Abstract
Purpose: To assess the relation between ankylosing spondylitis (AS) and degenerative disc disease emerging in association with various intrinsic and extrinsic factors and to evaluate the correlation between degree of degeneration in intervertebral discs and apparent diffusion coefficient (ADC) values. Methods: Thirty-five patients with AS and a control group of 35 patients were included in the study. Three hundred fifty intervertebral discs were assessed in terms of degeneration by analyzing signal intensities and morphologies on T2 weighted series of a 1.5 Tesla magnetic resonance scanner. ADC values were determined in diffusion weighted images (DWI) using a “b value of 500 s/mm2”. Patients in the AS and control groups were compared in terms of intervertebral disc degeneration, and association between degree of degeneration and ADC values was analyzed. Results: The mean of total degeneration degrees for five lumbar intervertebral discs was significantly higher in the patients with AS compared to the control group (16.77±4.67 vs 13.00±4.08, respectively; P=0.001). When intervertebral discs were analyzed separately, disc degeneration was again significantly higher in patients with AS compared to the control group, with the exception of L5-S1. Age, cholesterol level, triglyceride level, duration of disease and BASFI index were significantly associated with degree of degeneration in patients with AS. A negative correlation was determined between disc degeneration and ADC value. Conclusion: AS is a risk factor for degenerative disc disease due to its systemic effects, the fact it leads to posture impairment and its inflammatory effects on the vertebrae. A decrease in ADC values is observed as degeneration worsens in degenerative disc disease.
Keywords: Ankylosing spondylitis, apparent diffusion coefficient, intervertebral disc degeneration, magnetic resonance imaging
Introduction
Ankylosing spondylitis (AS) is an inflammatory disease of uncertain etiology that particularly affects the sacroiliac joints and axial skeleton and that leads to progressive bone fusion and thoracolumbar kyphotic deformity [1]. The typical clinical symptom is lower back pain, and this has an adverse impact on quality of life in many patients by causing both structural and functional problems. Bone marrow edema is observed in the vertebrae in the early period, while in later periods, destructive areas known as Romanus lesions and squaring of the vertebral corpus occur. Syndesmophytes develop as a result of calcification in the outer fibers in the annulus fibrosus.
The intervertebral discs are structures comprising 1/3 of the height of the spine at the lumbar level, located between each pair of vertebrae and whose basic function is to bear load and permit muscle movement. The intervertebral disc consists of three main components, the nucleus pulposus, annulus fibrosus and cartilage endplates and these components include different levels of collagen, proteoglycan and water [2,3]. Type I collagen is primarily found in the peripheral annulus and type II collagen in the nucleus pulposus. Type I collagen fibers are responsible for the annulus contractive power, while type II collagen is more hydrated and resists compression [2,4].
Disc composition changes with aging, and these changes affect the disc’s response to mechanical loading. It is almost impossible to distinguish the concept of degenerative disc, which may be regarded as physiological, from degenerative disc disease that appears at early ages as a result of accelerated degenerative changes [5]. Disc degeneration is a process involving different intrinsic and extrinsic factors. Spinal trauma and infections, spinal deformity, mechanical factors, smoking, vascular diseases and diabetes mellitus (DM) contribute to disc degeneration [6].
In this study, we evaluated the tendency to disc degeneration in patients with AS using signal and morphological characteristics of the intervertebral disc at magnetic resonance imaging (MRI). We also investigated whether or not there is an association between degrees of disc degeneration and ADC values in diffusion weighted series.
Materials and methods
Subjects
Thirty-five patients attending the Canakkale Onsekiz Mart University Faculty of Medicine Department of Physical Medicine and Rehabilitation, diagnosed with AS on the basis of ASAS diagnostic criterias and whose lumbar MRIs were available in the radiology archive were included in the study. The control group was also selected from patients without rheumatic disease and whose lumbar MRI results were available in the radiology archive. Subjects with DM, hypercholesterolemia, hypertriglyceridemia and kidney failure, cigarette smokers and patients using TNF-α blockers were excluded from the study. Subjects in both groups with vertebral fracture, spinal tumor and spondylodiscitis and with degenerative air or calcification in the disc space at radiography or MRI were also excluded. In addition, values for erythrocyte sedimentation rate (ESR), C reactive protein (CRP), Bath Ankylosing Spondylitis Activity Index (BASDAI), which measures disease activity, and Bath Ankylosing Spondylitis Functional Index (BASFI), which measures functional status, in patients with AS were also determined. Body mass index (BMI) was calculated in patients with AS using the formula weight divided by height squared (kg/m2). Ethical approval was granted by the Çanakkale Onsekiz Mart University Faculty of Medicine.
Radiological assessment and disc degeneration classification
Both the AS and control group patients were evaluated using a lumbar imaging protocol on a 1.5 Tesla MRI scanner (Signa Excite; GE Medical Systems, Wisconsin, USA). The imaging protocol in both groups involved a sagittal T1 weighted fast spin-echo sequence (500 ms/15.2 ms, repetition time [TR]/echo time [TE]; number of excitations [NEX], 2.0; 4 mm section thickness; 320×192 matrix; 27×27 cm field of view [FOV]), a sagittal T2 weighted fast spin-echo sequence (3000 ms/111 ms, TR/TE; NEX, 2.0; section thickness 4 mm; 320×224 matrix; 27×27 cm FOV), an axial T2 weighted fast spin-echo sequence (4600/90 ms, TR/TE; NEX, 2.0; section thickness 4 mm; 320×192 matrix; 20×20 cm FOV) and diffusion weighted imaging (3000/90 ms TR/TE; section thickness 4 mm; 128×128 matrix; 27×27 cm FOV). ADC values were measured on a workstation using GE software functool-AD. In ADC measurements, the region of interest (ROI) was located in such a way as to include the nucleus pulposus in intervertebral discs.
Degeneration in lumbar intervertebral discs was staged according to the classification modified by Pfirrmann et al. [7] (Table 1 and Figure 1). MRI findings were analyzed by the same radiologist blinded to the patients’ characteristics.
Table 1.
Classification of disc degeneration
| Grade | Structure | Nucleus and Annulus Distinction | Signal Intensity |
|---|---|---|---|
| I | Homogeneous, bright white | Clear | Hyperintense, isointense to cerebrospinal fluid |
| II | Inhomogeneous with or without horizontal bands | Clear | Hyperintense, isointense to cerebrospinal fluid |
| III | Inhomogeneous, gray | Unclear | Intermediate |
| IV | Inhomogeneous, gray to black | Lost | Intermediate to hypointense |
| V | Inhomogeneous, black | Lost | Hypointense and collapsed disc space |
Figure 1.
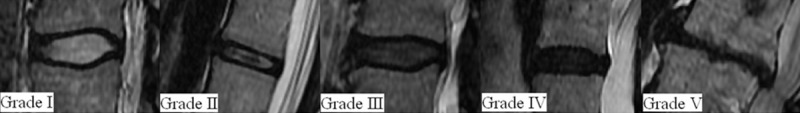
Grade I-V, Grading system for the assessment of lumbar disc degeneration.
Statistical analysis
Statistical analysis was performed on SPSS version 19.0 software. Normal distribution of data was assessed using the Kolmogorov-Smirnov test. Descriptive data were expressed as mean, standard deviation, frequency and percentage values. The independent samples T test was used to compares means between the groups and the chi square test to compare categoric variables. Relations between constant variables in the case and control groups were examined using Spearman’s correlation test and Pearson’s correlation test. ROC curve analyses was used to determine a cutoff value for ADC value in patients with ankylosing spondylitis. P values less than 0.05 were considered statistically significant.
Results
Thirty-five patients with AS (43.2±13.0 years) and 35 control group patients (41.9±12.1 years) were included. There was no significant difference between the patients with AS (13 women and 22 men) and the control group (15 women and 20 men) in terms of age and gender (P=0.671 Independent Samples T test and P=0.626 Chi-Square test, respectively). Furthermore, no significant difference was observed between the groups in terms of triglyceride, total cholesterol and LDL cholesterol levels at lipid profile analysis (P=0.711, and P=0.099, Independent Samples T test respectively). Total degeneration degrees for the five intervertebral discs at lumbar level were significantly higher in the patients with AS compared to the control group (16.77±4.67 vs 13.00±4.08, respectively; P=0.001 Independent Samples T test). When lumbar intervertebral discs were analyzed separately, mean degrees of degenerations at all levels, apart from the L5-S1 disc, were significantly higher in the patients with AS. Mean degeneration grade for each disc level in the patient and control groups is shown in Table 2. Age, cholesterol and triglyceride level were associated with disc degeneration grade in the control group (P<0.001 r=0.851 Pearson correlation, P<0.001 rho=0.704 Spearman correlation and P=0.013 r=416 Pearson correlation, respectively). Similarly, disc degeneration grade was correlated with between age, cholesterol and triglyceride level in the patients with AS (P<0.001 rho=0.649, P=0.015 rho=0.409 and P=0.030 rho=0.367 Spearman correlation, respectively). Disc degeneration in the AS group was also significantly associated with BASFI and duration of disease (P=0.010 rho=0.428 and P=0.001 rho=0.524 Spearman correlation respectively). BASDAI, CRP and BMI were not significantly associated with disc degeneration grade (P=0.410 r=0.144 Pearson correlation, P=0.696 rho=-0.069 and P=0.055 rho=0.327 Spearman correlation, respectively).
Table 2.
Comparison of disc degeneration in different intervertebral disc levels in patients with ankylosing spondylitis and the control group
| Disc Degeneration Degree | |||
|---|---|---|---|
|
|
|||
| Ankylosing Spondylitis (n=35) | Control Group (n=35) | P value | |
|
|
|||
| Disc Level | Mean±SD | Mean±SD | P value* |
| L1-2 | 3.3±0.9 | 2.4±0.7 | <0.001* |
| L2-3 | 3.2±0.9 | 2.3±0.8 | <0.001* |
| L3-4 | 3.2±1.0 | 2.2±0.9 | <0.001* |
| L4-5 | 3.5±1.1 | 2.7±1.2 | 0.006* |
| L5-S1 | 3.4±1.2 | 3.1±1.2 | 0.395 |
Statistically significant at P<0.05. Independent Samples T test.
Analysis of the total 350 discs in the 70 cases revealed a decrease in mean ADC value with increasing degeneration in discs at different degenerative stages (r=-0.636, P<0.001). Correlation between disc degeneration and ADC values is shown in Table 3. At receiver operating characteristic (ROC) analysis performed to identify normal and mildly degenerated discs (Grade I and Grade II), at a cutoff ADC value of 0.001605, area under curve (AUC) of 0.912 (95% confidence interval 0.882-0.943) exhibited sensitivity of 84%, specificity of 83%, positive predictive value of 75% (95% CI 70-84%) and negative predictive value of 87% (81%-92%).
Table 3.
Association between disc degeneration grade and apparent diffusion coefficient value
| Disc Degeneration Grade | No. | Mean Apparent Diffusion |
|---|---|---|
|
| ||
| Coefficient Value (mm2/s±SD) | ||
| Grade I | 32 | 1.84×10-3±0.09×10-3 |
| Grade II | 106 | 1.72×10-3±0.17×10-3 |
| Grade III | 79 | 1.53×10-3±0.19×10-3 |
| Grade IV | 104 | 1.26×10-3±0.63×10-3 |
| Grade V | 29 | 0.89×10-3±0.36×10-3 |
Discussion
Intervertebral discs, which contribute to the stabilization and flexibility of the spinal column, consist of three basic structures, the cartilage endplate, nucleus pulposus and the annulus fibrosus [2]. Disc tissue contains collagen, proteoglycan and water. While the level of collagen is high in the external part of the annulus, water levels in the nucleus are higher compared to those of collagen and aggrecan, the main proteoglycan of the nucleus [3,7]. Physiologically, disc water and glycoprotein levels of decrease with aging, and fibrosis and calcifications appear. The collagen content in the nucleus increases and changes occur in collagen type distribution. Amyloid accumulation is also seen in the annulus [8].
In addition to environmental factors such as mechanical factors, trauma, immobilization, smoking, DM, vascular diseases and infection, genetic factors have also been implicated in the etiology of disc degeneration. Miller et al. reported that the intervertebral disc was the connective tissue most subjected to age-associated dramatic changes [9]. Several studies have demonstrated that disc degeneration increases with aging [9,10]. In this study, we determined a significant association between disc degeneration and age in both the patients with AS and the control group (r=0.649, P< 0.001 and r=0.851, P<0.001, respectively). This finding is not surprising, due to the decrease in proteoglycan and water levels in the disc, changes in collagen type distribution and minor changes in structures such as fibronectin.
Recent studies have shown that expression of matrix degrading enzymes increases in disc degeneration. Matrix metalloproteinases (MMPs) in the extracellular matrix play a particular role in disc degeneration by degrading collagen and proteoglycans. Some studies have described an association between MMP1 and MMP3 and inflammatory cells in granulation tissue in herniated vertebral discs [11,12]. Neidhart et al. compared bone and soft tissue specimens from 30 patients with AS and 20 patients with degenerative disc disease, and detected elevated MMP1 and cathepsin K, involved in bone resorption and cartilage tissue destruction, in patients with AS [13]. Another recent study showed that aggrecan turnover and carboxypropeptide (CPII), a type II procollagen biomarker, increased in patients with AS [14].
An increased predisposition to atherosclerosis has been demonstrated in AS, and this constitutes a significant risk factor for degenerative disease in discs relying on diffusion for nutrition [6]. Inflammation in the vertebrae, spondylodiscitis and posture impairment affecting mechanical equilibrium are also risk factors for disc degeneration in these patients [6,15]. Disc degeneration was significantly higher in the patients with AS in our study compared with the control group. Mean total degeneration for the five discs at lumbar level was 16.77±4.67 in the AS patient group and 13.00±4.08 in the control group (P=0.001). We think that the significant association between disc degeneration and cholesterol and triglyceride levels in both groups is secondary to the atherosclerotic process and to compromise of disc nutrition [16]. There was a significant association between duration of disease and disc degeneration in patients with AS (r=0.524, P=0.001). This is an expected outcome due to AS’ association with atherosclerosis, impaired posture and inflammation following a progressive course. There was also a significant correlation between BASFI, the ability to perform daily activities, and degeneration (r=0.428, P=0.010). However, no correlation between BASDAI and degeneration was observed (r=0.144, P=0,410). This is also not surprising, since disc degeneration is a progressive process and BASDAI reflects disease activation in the most recent period. Additionally, these findings support Robertson’s report of a worsening in BASFI while BASDAI remains relatively stable in a 5-year follow-up of patients with AS [17].
Lumbar disc degeneration may be expected to appear more at the L4-5 and L5-S1 level due to the biomechanics and physical weight involved. Cadaver and MRI studies have shown increased degeneration in the L5-S1 level in particular [18]. One important finding in our study is that when lumbar disc levels were assessed separately, there was a significant increase in degeneration at all levels in patients with AS apart from L5-S1. We think that the absence of a significant difference in degeneration between the groups at the L5-S1 level is associated with predisposition to degeneration in both groups because of spinal biomechanics, and that in the other levels it stems from the systemic effects of AS in particular, as well as impaired posture and inflammatory effects on cytokines and vertebrae [19,20].
Diffusion-weighted imaging is exceedingly sensitive to changes in motion in water molecules. Water diffusion in tissue can be measured mathematically on ADC maps, and microscopic changes in tissue can be identified [21,22]. Kerttulla et al. first investigated diffusion imaging and ADC measurement in the context of discs [23]. However, 18 asymptomatic normal discs were investigated in that study. Kealey et al. investigated the association between ADC values and anatomical level in lumbar discs and reported that discs at higher levels had greater ADC levels [24]. Both studies reported a decrease in ADC values as disc degeneration increased. In agreement with the literature, we also determined a decrease in ADC values as disc degeneration increased. Changes occurring in basic molecules in the disc content, and particularly a decrease in water and glycoprotein levels, changes in collagen level and type distributions and fibrosis all affect this outcome with degeneration.
Disc degeneration in the patients with AS was significantly greater compared to the control group. Disc degeneration in both groups was correlated with age and cholesterol and triglyceride levels. It was also significantly correlated with disease duration and BASFI in the AS group. ADC values decrease as degeneration increases, in association with changes in water and basic constituents.
Disclosure of conflict of interest
None.
References
- 1.Bron JL, de Vries MK, Snieders MN, van der Horst-Bruinsma IE, van Royen BJ. Discovertebral (Andersson) lesions of the spine in ankylosing spondylitis revisited. Clin Rheumatol. 2009;28:883–892. doi: 10.1007/s10067-009-1151-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poole AR. Biologic markers and disc degeneration. J Bone Joint Surg Am. 2006;88(Suppl 2):72–75. doi: 10.2106/JBJS.E.01326. [DOI] [PubMed] [Google Scholar]
- 3.Yogandan N, Halliday A, Dicman C, Benzel E. Techniques, complication avoidance and management. In: Benzel E, editor. Practical anatomy and fundamental biomecanics spine surgery. 2nd edition. New York, NY: Churchill Livingstone; 1999. pp. 93–118. [Google Scholar]
- 4.Antoniou J, Steffen T, Nelson F, Winterbottom N, Hollander AP, Poole RA, Aebi M, Alini M. The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J Clin Invest. 1996;98:996–1003. doi: 10.1172/JCI118884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fardon DF, Milette PC Combined Task Forces of the North American Spine Society, American Society of Spine Radiology, and American Society of Neuroradiology. Nomenclature and classification of lumbar disc pathology. Recommendations of the Combined task Forces of the North American Spine Society, American Society of Spine Radiology, and American Society of Neuroradiology. Spine (Phila Pa 1976) 2001;26:93–113. doi: 10.1097/00007632-200103010-00006. [DOI] [PubMed] [Google Scholar]
- 6.Holm S. Pathophysiology of disc degeneration. Acta Orthop Scand Suppl. 1993;251:13–15. doi: 10.3109/17453679309160105. [DOI] [PubMed] [Google Scholar]
- 7.Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine (Phila Pa 1976) 2001;26:1873–1878. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 8.Yasuma T, Arai K, Suzuki F. Age-related phenomena in the lumbar intervertebral discs. Lipofuscin and amyloid deposition. Spine (Phila Pa 1976) 1992;17:1194–1198. doi: 10.1097/00007632-199210000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Miller JA, Schmatz C, Schultz AB. Lumbar disc degeneration: correlation with age, sex, and spine level in 600 autopsy specimens. Spine (Phila Pa 1976) 1988;13:173–178. [PubMed] [Google Scholar]
- 10.Kang JD, Stefanovic-Racic M, McIntyre LA, Georgescu HI, Evans CH. Toward a biochemical understanding of human intervertebral disc degeneration and herniation. Contributions of nitric oxide, interleukins, prostaglandin E2, and matrix metalloproteinases. Spine (Phila Pa 1976) 1997;22:1065–1073. doi: 10.1097/00007632-199705150-00003. [DOI] [PubMed] [Google Scholar]
- 11.Maksymowych WP, Landewe R, Conner-Spady B, Dougados M, Mielants H, van der Tempel H, Poole AR, Wang N, van der Heijde D. Serum matrix metalloproteinase 3 is an independent predictor of structural damage progression in patients with ankylosing spondylitis. Arthritis Rheum. 2007;56:1846–1853. doi: 10.1002/art.22589. [DOI] [PubMed] [Google Scholar]
- 12.Matsui Y, Maeda M, Nakagami W, Iwata H. The involvement of matrix metalloproteinases and inflammation in lumbar disc herniation. Spine (Phila Pa 1976) 1998;23:863–868. doi: 10.1097/00007632-199804150-00005. [DOI] [PubMed] [Google Scholar]
- 13.Neidhart M, Baraliakos X, Seemayer C, Zelder C, Gay RE, Michel BA, Boehm H, Gay S, Braun J. Expression of cathepsin K and matrix metalloproteinase 1 indicate persistent osteodestructive activity in long-standing ankylosing spondylitis. Ann Rheum Dis. 2009;68:1334–1339. doi: 10.1136/ard.2008.092494. [DOI] [PubMed] [Google Scholar]
- 14.Kim TH, Stone M, Payne U, Zhang X, Ionescu M, Lobanok T, King L, Poole AR, Inman RD. Cartilage biomarkers in ankylosing spondylitis: relationship to clinical variables and treatment response. Arthritis Rheum. 2005;52:885–891. doi: 10.1002/art.20870. [DOI] [PubMed] [Google Scholar]
- 15.Resorlu H, Akbal A, Resorlu M, Gokmen F, Ates C, Uysal F, Adam G, Aylanc N, Arslan M, Inceer BS. Epicardial adipose tissue thickness in patients with ankylosing spondylitis. Clin Rheumatol. 2015;34:295–9. doi: 10.1007/s10067-014-2568-4. [DOI] [PubMed] [Google Scholar]
- 16.Appel H, Loddenkemper C, Grozdanovic Z, Ebhardt H, Dreimann M, Hempfing A, Stein H, Metz-Stavenhagen P, Rudwaleit M, Sieper J. Correlation of histopathological findings and magnetic resonance imaging in the spine of patients with ankylosing spondylitis. Arthritis Res Ther. 2006;8:R143. doi: 10.1186/ar2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robertson LP, Davis MJ. A longitudinal study of disease activity and functional status in a hospital cohort of patients with ankylosing spondylitis. Rheumatology (Oxford) 2004;43:1565–1568. doi: 10.1093/rheumatology/keh386. [DOI] [PubMed] [Google Scholar]
- 18.Siemionow K, An H, Masuda K, Andersson G, Cs-Szabo G. The effects of age, sex, ethnicity, and spinal level on the rate of intervertebral disc degeneration: a review of 1712 intervertebral discs. Spine (Phila Pa 1976) 2011;36:1333–1339. doi: 10.1097/BRS.0b013e3181f2a177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keller TS, Colloca CJ, Harrison DE, Harrison DD, Janik TJ. Influence of spine morphology on intervertebral disc loads and stresses in asymptomatic adults: implications for the ideal spine. Spine J. 2005;5:297–309. doi: 10.1016/j.spinee.2004.10.050. [DOI] [PubMed] [Google Scholar]
- 20.Zhu J, Yu DT. Matrix metalloproteinase expression in the spondyloarthropathies. Curr Opin Rheumatol. 2006;18:364–368. doi: 10.1097/01.bor.0000231904.04548.09. [DOI] [PubMed] [Google Scholar]
- 21.Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology. 1986;161:401–407. doi: 10.1148/radiology.161.2.3763909. [DOI] [PubMed] [Google Scholar]
- 22.Zhang W, Ma X, Wang Y, Zhao J, Zhang X, Gao Y, Li S. Assessment of apparent diffusion coefficient in lumbar intervertebral disc degeneration. Eur Spine J. 2014;23:1830–1836. doi: 10.1007/s00586-014-3285-z. [DOI] [PubMed] [Google Scholar]
- 23.Kerttula LI, Jauhiainen JP, Tervonen O, Suramo IJ, Koivula A, Oikarinen JT. Apparent diffusion coefficient in thoracolumbar intervertebral discs of healthy young volunteers. J Magn Reson Imaging. 2000;12:255–260. doi: 10.1002/1522-2586(200008)12:2<255::aid-jmri7>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 24.Kealey SM, Aho T, Delong D, Barboriak DP, Provenzale JM, Eastwood JD. Assessment of apparent diffusion coefficient in normal and degenerated intervertebral lumbar disks: initial experience. Radiology. 2005;235:569–574. doi: 10.1148/radiol.2352040437. [DOI] [PubMed] [Google Scholar]


