Abstract
Red blood distribution width (RDW) is a novel prognostic marker that reflects oxidative stress and inflammation in patients. Chronic inflammation has been proposed as a candidate mechanism between benign prostatic hyperplasia (BPH) and metabolic syndrome (MetS). However, the relationship between RDW and MetS in BPH patients is unclear. Men aged 50 year-old or older with BPH were recruited into the study. The BPH patients were classified as MetS group and non-MetS group. 69 patients without BPH and MetS were as the control group. The clinical information and RDW were measured to identify their relationship. MetS was diagnosed in 34% of the patients. The RDW values were found to be higher in the BPH group than in the control group [(13.3 ± 0.8) vs. (12.6 ± 0.8), P < 0.001]. The total prostate volume (TPV) and post void residual (PVR) urine volume were significantly higher in subject with MetS than in non-MetS and related with the number of metabolic abnormalities. High serum triglyceride and low serum high- density lipoprotein cholesterol (HDL-C) levels were significantly associated with TPV even adjusting for age (adjusted r = 0.373, P < 0.001, and adjusted r = -0.425, P < 0.001, respectively).There was a significant correlation between RDW and TPV (r = 0.370, P < 0.001), Body mass index (BMI) (r = 0.367, P < 0.001) and MetS (r = 0.276, P < 0.001). The data indicated that RDW was independently correlated with the presence of MetS (odd ratio 1.226, 95% confidence intervals 0.89-1.87, P < 0.001). MetS is associated with BPH development in men. The RDW level is significantly higher in patients with BPH than that in control. RDW is an independent predictor of MetS in BPH patients.
Keywords: Red blood cell distribution width, benign prostatic hyperplasia, metabolic syndrome, inflammation
Introduction
Benign prostatic hyperplasia (BPH), a common nonmalignant urologic condition among older man, is characterized by prostate enlargement, bladder outlet obstruction, and lower urinary tract symptoms (LUTS). Emerging data indicate that many age-related disorders, such as metabolic syndrome (MetS), type 2 diabetes, cardiovascular disease, or a combination thereof, are associated with BPH development and progression [1,2]. The relationship between BPH and MetS are complex. Chronic inflammation has been proposed as a candidate mechanism between these two clinical diseases [3-5].
Red blood cell distribution width (RDW) is a measure of the variability in the size of circulating erythrocytes. It is routinely reported to physicians in clinical practice as part of the automated complete blood count and is currently mainly used as an index in the differential diagnosis of anemia [6]. Recently, studies have demonstrated that RDW might provide useful information for the prognosis of patients with cardiovascular diseases, including MetS, heart failure, coronary artery diseases, and chronic heart disease. Although the underlying biological mechanisms remain understand. RDW is being recognized as a global marker of chronic inflammation and oxidative stress [7-11].
To our knowledge, the RDW in BPH patients has never been investigated. In this study, we aimed to investigate the association of serum RDW levels with the development of BPH, especially in BPH patients with MetS.
Methods
Study subjects
From January 2012 to December 2012, men aged 50 year-old or older with BPH, significant LUTS (International Prostate Symptom Score [IPSS] ≥ 8) and without palpable prostatic nodules were recruited into the study. This study excluded cases that had been diagnosed with urinary tract infection, prostatitis, known malignant diseases including prostate cancer or cases who had been administrated related drugs, including-blockers, anticholinergics, 5-reductase inhibitors, and phosphodiesterase-5 inhibitors. The BPH patients were classified as MetS group and non-MetS group. At the same time, 69 patients without BPH and MetS were chose as the control group. The study was approved by the local ethics committee, and complied with the Declaration of Helsinki.
LUTS/BPH assessment
The patients’ medical history was collected using a standardized structured questionnaire. IPSS was administered to the patients to evaluate the urinary symptoms. The total prostate volume (TPV) and post void residual (PVR) urine volume were calculated using ultrasonography. The serum prostate-specific antigen (PSA) levels were collected in the morning after an overnight fast and determined using radioimmunoassay.
Definition of MetS
Body height (m), weight (kg), waist circumference (cm) and blood pressure (mmHg), were measured by trained personnel using a standardized protocol. Body mass index (BMI) was calculated by dividing the weight (kg) by the square of height (m). The biochemical analyses included serum glucose, total cholesterol, triglycerides, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C).
A diagnosis of MetS had to satisfy three or more of the criteria [12,13]. Which are as follows: (1) waist circumference ≥ 85 cm (Recommended waist circumference thresholds for abdominal obesity of Chinese men), (2) triglycerides ≥ 150 mg/dl or treatment for hypertriglyceridemia, (3) high-density lipoprotein-cholesterol (HDL-C) < 40 mg/dl or treatment for reduced HDL-C, (4) blood pressure ≥ 130/85 mmHg or current use of antihypertensive medications, and (5) fasting blood glucose ≥ 100 mg/dl or previous diagnosis of type 2 diabetes mellitus.
Determination of RDW
Erythrocyte count, hemoglobin, white blood cell (WBC) count, RDW, and C-reactive protein (CRP) were determined by the automated hematology analyzer. The normal range of RDW (%) in our laboratory was 10.9%-15.4%.
Statistical analysis
Continuous variables are demonstrated as means ± standard deviation (SD) and categorical variables as numbers and percentages. The baseline characteristics of the groups were compared using analysis of variance (ANOVA) for continuous variables and by the X2 statistic for non-continuous variables. The Spearman test was used to assess the correlation between variables. Univariate and multivariate logistic regression were used to identify the risk factors influencing MetS. The strength of the association between risk factors and MetS was expressed as odd ratios (OR) with 95% confidence intervals (CI). Only variables with P < 0.1 in the univariate logistic regression analyses were used in the multivariate logistic regression analysis. Differences were considered statistically significant by a two tailed p-value of < 0.05. Analyses were performed with SPSS statistical software (v. 17.0).
Results
Baseline clinical characteristics
Baseline clinical characteristics of the study population are given in Table 1. Of the total 426 men, 144 (33.8%) had MetS. In particular, 105 (24.6%) presented with 3/5 parameters of Mets, 34 (8%) 4/5, and 5 (1.2%) 5/5. There were no differences in age and smoking history. However, body weight, BMI, and waist circumference were significantly higher in subjects with MetS. The percentage with a history of diabetes and hypertension were significantly higher in patients with MetS among the three groups.
Table 1.
Baseline clinical characteristics
| Variables | MetS (n = 144) | Non-MetS (n = 282) | Control (n = 69) | P value |
|---|---|---|---|---|
| Age (year) | 70.5 ± 10.7 | 71.1 ± 11.7 | 70.8 ± 9.8 | 0.612 |
| Weight (kg) | 73.3 ± 7.2 | 64.9 ± 9.7 | 68.6 ± 8.1 | 0.001 |
| Waist circumference (cm) | 92.1 ± 3.3 | 86.3 ± 3.3 | 85.2 ± 5.4 | 0.045 |
| BMI (kg/m2) | 26.0 ± 7.2 | 23.1 ± 2.8 | 22.3 ± 1.8 | 0.001 |
| Hypertension (%) | 74.7 | 40.3 | 19.3 | 0.001 |
| Diabetes mellitus (%) | 69.4 | 20.8 | 13.7 | 0.001 |
| Smoking (%) | 47.2 | 53.1 | 48.3 | 0.260 |
| RDW (%) | 13.8 ± 0.6 | 13.0 ± 0.6 | 12.6 ± 0.8 | 0.001 |
| Hemoglobin (g/L) | 136.3 ± 11.8 | 132.5 ± 13.1 | 135.6 ± 11.1 | 0.032 |
| WBC (G/L) | 7.5 ± 1.8 | 7.5 ± 1.7 | 7.8 ± 1.2 | 0.613 |
| Triglyceride (mg/dl) | 185.3 ± 19.6 | 155.8 ± 18.6 | 142.5 ± 23.3 | 0.001 |
| HDL-C (mg/dl) | 37.4 ± 11.9 | 57.3 ± 13.4 | 64.8 ± 11.9 | 0.001 |
| CRP (mg/L) | 17.9 ± 3.8 | 21.3 ± 4.5 | 19.3 ± 6.9 | 0.430 |
BMI, body mass index; RDW, red blood cell distribution width; WBC, white blood cell; HDL-C, high-density lipoprotein cholesterol; CRP, C-reactive protein.
In laboratory findings, the RDW values were found to be higher in the BPH group than in the control group [(13.3 ± 0.8) vs. (12.6 ± 0.8), P < 0.001]. In addition, in BPH patients, RDW values were higher in MetS group than in non-MetS group [(13.8 ± 0.6) vs. (13.0 ± 0.6), P < 0.001]. There were significant differences in triglyceride and HDL-C levels. However, there were no significant differences among the three groups with respect to WBC, hemoglobin and CRP levels.
Comparison of LUT/BPH between the patients with and without MetS
As shown in Table 2, the IPSS and PSA levels were not significantly different from the two groups. However, TPV and PVR were significantly higher in subject with MetS.
Table 2.
Comparison of lower urinary tract symptoms/benign prostatic hyperplasia between the patients with and without metabolic syndrome
| Variables | MetS (n = 144) | Non-MetS (n = 282) | P-value |
|---|---|---|---|
| TPV (cm3) | 37.1 ± 5.7 | 33.1 ± 3.7 | 0.001 |
| PVR (mL) | 36.1 ± 5.2 | 30.5 ± 3.7 | 0.001 |
| PSA (ng/ml) | 1.43 ± 0.2 | 1.3 ± 0.3 | 0.811 |
| IPSS | 21.2 ± 3.2 | 20.1 ± 3.4 | 0.801 |
TPV, total prostate volume; PVR, postvoid residual; PSA, prostate-specific antigen; IPSS, International Prostate Symptom Score.
Correlation between the numbers of MetS components with LUT/BPH
There was a gradual but significant increase in the TPV and PVR as the number of MetS components increased (P < 0.001) (Figure 1). TPV and PVR were significantly related with the number of metabolic abnormalities after adjustment for age (adjusted r = 0.251, P < 0.001, and adjusted r = 0.275, P < 0.001, respectively) (Figure 1). Among MetS parameters, high serum triglyceride and low serum HDL-C levels were significantly associated with prostatic volume even adjusting for age (adjusted r = 0.373, P < 0.001, and adjusted r = 0.425, P < 0.001, respectively).
Figure 1.
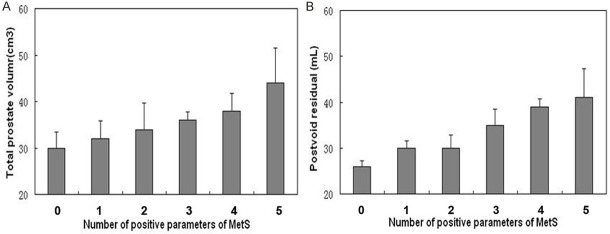
Correlation between the numbers of positive metabolic syndrome (MetS) components with benign prostatic hyperplasia (BPH) (A and B). TPV and PVR significantly increase with the increasing number of components of MetS (P < 0.001).
Correlations of RDW
Spearman correlations analysis showed a significant correlation between RDW and TPV (r = 0.370, P < 0.001), BMI (r = 0.367, P < 0.001) and MetS (r = 0.276, P < 0.001) in the study population. There were no correlation between RDW and hemoglobin (r = -0.156, P = 0.263) and CRP (r = 0.176, P = 0.053). All seven variables associated with MetS were presented in Table 3. Variables including history of hypertension, diabetes, BMI, HDL-C, and RDW found to be statistically significant in univariate analyses were entered into multivariate Logistic regression analysis. The data indicated that history of hypertension, diabetes, BMI, and RDW were independently correlated with the presence of MetS (Table 4).
Table 3.
Risk factors of metabolic syndrome in the benign prostatic hyperplasia patients
| Univariable predictor OR (95% CI) | P-value | |
|---|---|---|
| Age, years ≥ 75 y | 2.47 (1.32-4.63) | 0.07 |
| History of hypertension | 15.90 (7.58-23.60) | 0.001 |
| diabetes | 8.11 (5.05-10.26) | 0.001 |
| BMI | 2.80 (1.34-5.85) | 0.001 |
| triglycerides | 1.549 (1.01-3.85) | 0.524 |
| HDL-C | 1.324 (0.87-3.05) | 0.001 |
| RDW | 3.326 (1.953-4.658) | 0.001 |
OR, odd ratio; CI, confidence intervals; BMI, body mass index; RDW, red blood cell distribution width; HDL-C, high-density lipoprotein cholesterol.
Table 4.
Multivariable risk factors of metabolic syndrome in the benign prostatic hyperplasia patients
| Multivariable predictor OR (95% CI) | P-value | |
|---|---|---|
| History of hypertension | 5.66 (3.11-7.79) | 0.001 |
| diabetes | 3.61 (2.05-5.26) | 0.001 |
| BMI | 2.12 (1.39-3.32) | 0.001 |
| HDL-C | 1.224 (0.93-1.85) | 0.051 |
| RDW | 1.226 (0.89-1.87) | 0.001 |
OR, odd ratio; CI, confidence intervals; BMI, body mass index; RDW, red blood cell distribution width; HDL-C, high-density lipoprotein cholesterol.
Discussion
Our study demonstrated the existence of an association among MetS features, BPH development and RDW. In the present study, we found that the MetS correlated with the BPH development in men. The RDW level was significantly higher in patients with BPH than that in control. RDW was an independent predictor of MetS in BPH patients. We speculate that inflammation has a relevant impact on LUTS severity. It suggest that paying attention to RDW might provide valuable clinical information among BPH patients without additional cost because RDW is available as part of a complete blood count.
Clinical evidence reported that chronic inflammation presented a key condition leading to prostate enlargement and to an increased symptoms score as well as a major risk of complications. The pioneering study [14] showed a positive association between inflammation and prostate volume, indeed patients with chronic inflammation at biopsy had higher volumes than those without inflammation (46.5 vs. 43.4 mL, respectively; P < 0.001). Robert [15] et al. assessed the degree of prostatic inflammation using cytological and immunohistochemical parameters in 282 patients treated with surgery for complicated or symptomatic BPH. Interestingly, the grade of prostatic inflammation was strongly associated with LUTS severity, and patients with chronic inflammation had higher IPSS than those without inflammation (21 vs. 12 respectively; P = 0.02). Moreover, prostate volume was significantly higher in patients with high-grade inflammatory pattern (77 vs. 62 mL; P = 0.002). Burris [16] et al. showed that LUTS improvement after radical prostatectomy correlated with degree of prostatic inflammation. On multivariate analysis, only patients with severe prostatic inflammation had significant improvement in their IPSS. They concluded that inflammation was an independent predictor of IPSS. All these data showed a statistically and clinically significant association between chronic inflammation and LUTS severity, supporting the hypothesis that inflammation could contribute development of BPH.
Recently, it has been shown that patients affected by MetS are at higher risk of BPH [1]. It is known that insulin resistance with secondary hyperinsulinemia, the core pathophysiology of MetS, is related to prostatic enlargement [17]. Park [1] and his colleagues have found that BPH ratio, TPV ≥ 30 mL, and PVR ≥ 50 mL significantly increased with an increasing number of metabolic abnormalities. Then, the same teamwork [2] has proved that the MetS was significantly associated with the predictors of the risk of clinical progression of BPH. Based on those results, Gacci’ study [3] was to examine the correlation among pre-operatory LUTS/BPH severity, MetS features and inflammatory infiltrates in prostatectomy specimens. A significant positive correlation between the presence of MetS and the inflammatory score (IS) was observed, a positive correlation among both IS and IPSS was also observed. Therefore, MetS can be relevant for the development of a remarkable intraprostatic inflammation that could be a predictor, or even a drive, of BPH progression. The consistency of the correlation between BPH and MetS, reported in our study, is in agreement with other previously reported experiments.
Obviously, chronic prostatic inflammation can be histologically detected only in patients who undergo prostate biopsies. However, most patients with LUTS/BPH do not want to do that. Although a number of potential markers (CRP, interleukin (IL)-8, and markers of oxidative stress) have been evaluated, these markers are generally nonspecific for BPH. Also, some are expensive and probably require further clinical evaluation before introduction into daily clinical practice. However, it opens the search for biomarkers that could be used to stratify patients as to the risk of developing BPH or related BPH adverse outcomes, or to monitor symptoms and response to medical therapy for BPH.
In recent years, RDW, a new marker, has been suggested as the final common pathway of multiple pathologic processes, including inflammation and therefore reflecting more than certain forms of anaemia [6]. Reliable data emerged from a variety of clinical studies have supported the hypothesis that RDW might be a useful parameter for gathering meaningful clinical information, either diagnostic or prognostic, on a variety of cardiovascular disorders. Highly significant associations have been described between RDW value and all-cause, non-cardiac and cardiac mortality in patients with coronary artery disease (CAD), acute and chronic heart failure, peripheral artery disease, stroke [6]. It is however still unclear whether anisocytosis might be the cause, or a simple epiphenomenon of an underlying disease. Recently years, researchers paid more attention to the metabolic abnormalities. They have found that elevated RDW was related to patients with arterial hypertension [18], diabetes mellitus [7] overweight [8], and MetS [9]. Our study supported that elevated RDW was related to the MetS in BPH patients. To our knowledge, this was the first time to evaluate the association of serum RDW levels with the development of BPH, especially in BPH patients with MetS.
A possible explanation for the observed association between RDW and MetS in BPH patients is that high RDW reflects an underlying inflammatory state that impairs erythrocyte maturation and consequent inadequate production of the hormone erythropoietin, undernutrition (i.e., deficiencies in nutrients, such as iron, vitamin B12, and folate), or oxidative damage. Among these variables, oxidative stress and inflammation have been hypothesized as the important determinants of RDW [19]. Inflammatory states are strongly related to ineffective erythropoiesis, and it has been demonstrated that inflammatory cytokines, such as tumor necrosis factor (TNF)-α, IL-1β and IL-6, desensitize bone marrow erythroid progenitors to erythropoiesis, inhibit red blood cell (RBC) maturation and thereby promote anisocytosis. In some clinical investigations, significant and positive associations were found between RDW and a variety of inflammatory markers, such as hsCRP, erythrocyte sedimentation rate, IL-6, soluble transferrin receptor, soluble TNF receptor I, and soluble TNF receptor II [20]. Fukuta [21] et al. observed a significant correlation between B-type natriuretic Peptide (BNP) and RDW in patients undergoing cardiac catheterization for CAD, thereby suggesting the existence of a potential interplay between anisocytosis and neurohumoral mediators.
But some researchers presented other opinions. They found that individuals with low RDW had significantly higher risk of developing DM [22]. They proposed that rise in fasting glucose and postprandial hyperglycaemia could be sufficient to change the mechanical properties of the RBCs, to reduce survival and create a more homogenous population of cells. But in our study, we suggested that elevated RDW was independently associated with MetS in patients with BPH. We believed the different type of diseases leading to the different results. We chose the patients with MetS in BPH, not just diabetes. Secondly, maybe inflammatory state resulted in ineffective erythropoiesis was the leading mechanism in our study.
Some limitations in the present study must be considered. First, this was a single center, retrospective study with a small sample size. Residual confounding factors might thus have affected the results, regardless of the adjusted analysis. Second, the other features, including iron, vitamin B12 and folate was not mentioned in this study.
Conclusions
The presence of MetS is associated with a substantial increase of BPH. The RDW level is significantly higher in patients with BPH than that in control. RDW is independently associated with MetS in patients with BPH. In conclusion, MetS can be relevant for the development of BPH via inflammation.
Acknowledgements
We wish to thank Yong-Ming Wu and Xiu-Qiang Lian for their assistance with the manuscript.
Disclosure of conflict of interest
None.
References
- 1.Park YW, Kim SB, Kwon H, Kang HC, Cho K, Lee KI, Kim YJ, Lee JH. The relationship between lower urinary tract symptoms/benign prostatic hyperplasia and the number of components of metabolic syndrome. Urology. 2013;82:674–679. doi: 10.1016/j.urology.2013.03.047. [DOI] [PubMed] [Google Scholar]
- 2.Kwon H, Kang HC, Lee JH. Relationship between predictors of the risk of clinical progression of benign prostatic hyperplasia and metabolic syndrome in men with moderate to severe lower urinary tract symptoms. Urology. 2013;81:1325–1329. doi: 10.1016/j.urology.2013.01.042. [DOI] [PubMed] [Google Scholar]
- 3.Gacci M, Vignozzi L, Sebastianelli A, Salvi M, Giannessi C, De Nunzio C, Tubaro A, Corona G, Rastrelli G, Santi R, Nesi G, Serni S, Carini M, Maggi M. Metabolic syndrome and lower urinary tract symptoms: The role of inflammation. Prostate Cancer Prostatic Dis. 2013;16:101–106. doi: 10.1038/pcan.2012.44. [DOI] [PubMed] [Google Scholar]
- 4.Bostanci Y, Kazzazi A, Momtahen S, Laze J, Djavan B. Correlation between benign prostatic hyperplasia and inflammation. Curr Opin Urol. 2013;23:5–10. doi: 10.1097/MOU.0b013e32835abd4a. [DOI] [PubMed] [Google Scholar]
- 5.Gandaglia G, Briganti A, Gontero P, Mondaini N, Novara G, Salonia A, Sciarra A, Montorsi F. The role of chronic prostatic inflammation in the pathogenesis and progression of benign prostatic hyperplasia (BPH) Bju Int. 2013;112:432–441. doi: 10.1111/bju.12118. [DOI] [PubMed] [Google Scholar]
- 6.Montagnana M, Cervellin G, Meschi T, Lippi G. The role of red blood cell distribution width in cardiovascular and thrombotic disorders. Clin Chem Lab Med. 2012;50:635–641. doi: 10.1515/cclm.2011.831. [DOI] [PubMed] [Google Scholar]
- 7.Tsuboi S, Miyauchi K, Kasai T, Ogita M, Dohi T, Miyazaki T, Yokoyama T, Kojima T, Yokoyama K, Kurata T, Daida H. Impact of red blood cell distribution width on long-term mortality in diabetic patients after percutaneous coronary intervention. Circ J. 2013;77:456–461. doi: 10.1253/circj.cj-12-0730. [DOI] [PubMed] [Google Scholar]
- 8.Fujita B, Strodthoff D, Fritzenwanger M, Pfeil A, Ferrari M, Goebel B, Figulla HR, Gerdes N, Jung C. Altered red blood cell distribution width in overweight adolescents and its association with markers of inflammation. Pediatr Obes. 2013;8:385–391. doi: 10.1111/j.2047-6310.2012.00111.x. [DOI] [PubMed] [Google Scholar]
- 9.Sanchez-Chaparro MA, Calvo-Bonacho E, Gonzalez-Quintela A, Cabrera M, Sainz JC, Fernandez-Labandera C, Aguado LQ, Meseguer AF, Valdivielso P, Roman-Garcia J. Higher red blood cell distribution width is associated with the metabolic syndrome: Results of the Ibermutuamur CArdiovascular RIsk assessment study. Diabetes Care. 2010;33:e40. doi: 10.2337/dc09-1707. [DOI] [PubMed] [Google Scholar]
- 10.Ma FL, Li S, Li XL, Liu J, Qing P, Guo YL, Xu RX, Zhu CG, Jia YJ, Liu G, Dong Q, Wu NQ, Jiang LX, Li JJ. Correlation of red cell distribution width with the severity of coronary artery disease: A large Chinese cohort study from a single center. Chin Med J (Engl) 2013;126:1053–1057. [PubMed] [Google Scholar]
- 11.Mucsi I, Ujszaszi A, Czira ME, Novak M, Molnar MZ. Red cell distribution width is associated with mortality in kidney transplant recipients. Int Urol Nephrol. 2014;46:641–651. doi: 10.1007/s11255-013-0530-z. [DOI] [PubMed] [Google Scholar]
- 12.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SJ, Spertus JA, Costa F. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 13.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SJ. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; And International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 14.Nickel JC, Roehrborn CG, O’Leary MP, Bostwick DG, Somerville MC, Rittmaster RS. The relationship between prostate inflammation and lower urinary tract symptoms: Examination of baseline data from the REDUCE trial. Eur Urol. 2008;54:1379–1384. doi: 10.1016/j.eururo.2007.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robert G, Descazeaud A, Nicolaiew N, Terry S, Sirab N, Vacherot F, Maille P, Allory Y, de la Taille A. Inflammation in benign prostatic hyperplasia: A 282 patients’ immunohistochemical analysis. Prostate. 2009;69:1774–1780. doi: 10.1002/pros.21027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burris MB, Cathro HP, Kowalik CG, Jensen D, Culp SH, Steers WD, Krupski TL. Lower urinary tract symptom improvement after radical prostatectomy correlates with degree of prostatic inflammation. Urology. 2014;83:186–190. doi: 10.1016/j.urology.2013.07.080. [DOI] [PubMed] [Google Scholar]
- 17.De Nunzio C, Kramer G, Marberger M, Montironi R, Nelson W, Schroder F, Sciarra A, Tubaro A. The controversial relationship between benign prostatic hyperplasia and prostate cancer: The role of inflammation. Eur Urol. 2011;60:106–117. doi: 10.1016/j.eururo.2011.03.055. [DOI] [PubMed] [Google Scholar]
- 18.Ozcan F, Turak O, Durak A, Isleyen A, Ucar F, Ginis Z, Ucar F, Basar FN, Aydogdu S. Red cell distribution width and inflammation in patients with non-dipper hypertension. Blood Press. 2013;22:80–85. doi: 10.3109/08037051.2012.707336. [DOI] [PubMed] [Google Scholar]
- 19.Patel KV, Semba RD, Ferrucci L, Newman AB, Fried LP, Wallace RB, Bandinelli S, Phillips CS, Yu B, Connelly S, Shlipak MG, Chaves PH, Launer LJ, Ershler WB, Harris TB, Longo DL, Guralnik JM. Red cell distribution width and mortality in older adults: A meta-analysis. J Gerontol A Biol Sci Med Sci. 2010;65:258–265. doi: 10.1093/gerona/glp163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lippi G, Targher G, Montagnana M, Salvagno GL, Zoppini G, Guidi GC. Relation between red blood cell distribution width and inflammatory biomarkers in a large cohort of unselected outpatients. Arch Pathol Lab Med. 2009;133:628–632. doi: 10.5858/133.4.628. [DOI] [PubMed] [Google Scholar]
- 21.Fukuta H, Ohte N, Mukai S, Saeki T, Asada K, Wakami K, Kimura G. Elevated plasma levels of B-type natriuretic Peptide but not C-reactive protein are associated with higher red cell distribution width in patients with coronary artery disease. Int Heart J. 2009;50:301–312. doi: 10.1536/ihj.50.301. [DOI] [PubMed] [Google Scholar]
- 22.Engstrom G, Smith JG, Persson M, Nilsson PM, Melander O, Hedblad B. Red cell distribution width, haemoglobin A1c and incidence of diabetes mellitus. J Intern Med. 2014;276:174–183. doi: 10.1111/joim.12188. [DOI] [PubMed] [Google Scholar]


