patients. J Clin Electroneurophysiology. 2007;16:23–25. [Google Scholar]
- 19.Niu XT, Shao B, Ni PQ, Wang X, Lv ZK, Zheng JH, Jin MY. Study of BCR and SSEP in Sphincter dysfunction of female. Chinese J Phys Med Rehabil. 2010;32:691–692. [Google Scholar]
- 20.Niu X, Shao B, Ni P, Wang X, Chen X, Zhu B, Wang Z, Teng H, Jin K. Bulbocavernosus reflex and pudendal nerve somatosensory-evoked potentials responses in female patients with nerve system diseases. J Clin Neurophysiol. 2010;27:207–11. doi: 10.1097/WNP.0b013e3181dd4fca. [DOI] [PubMed] [Google Scholar]
- 21.Wei ZJ, Jin FS, Li QS, Fang YH. The clinical application of pelvic floor muscle electrical stimulation in the female patients with urinary incontinence. Chinese J Phys Med Rehabil. 2004;26:344–346. [Google Scholar]
- 22.Vodusek DB. Pudendal SEP and bulbocavernosus reflex in women. Electroencephalogr Clin Neurophysiol. 1990;77:134–6. doi: 10.1016/0168-5597(90)90027-b. [DOI] [PubMed] [Google Scholar]


