Abstract
Background: The regenerating gene (Reg), encoding lectin-related protein, was originally isolated from a rat regenerating pancreatic islets. Interleukin-22 (IL-22), a recently identified cytokine, is produced by Th 17 cells and natural killer cells. Both of them have been shown to play an important role in controlling tissue repair. But, it is unclear whether the IL-22/Reg axis is involved in liver regeneration and the improvement of liver function in a rat model of acute liver injury. Aims: We investigated the expression levels of Reg proteins after IL-22 stimulation in a rat model of acute liver injury, and estimated the effects of Reg proteins ameliorating acute liver injury. Methods: Western blot was used to measure the expressions of Reg I, Reg III, Reg IV proteins after treatment with recombinant lentivirus IL-22. At the same time, the expression levels of TB, ALT, AST, endotoxin (ETM), superoxide dismutase (SOD), malondialdehyde (MDA) were detected by related reagents. Results: In a rat model of acute liver injury, the expression levels of Reg I, Reg III, Reg IV proteins were increased after treatment with IL-22 recombinant lentivirus compared with treatment with lentivirus-empty vector, especially, Reg IV protein expression. Meanwhile, treatment with IL-22 recombinant lentivirus reduced serum levels of TB, ALT, AST, ETM, and decreased MAD levels in rat liver tissues, but increased SOD levels in rat liver tissues. Conclusion: IL-22 stimulation enhanced the expressions of Reg proteins in liver cell, especially, Reg IV protein, and ameliorated liver injury in a rat model of acute liver injury. Reg protein, especially Reg IV protein, might act as a biological mediator of immune cell-derived IL-22 in the recovering mechanism of liver injury.
Keywords: Reg protein, interleukin-22 (IL-22), liver injury, liver regeneration
Introduction
The liver has great regenerative potential in that it has the ability to regeneration after injury despite the fact that hepatocytes do not actively divide in normal condition [1]. After partial hepatectomy, mature hepatocytes will replicate until the normal hepatic mass is restored. A complex network of signaling pathways refers to liver regeneration: a cytokine pathway, responsible for hepatocyte priming; a growth factor pathway, responsible for cell cycle progression [2]. The regenerating gene (Reg), encoding lectin-related protein, was originally isolated from a rat regenerating pancreatic islets [3]. Reg family includes four subtypes. However, there are just three subtypes to express in human tissue and rat tissue, and those are Reg I, Reg III, Reg IV protein, respectively [4]. Then, many results demonstrated that Reg proteins were involved in cell regeneration, anti-infection, anti-apoptosis, promoting cell adhesion, and so on [5-15]. It was suggested that IL-6 and IFN-γ were possible stimulators of Reg Iαgene expression under inflammatory conditions [16,17]. However, the factors involved in ectopic expression of the Reg gene in the liver tissue are still unclear.
Interleukin-22 (IL-22) is a member of the interleukin-10 (IL-10) family of cytokines. It is produced by activated T cells and natural killer (NK) cells [18,19] and acts via a heterodimeric receptor complex consisting of IL-22 receptor-α (IL-22Rα) and IL-10 receptor-β (IL-10Rβ). IL-22Rs are expressed on a variety of tissues, including the kidney, pancreas, and liver [20]. It has been identified that IL-22 promoted liver cell regeneration by increasing hepatic proliferation and hepatocyte migration [21]. In this study, therefore, we attempted to clarify whether the IL-22/Reg axis is involved in the recovering of acute liver injury.
Materials and methods
A rat model of acute liver injury
Rats with 180±5 g weight were purchased from Animal Center of Chongqing Medical University. After adaptive feed for 7 days, the rats were injected with a single dose of D-gal (1.8 g/kg), and then divided into two groups. They were treatment group and control group. The rats were administrated after injection for 2 hours when the serum levels of ALT and AST were marked increase.
Administration of rats
The rats were treated with IL-22 recombinant lentivirus or lentivirus-empty vector at 2 hours after injected with a single dose of D-gal. The treatment group were injected with IL-22 recombinant lentivirus (2×108 pfu/ml), and the control group were injected with lentivirus-empty vector (2×108 pfu/ml). All the administrated rats were euthanized at 1 day, 3 day, 5 day after injection as experimental procedure.
Western blot analysis
Liver tissues were lysed in protein extraction buffer containing 20 mM Tris-HCL (pH 7.4), 150 mM NaCl, 2 mM EDTA, 1% Nonidet P-40, 50 mM NaF, and 1× proteinase inhibitor. Protein extract was fractionated by SDS-polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane. The membrane was incubated with a primary antibody and then with a peroxidase-conjugated secondary antibody. Proteins were detected using an enhanced chemiluminescence system.
Blood chemistry, endotoxin and lipid peroxidation assay
The rat blood was sent to the Clinical Laboratory, The First Affiliated Hospital of Chongqing Medical University for assaying the level of TB, ALT and AST. The serum level of endotoxin was detected by chromogenic substrate limulus reagent kit. Then, the MDA and SOD levels of rat liver tissues were detected by glucosinolates barbituric acid colorimetric method and xanthine oxidase assay, according to the manuals.
Statistical analysis
Data were expressed as means±SD. To compare values obtained from two groups, t-Test was performed by statistical software SPSS 13.0. P < 0.05 was considered statistically significant.
Results
Reg protein expressions in rat liver tissues
To investigate whether the IL-22/Reg axis is involved in liver regeneration, we examined the expressions of Reg I, Reg III, Reg IV in rat liver tissues after administration by western blot. IL-22 stimulation enhances the expressions of Reg proteins in liver tissue. In the treatment group, the expression levels of Reg proteins were significantly greater at 1 day after IL-22 recombinant lentivirus stimulation than at other time points, and then were decreased gradually from 1 day to 5 days. In the control group, the expression levels of Reg proteins were minimal at 1 day after lentivirus-empty vector stimulation compared with other time points, and were increased gradually from 1 day to 5 days. In each group, Reg IV expression was obviously stronger than Reg I or Reg III expression. Meanwhile, Reg IV expressions at all time points were much higher in the treatment group than in the control group. Nevertheless, Reg I expressions at 1 day, 3 day were markedly stronger in the treatment group than in the control group, and Reg I expression at 5 day in the treatment group was similar to that in the control group; Reg III expression at 1 day was significantly greater in the treatment group than in the control group. But, Reg III expressions at 3 day, 5 day in the treatment group were as many as those were in the control group (Figure 1).
Figure 1.
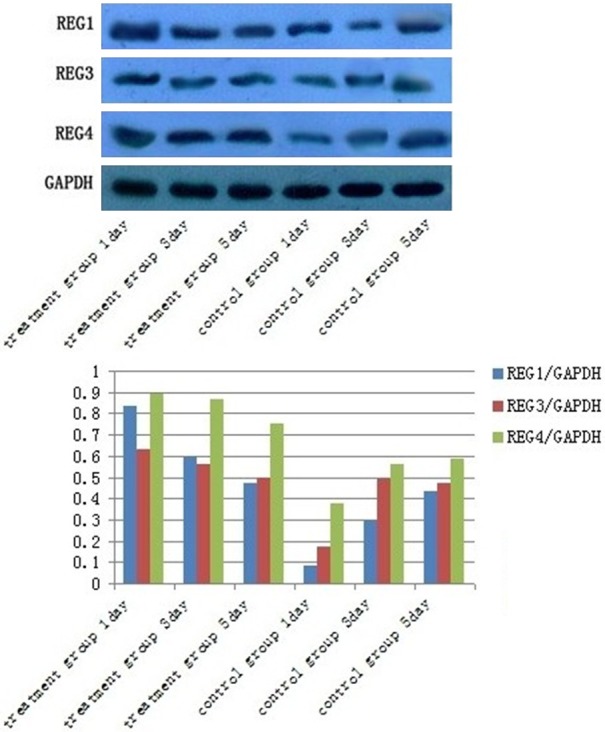
Reg IV expressions at all time points were much higher in the treatment group compared with the control group (P < 0.05); Reg I expressions at 1 day, 3 day were markedly stronger in the treatment group compared with the control group (P < 0.05); Reg III expression at 1 day was significantly greater in the treatment group compared with the control group (P < 0.05).
Blood chemistry, the levels of endotoxin and lipid peroxidation in the treatment group and the control group
To explore the therapeutic potential of IL-22 in acute liver injury, blood chemistry, the levels of endotoxin and lipid peroxidation were assessed in both groups. According to our data (Table 1), the serum levels of TB, ALT, AST were lower after injection with IL-22 recombinant lentivirus compared with control group, especially, after injection for 3 days (P < 0.05). Meanwhile, the level of ETM was significantly decreased after treatment with IL-22 compared with control group (P < 0.05). In contrast to the control group, the levels of SOD in rat liver tissue were obviously higher in the treatment group (P < 0.05), however, MAD levels were significantly decreased in the treatment group from 1 day (P < 0.05). In a word, the treatment of IL-22 recombinant lentivirus ameliorated liver function and lipid peroxidation in a rat model of acute liver injury.
Table 1.
Blood Chemistry, the levels of endotoxin and lipid peroxidation in two groups
| Groups | Items | 1 day | 3 day | 5 day |
|---|---|---|---|---|
| Treatment group | TB | 0.82±0.05 | 40.21±3.04 | 60.63±5.42 |
| ALT | 496.87±10.03 | 633.52±13.21 | 896.65±15.85 | |
| AST | 600.42±10.65 | 953.66±12.45 | 1963.78±41.23 | |
| ETM | 2.82±0.42 | 5.37±0.31 | 1.12±0.12 | |
| SOD | 122.67±2.42 | 101.849±1.23 | 140.21±2.71 | |
| MAD | 10.48±0.62 | 13.45±0.96 | 4.25±0.35 | |
| Control group | TB | 1.38±0.32 | 69.86±7.20 | 127.23±11.64 |
| ALT | 663.56±13.32 | 1132.11±56.47 | 2564.38±66.85 | |
| AST | 798.56±16.25 | 1675.98±52.24 | 3012.24±68.32 | |
| ETM | 3.75±0.39 | 9.53±0.49 | 4.15±0.61 | |
| SOD | 101.28±3.53 | 81.28±2.57 | 105.78±0.52 | |
| MAD | 14.86±0.83 | 19.06±0.75 | 11.68±0.93 |
Discussion
Although the recovering mechanism of liver injury is still unclear, accumulating evidences suggest the complex process of liver regeneration, including the precise timing and coordination of DNA replication, is controlled by multiple hormonal and cytokine signals. Cytokines and growth factor such as TNF-α, IL-6, transforming growth factor-β, and hepatocyte growth factor have been shown to be involved in the priming and progression of hepatocyte proliferation after liver injury [22-26]. Recently, some researchers have found IL-22 could promote liver regeneration and improve liver function after liver injury [27,28]. In our study, we identified the role of IL-22 in liver cell regeneration again. Our results showed treatment with IL-22 recombinant lentivirus reduced serum levels of TB, ALT, AST, ETM, meanwhile, decreased MAD levels in rat tissues, but increased SOD levels in rat tissues. These data illustrated IL-22 had an important effect on the recovery of acute liver injury.
Interestingly, Akira Sekikawa et al [29] have demonstrated IL-22 is responsible for Reg Iα overexpression in the colonic epithelium of UC mucosa. Therefore, in this study, we examined whether IL-22 stimulation enhances the expressions of Reg proteins in liver cell after liver injury, because in the early stage of our work, we discovered Reg proteins, including Reg I, Reg III, Reg IV, in liver tissues were distinctly upregulated in the process of liver regeneration. In term of our results, the expression levels of Reg I, Reg III, Reg IV proteins were increased after treatment with IL-22 recombinant lentivirus compared with treatment with lentivirus-empty vector. Among the three subtypes of Reg proteins, the upregulation of Reg IV protein was most obvious. So, it appears reasonable to speculate that IL-22 stimulation is responsible for Reg protein overexpression in liver tissue. Moreover, several studies have emphasized that IL-22 mediates host defense against bacterial pathogens [30,31] and promotes wound healing by exerting cell proliferative and anti-apoptotic effects [32,33]. Zhang et al [30] have reported that Reg family proteins mediated the antibacterial effect of IL-22 in murine experimental colitis. Along with others, we have suggested that Reg family protein are importantly involved in the regeneration of tissues [34-36]. Accordingly, together with our present data, it is tempting to speculate that Reg IV protein is most pivotal target for IL-22 in protecting liver tissue from inflammation-associated injury, although further studies will be needed to test this hypothesis.
In summary, we have shown that the expression levels of Reg proteins were stronger, especially Reg IV protein, and liver injury was ameliorated after treatment with IL-22 recombinant lentivirus in a rat model of acute liver injury. Taken together, our data suggested that Reg protein, especially Reg IV protein, might act as a biological mediator of immune cell-derived IL-22 in the recovering mechanism of liver injury.
Acknowledgements
The authors thank professor Bo Qin for helpful advice. This work was supported by natural Science Foundation Project of Chongqing Science And Technology Committee (No. cstc2013jcyjA10078) and the Projects of Municipal Health Bureau of Chongqing (No. 2012-02-22).
Disclosure of conflict of interest
None.
References
- 1.Koniaris LG, McKillop IH, Schwartz SI, Zimmers TA. Liver regeneration. J Am Coll Surg. 2003;197:634–659. doi: 10.1016/S1072-7515(03)00374-0. [DOI] [PubMed] [Google Scholar]
- 2.Fausto N, Riehle KJ. Mechanisms of liver regeneration and their clinical implications. J Hepatobiliary Pancreat Surg. 2005;12:181–189. doi: 10.1007/s00534-005-0979-y. [DOI] [PubMed] [Google Scholar]
- 3.Terazono K, Yamamoto H, Takasawa S, Shiga K, Yonemura Y, Tochino Y, Okamoto H. A novel gene activated in regenerating islets. J Biol Chem. 1988;263:2111–2114. [PubMed] [Google Scholar]
- 4.Liu JL, Cui W. Which gene, Reg2 or Reg3beta, was targeted that affected liver regeneration? Hepatology. 2007;45:1584–1585. doi: 10.1002/hep.21693. [DOI] [PubMed] [Google Scholar]
- 5.Sanchez D, Mueller CM, Zenilman ME. Pancreatic regeneration gene I and acinar cell differentiation: influence on cellular lineage. Pancreas. 2009;38:572–577. doi: 10.1097/mpa.0b013e3181a1d9f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fukuhara H, Kadowaki Y, Ose T, Monowar A, Imaoka H, Ishihara S, Takasawa S, Kinoshita Y. In vivo evidence for the role of Reg I in gastric regeneration: transgenic overexpression of Reg I accelerates the healing of experimental gastric ulcers. Lab Invest. 2010;90:556–565. doi: 10.1038/labinvest.2010.42. [DOI] [PubMed] [Google Scholar]
- 7.Pittenger GL, Taylor-Fishwick D, Vinik AI. The role of islet neogeneis-associated protein (INGAP) in pancreatic islet neogenesis. Curr Protein Pept Sci. 2009;10:37–45. doi: 10.2174/138920309787315211. [DOI] [PubMed] [Google Scholar]
- 8.Vasseur S, Folch-Puy E, Hlouschek V, Garcia S, Fiedler F, Lerch MM, Dagorn JC, Closa D, Iovanna JL. p8 improves pancreatic response to acute pancreatitis by enhancing the expression of the anti-inflammatory protein pancreatitis-associatec protein I. J Biol Chem. 2004;279:7199–7207. doi: 10.1074/jbc.M309152200. [DOI] [PubMed] [Google Scholar]
- 9.Zhang H, Kandil E, Lin YY, Levi G, Zenilman ME. Targeted inhibition of gene expression of pancreatitis-associated proteins exacerbates the severity of acute pancreatitis in rats. Scand J Gastroenterol. 2004;39:870–81. doi: 10.1080/00365520410006477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang X, Wang B, Wu J. Pancreatitis-associated protein-I mRNA expression in mouse pancreas is upregulated by lipopolysaccharide independent of cerulean-pancreatitis. J Gastroenterol Hepatol. 2001;16:79–86. doi: 10.1046/j.1440-1746.2001.02389.x. [DOI] [PubMed] [Google Scholar]
- 11.Mueller M, Zhang H, Zenilman ME. Pancreatic reg I binds MKP-1 and regulates cyclin D in pancreatic-derived cell. J Surg Res. 2008;150:137–143. doi: 10.1016/j.jss.2008.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sekikawa A, Fukui H, Fujii S, Ichikawa K, Tomita S, Imura J, Chiba T, Fujimori T. REG I protein mediates an anti-apoptotic effect of STAT3 signaling in gastric cancer cells. Carcinogenesis. 2008;29:76–83. doi: 10.1093/carcin/bgm250. [DOI] [PubMed] [Google Scholar]
- 13.Malka D, Vasseur S, Bodeker H, Ortiz EM, Dusetti NJ, Verrando P, Dagorn JC, Iovanna JL. Tumor necrosis factor α triggers antiapoptotic mechanisms in rat pancreatic cells through pancreatitis-associated protein I activation. Gastroenterology. 2000;119:816–828. doi: 10.1053/gast.2000.16491. [DOI] [PubMed] [Google Scholar]
- 14.Valery C, Vasseur S, Sabatier F, Iovanna JL, Dagorn JC, Grob JJ, Verrando P. Pancreatitis associated protein I (PAP-I) alters adhesion and motility of human melanocytes and melanoma cells. J Invest Dermatol. 2001;116:426–433. doi: 10.1046/j.1523-1747.2001.01278.x. [DOI] [PubMed] [Google Scholar]
- 15.Christa L, Carnot F, Simon MT, Levavasseur F, Stinnakre MG, Lasserre C, Thepot D, Clement B, Devinoy E, Brechot C. HIP/PAP is an adhesive protein expressed in hepatocarcinoma, normal paneth, and pancreatic cells. Am J Physiol. 1996;271:G993–1002. doi: 10.1152/ajpgi.1996.271.6.G993. [DOI] [PubMed] [Google Scholar]
- 16.Sekikawa A, Fukui H, Fujii S, Nanakin A, Kanda N, Uenoyama Y, Sawabu T, Hisatsune H, Kusaka T, Ueno S, Nakase H, Seno H, Fujimori T, Chiba T. Possible role of REG Iα protein in ulcerative colitis and colitic cancer. Gut. 2005;54:1437–1444. doi: 10.1136/gut.2004.053587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sekikawa A, Fukui H, Fujii S, Takeda J, Nanakin A, Hisatsune H, Seno H, Takasawa S, Okamoto H, Fujimori T, Chiba T. REG I α protein may function as a trophic and/or anti-apoptotic factor in the development of gastric cancer. Gastroenterology. 2005;128:642–653. doi: 10.1053/j.gastro.2004.12.045. [DOI] [PubMed] [Google Scholar]
- 18.Wolk K, Kunz S, Asadullah K, Sabat R. Cutting edge: immune cells as sources and targets of the IL-10 family members? J Immunol. 2002;168:5397–5402. doi: 10.4049/jimmunol.168.11.5397. [DOI] [PubMed] [Google Scholar]
- 19.Xie MH, Aggarwal S, Ho WH, Foster J, Zhang Z, Stinson J, Wood WI, Goddard AD, Gurney AL. Interleukin (IL)-22, a novel human cytokine that signals through the interferon receptor-related proteins CRF2-4 and IL-22R. J Biol Chem. 2000;275:31335–31339. doi: 10.1074/jbc.M005304200. [DOI] [PubMed] [Google Scholar]
- 20.Kotenko SV, Izotova LS, Mirochnitchenko OV, Esterova E, Dickensheets H, Donnelly RP, Pestka S. Identification of the functional interleukin-22 (IL-22) receptor complex: the IL-10R2 chain (IL-10Rbeta) is a common chain of both the IL-10 and IL-22 (IL-10-related T cell-derived inducible factor, IL-TIF) receptor complexes. J Biol Chem. 2001;276:2725–2732. doi: 10.1074/jbc.M007837200. [DOI] [PubMed] [Google Scholar]
- 21.Brand S, Dambacher J, Beigel F, Zitzmann K, Heeg MH, Weiss TS, Prüfer T, Olszak T, Steib CJ, Storr M, Göke B, Diepolder H, Bilzer M, Thasler WE, Auernhammer CJ. IL-22-mediated liver cell regeneration is abrogated by SOCS-1/3 overexpression in vitro. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1019–G1028. doi: 10.1152/ajpgi.00239.2006. [DOI] [PubMed] [Google Scholar]
- 22.Campbell JS, Prichard L, Schaper F, Schmitz J, Stephenson-Famy A, Rosenfeld ME, Argast GM, Heinrich PC, Fausto N. Expression of suppressors of cytokine signaling during liver regeneration. J Clin Invest. 2001;107:1285–1292. doi: 10.1172/JCI11867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cressman DE, Greenbaum LE, DeAngelis RA, Ciliberto G, Furth EE, Poli V, Taub R. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science. 1996;274:1379–1383. doi: 10.1126/science.274.5291.1379. [DOI] [PubMed] [Google Scholar]
- 24.Fausto N. Liver regeneration and repair: hepatocytes, progenitor cells, and stem cells. Hepatology. 2004;39:1477–1487. doi: 10.1002/hep.20214. [DOI] [PubMed] [Google Scholar]
- 25.Trautwein C, Rakemann T, Niehof M, Rose-John S, Manns MP. Acute-phase response factor, increased binding, and target gene transcription during liver regeneration. Gastroenterology. 1996;110:1854–1862. doi: 10.1053/gast.1996.v110.pm8964411. [DOI] [PubMed] [Google Scholar]
- 26.Yamada Y, Kirillova I, Peschon JJ, Fausto N. Initiation of liver growth by tumor necrosis factor: deficient liver regeneration in mice lacking type I tumor necrosis factor receptor. Proc Natl Acad Sci U S A. 1997;94:1441–1446. doi: 10.1073/pnas.94.4.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ren X, Hu B, Colletti LM. IL-22 is involved in liver regeneration after hepatectomy. Am J Physiol Gastrointest Liver Physiol. 2010;298:G74–G80. doi: 10.1152/ajpgi.00075.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ki SH, Park O, Zheng M, Morales-Ibanez O, Kolls JK, Bataller R, Gao B. Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: role of signal transducer and activator of transcription 3. Hepatology. 2010;52:1291–1300. doi: 10.1002/hep.23837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sekikawa A, Fukui H, Suzuki K, Karibe T, Fujii S, Ichikawa K, Tomita S, Imura J, Shiratori K, Chiba T, Fujimori T. Involvement of the IL-22/REG Ialpha axis in ulcerative colitis. Lab Invest. 2010;90:496–505. doi: 10.1038/labinvest.2009.147. [DOI] [PubMed] [Google Scholar]
- 30.Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ, Ouyang W. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 31.Wolk K, Witte E, Wallace E, Döcke WD, Kunz S, Asadullah K, Volk HD, Sterry W, Sabat R. IL-22 regulates the expression of genes responsible for antimicrobial defense, celluar differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur J Immunol. 2006;36:1309–1323. doi: 10.1002/eji.200535503. [DOI] [PubMed] [Google Scholar]
- 32.Brand S, Beigel F, Olszak T, Zitzmann K, Eichhorst ST, Otte JM, Diepolder H, Marquardt A, Jagla W, Popp A, Leclair S, Herrmann K, Seiderer J, Ochsenkühn T, Göke B, Auernhammer CJ, Dambacher J. IL-22 is increased in active Crohn’s disease and promotes proinflammatory gene expression and intestinal epithelial cell migration. Am J Physiol Gastrointest Liver Physiol. 2006;290:G827–G838. doi: 10.1152/ajpgi.00513.2005. [DOI] [PubMed] [Google Scholar]
- 33.Boniface K, Bernard FX, Garcia M, Gurney AL, Lecron JC, Morel F. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J Immunol. 2005;174:3695–3702. doi: 10.4049/jimmunol.174.6.3695. [DOI] [PubMed] [Google Scholar]
- 34.Fukui H, Kinoshita Y, Maekawa T, Okada A, Waki S, Hassan S, Okamoto H, Chiba T. Regenerating gene protein may mediate gastric mucosal proliferation induced by hypergastrinemia in rats. Gastroenterology. 1998;115:1483–1493. doi: 10.1016/s0016-5085(98)70027-7. [DOI] [PubMed] [Google Scholar]
- 35.Nanakin A, Fukui H, Fujii S, Sekikawa A, Kanda N, Hisatsune H, Seno H, Konda Y, Fujimori T, Chiba T. Expression of the REG IV gene in ulcerative colitis. Lab Invest. 2007;87:304–314. doi: 10.1038/labinvest.3700507. [DOI] [PubMed] [Google Scholar]
- 36.Ose T, Kadowaki Y, Fukuhara H, Kazumori H, Ishihara S, Udagawa J, Otani H, Takasawa S, Okamoto H, Kinoshita Y. Reg I-knockout mice reveal its role in regulation of cell growth that is required in generation and maintenance of the villous structure of small intestine. Oncogene. 2007;26:349–359. doi: 10.1038/sj.onc.1209799. [DOI] [PubMed] [Google Scholar]


