Abstract
Impulse oscillometry (IOS) is a good method for measuring airway resistance. The aim of this study was to assess the diagnostic contribution of IOS combined with bronchial dilation test (BDT) when distinguishing between patients with asthma and those with chronic obstructive pulmonary disease (COPD). 870 were enrolled in the study including 561 patients with asthma, 100 patients with COPD and 209 patients with chronic coughing or normal subjects. All the participants underwent routine pulmonary function tests, IOS and BDT examination. And IOS examination was before and after BDT. IOS parameters (R5, R20, R25, R35, X5, X20, X25, X35, Fres, Zrs & RP) and forced expiratory volume in one second (FEV1) were recorded. Receiver operating characteristics (ROC) curve analysis was performed to evaluate the diagnostic ability to differentiate asthma and COPD. The discriminative power of the various parameters studied was determined by means of ROC curves: the area under the curve (AUC), sensitivity and specificity. The X5, X20, X25, X35, Fres, Zrs and Rp correlated better with COPD. In particular, X5, Fres and X25 have been found to be significantly correlated with COPD. The diagnostic efficiency of X5, Fres and X25 when diagnosis COPD, expressed by ROC curve parameters, was as follows: AUC (0.725, 0.730, 0.724), sensitivity (67%, 77%, 83%) and specificity (68%, 65%, 58%), respectively. The diagnostic efficiency of Zrs, R5 and X35 when diagnosis asthma, expressed by ROC curve parameters, was as follows: AUC (0.721, 0.710, 0.695), sensitivity (62%, 72%, 53%) and specificity (72%, 61%, 76%), respectively. Our findings show, that X5, X25 and Fres may be useful for predictions and evaluations for COPD. And R5, X35 and Zrs may provide useful IOS parameters for asthma. IOS combined BDT could be useful diagnostic and differential diagnosis between asthma and COPD.
Keywords: Impulse oscillometry system (IOS), chronic obstructive pulmonary disease (COPD), asthma
Introduction
Asthma and chronic obstructive pulmonary disease (COPD) are chronic inflammatory disease of the airways and cause a major public health concern [1,2]. Asthma is a chronic inflammatory disease of the airways, usually starting in childhood, characterized by reversible airflow obstruction [3]. On the contrary, COPD is typically caused by tobacco smoking and displays incompletely airflow obstruction [4]. Obstruction is usually intermittent and reversible in asthma, but is progressive and irreversible in COPD [5]. Therefore, asthma and COPD may overlap and converge, especially in older people [6].
Bronchial dilation test (BDT) was used for the diagnosis and differential diagnosis between asthma and COPD [7]. In BDT, forced expiratory volume in the first second (FEV1) was used as a measure of evaluation [8]. However, BDT may be affected by many factors, such as the state of systemic in patients, patients’ cooperation. Sometimes, the result does not fully reflect the degree of patients’ airway obstruction.
Impulse oscillometry (IOS) is a good method for measuring airway resistance. In contrast to BDT, a major advantage in IOS is its ability to perform these measurements in a noninvasive and relatively effort independent during spontaneous normal tidal breathing [9,10]. Therefore, the advantage of IOS is that requires minimal patient cooperation. It measures both small and large airways resistance and resonance capacitance of the lung [11]. Due to detecting large and small airways diseases separately, IOS has been applied in asthma diagnosis and management [12-14].
In this study, we used IOS before and after BDT to examine whether IOS parameter differ between patients with asthma and patients with COPD and to evaluate the value of diagnosis and differential diagnosis.
Materials and methods
Study population
870 participants (561 patients with asthma, 100 patients with COPD and 209 patients with chronic coughing or normal subjects) who visited the Department of Respiratory Medicine of the 306th Hospital of PLA between February 2002 and December 2009 were enrolled in the study. This study included 552 males and 318 females, ranging from 15 years to 98 years old (mean age, 50.5 ± 18.8 years old). The diagnosis of asthma and COPD were established according to American Thoracic Society (ATS) criteria. This study was conducted with approval from the Ethics Committee of 306 Hospital of PLA. Written informed consent was obtained from all participants.
Bronchial dilation test (BDT)
All subjects withheld short- and long-acting bronchodilators 6 and 24 hours, respectively, prior to study visits. FEV1 at 15 minutes after inhaling 200 μg salbutamol is the best choice as it is highly efficient and causes less side effects. An absolute increase of FEV1 200 ml or improving rate FEV1 12% should be the positive standard of diagnosing asthma after patients inhaling 200 μg salbutamol. Without the positive results, combined with the clinical symptoms, patients were diagnosed COPD (FEV1 < 200 ml or improving rate FEV1 < 12%).
Impulse oscillometry (IOS)
IOS parameters were collected before conventional spirometry using the MasterLab IOS System (Erich Jaeger Co., Würzburg, Germany). The pneumotachometer was calibrated daily using a 3 litre syringe, and pressure calibration was checked weekly with a reference resistance (0.2 kPa/l/s). The individuals wore a nose clip and a manufacturer-provided oval hard plastic mouthpiece to prevent expired air from escaping. They were also asked to support their cheeks with their hands to decrease shunt compliance. Artifacts caused by coughing, breath-holding, swallowing, and vocalization were not included. The impedance (Zrs) representing a complex airway resistance, which includes two components, the real resistance (Rrs) and the imaginary reactance (Xrs), has also been determined. The frequency range of the signal was from 0 to 100 Hz, and we recorded R5-35 and X5-35. Rrs at 5 and 20 Hz represent the low (total resistance) and high (central resistance) frequency range, respectively. The parameters evaluated were R5, R20, R25, R35, X5, X20, X25, X35, resonant frequency (Fres) and peripheral resistance (Rp). Reported results are averages of 3-4 technically acceptable periods of 40-60 s of tidal breathing.
Statistics
Data were analyzed using version 11 of the SPSS statistical software. Data are given as mean ± standard deviation (SD). Receiver operating characteristics (ROC) curve analysis was performed to evaluate the diagnostic ability to differentiate asthma and COPD. The discriminative power of the various parameters studied was determined by means of ROC curves: the area under the curve (AUC), sensitivity and specificity. The ROC curves with AUC were plotted to demonstrate sensitivity and specificity of the evaluated IOS. P-value < 0.05 were considered statistically significant.
Results
A total of 870 participants were enrolled into this study. Among 870 patients, 561 were diagnosed with asthma, 100 were diagnosed with COPD and 209 patients with chronic coughing or normal subjects. ROC curves were constructed for each of the IOS measurements. For 209 patients with chronic coughing or normal subjects, were just negative controls when established the optimal cut-off values for IOS parameters in ROC curves to diagnose asthma and COPD.
The X5, X20, X25, X35, Fres, Zrs and Rp correlated better with COPD. In particular, X5, Fres and X25 have been found to be significantly correlated with COPD. The X5 had AUC of 0.725 and produced a sensitivity of 67% and a specificity of 68% for the diagnosis of COPD. The Fres had AUC of 0.730 and produced a sensitivity of 77% and a specificity of 65% for the diagnosis of COPD. The X25 had AUC of 0.724 and produced a sensitivity of 83% and a specificity of 58% for the diagnosis of COPD (Tables 1 and 2).
Table 1.
AUC about IOS parameters: predictive value of asthma and COPD
| IOS parameters | Before BDT | After BDT | BDT difference§ | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Asthma | COPD | Asthma | COPD | Asthma | COPD | |
| R5 | 0.617 | 0.593 | 0.505 | 0.686 | 0.710* | 0.613 |
| R20 | 0.518 | 0.497 | 0.534 | 0.576 | 0.580 | 0.621 |
| X5 | 0.615 | 0.656 | 0.492 | 0.725* | 0.684 | 0.517 |
| Fres | 0.656 | 0.627 | 0.533 | 0.730* | 0.694 | 0.616 |
| Zrs | 0.617 | 0.614 | 0.505 | 0.713* | 0.721* | 0.600 |
| R25 | 0.507 | 0.512 | 0.536 | 0.553 | 0.572 | 0.589 |
| R35 | 0.498 | 0.526 | 0.546 | 0.546 | 0.580 | 0.582 |
| X20 | 0.650 | 0.625 | 0.533 | 0.717* | 0.692 | 0.607 |
| X25 | 0.644 | 0.627 | 0.536 | 0.724* | 0.679 | 0.622 |
| X35 | 0.659 | 0.622 | 0.543 | 0.706* | 0.695 | 0.606 |
| Rp | 0.592 | 0.655 | 0.506 | 0.715* | 0.669 | 0.601 |
AUC > 0.7.
BDT difference: the difference between after BDT and before BDT.
Table 2.
Sensitivity and specificity of IOS predictive indexs for asthma and COPD
| IOS predictive indexs | Sensitivity | Specificity |
|---|---|---|
| Asthma | ||
| R5 | 0.72 | 0.61 |
| X35 | 0.53 | 0.76 |
| Zrs | 0.62 | 0.72 |
| COPD | ||
| X5 | 0.67 | 0.68 |
| X25 | 0.83 | 0.58 |
| Fres | 0.77 | 0.65 |
The Zrs had AUC of 0.721 and produced a sensitivity of 62% and a specificity of 72% for the diagnosis of asthma. The R5 had AUC of 0.710 and produced a sensitivity of 72% and a specificity of 61% for the diagnosis of asthma. The X35 had AUC of 0.695 and produced a sensitivity of 53% and a specificity of 76% for the diagnosis of asthma (Tables 1 and 2). IOS parameters had predictive effects if AUC is higher than 0.7. Diagnostic performance of IOS parameters were shown in Figure 1. Therefore, X5, X20, X25, X35, Fres, Zrs and Rp correlated better with COPD. The X5, X25 and Fres have clearly the best predictive value for COPD. For asthma, R5, X35 and Zrs have clearly the best predictive value.
Figure 1.
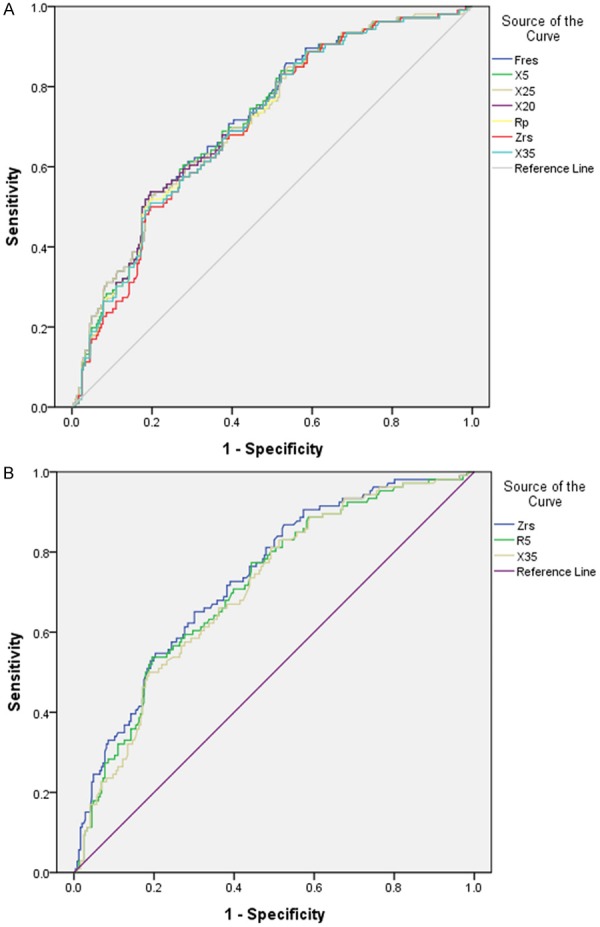
Receiver Operating Characteristic (ROC) curves for distinguishing COPD (A) and asthma (B). The diagnostic efficiency of X5, Fres and X25 when diagnosis COPD, expressed by ROC curve parameters, was as follows: AUC (0.725, 0.730, 0.724), sensitivity (67%, 77%, 83%) and specificity (68%, 65%, 58%), respectively. The diagnostic efficiency of Zrs, R5 and X35 when diagnosis asthma, expressed by ROC curve parameters, was as follows: AUC (0.721, 0.710, 0.695), sensitivity (62%, 72%, 53%) and specificity (72%, 61%, 76%), respectively.
Discussion
Many clinical studies have reported that IOS has reference values and high reproducibility in chronic pulmonary diseases [15]. Asthma and COPD are prevalent chronic pulmonary diseases [16]. COPD is characterized by chronic airway inflammation and/or airflow limitation and asthma is characterized by airway obstruction [17]. Generally, reversibility of airflow limitation is incomplete in COPD, while that in asthma can be complete [18]. Therefore, asthma and COPD may overlap and converge [19,20]. Our study was to explore the application and clinical value of combining IOS and BDT for differential diagnosis between asthma and COPD.
In the present study, 870 participants were categorized in three groups: 1) 561 patients with asthma; 2) 100 patients with COPD; 3) 209 patients with chronic coughing or normal subjects. After inhaling salbutamol, IOS and spirometry measurement was detected by devices. Compared with the control group (chronic coughing or normal subjects), the results showed that IOS was useful to distinguish between patients with asthma and those with COPD. So that it seems more to apply for the target populations in medical procedures.
In IOS parameters, X5, X20, X25, X35, Fres, Zrs and Rp correlated better with COPD. In particular, X5, X25 and Fres have been found to be significantly correlated with COPD. And these IOS parameters of sensitivity and specificity are good for COPD. For asthma, there was no significant difference in X5, X25 and Fres. But R5, X35 and Zrs had excellent correlations in asthma with high sensitivity and specificity. These parameters could be predicted COPD and asthma, respectively.
The results were an initial exploration of IOS parameters differences in resistance and reactance in patients with asthma compared with patients with COPD. We observed that peripheral airway resistance and airway elastic resistance increased in patients with COPD but not in patients with asthma. The measurement of IOS parameters showed that total airway resistance and viscous resistance were significantly elevated in asthma. The differences between the two diseases are consistent with the cellular and molecular features of airway inflammation and the degree of reversibility of airway flow limitation.
In conclusion, IOS parameters, X5, X25 and Fres may be useful for predictions and evaluations for COPD. And R5, X35 and Zrs may be as diagnostic markers in patients for asthma. Thus, IOS combined BDT could be useful diagnostic and differential diagnosis between asthma and COPD. In particularly, it suits for old people because IOS requires little patient cooperation and noninvasive and quick method.
Disclosure of conflict of interest
None.
References
- 1.Leuppi JD, Ott SR. [Management of an acute exacerbation of asthma and COPD] . Ther Umsch. 2014;71:289–293. doi: 10.1024/0040-5930/a000515. [DOI] [PubMed] [Google Scholar]
- 2.Andersen H, Lampela P, Nevanlinna A, Sayna-jakangas O, Keistinen T. High hospital burden in overlap syndrome of asthma and COPD. Clin Respir J. 2013;7:342–346. doi: 10.1111/crj.12013. [DOI] [PubMed] [Google Scholar]
- 3.Croisant S. Epidemiology of asthma: prevalence and burden of disease. Adv Exp Med Biol. 2014;795:17–29. doi: 10.1007/978-1-4614-8603-9_2. [DOI] [PubMed] [Google Scholar]
- 4.Lopez-Giraldo A, Rodriguez-Roisin R, Agusti A. [Chronic obstructive pulmonary disease: The golden decade. Implications for the diagnosis, prevention and treatment of chronic obstructive pulmonary disease.] . Med Clin (Barc) 2014 doi: 10.1016/j.medcli.2014.03.009. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 5.Postma DS, Reddel HK, ten Hacken NH, van den Berge M. Asthma and chronic obstructive pulmonary disease: similarities and differences. Clin Chest Med. 2014;35:143–156. doi: 10.1016/j.ccm.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 6.Nakawah MO, Hawkins C, Barbandi F. Asthma, chronic obstructive pulmonary disease (COPD), and the overlap syndrome. J Am Board Fam Med. 2013;26:470–477. doi: 10.3122/jabfm.2013.04.120256. [DOI] [PubMed] [Google Scholar]
- 7.Takemura M, Niimi A, Minakuchi M, Matsumoto H, Ueda T, Chin K, Mishima M. Bronchial dilatation in asthma: relation to clinical and sputum indices. Chest. 2004;125:1352–1358. doi: 10.1378/chest.125.4.1352. [DOI] [PubMed] [Google Scholar]
- 8.Bellocchia M, Masoero M, Ciuffreda A, Croce S, Vaudano A, Torchio R, Boita M, Bucca C. Predictors of cardiovascular disease in asthma and chronic obstructive pulmonary disease. Multidiscip Respir Med. 2013;8:58. doi: 10.1186/2049-6958-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winkler J, Hagert-Winkler A, Wirtz H, Hoheisel G. [Modern impulse oscillometry in the spectrum of pulmonary function testing methods] . Pneumologie. 2009;63:461–469. doi: 10.1055/s-0029-1214938. [DOI] [PubMed] [Google Scholar]
- 10.Singh D, Tal-Singer R, Faiferman I, Lasenby S, Henderson A, Wessels D, Goosen A, Dallow N, Vessey R, Goldman M. Plethysmography and impulse oscillometry assessment of tiotropium and ipratropium bromide; a randomized, double-blind, placebo-controlled, cross-over study in healthy subjects. Br J Clin Pharmacol. 2006;61:398–404. doi: 10.1111/j.1365-2125.2006.02594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olaguibel JM, Alvarez MJ, Uribe P, Garcia BE, Tabar AI. [New techniques in the study of asthma] . An Sist Sanit Navar. 2003;26(Suppl 2):57–63. [PubMed] [Google Scholar]
- 12.Saadeh C, Cross B, Gaylor M. Retrospective observations on the ability to diagnose and manage patients with asthma through the use of impulse oscillometry: comparison with spirometry and overview of the literature. Pulm Med. 2014;2014:376890. doi: 10.1155/2014/376890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi Y, Aledia AS, Tatavoosian AV, Vijayalakshmi S, Galant SP, George SC. Relating small airways to asthma control by using impulse oscillometry in children. J Allergy Clin Immunol. 2012;129:671–678. doi: 10.1016/j.jaci.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pisi R, Tzani P, Aiello M, Martinelli E, Marangio E, Nicolini G, Olivieri D, Chetta A. Small airway dysfunction by impulse oscillometry in asthmatic patients with normal forced expiratory volume in the 1st second values. Allergy Asthma Proc. 2013;34:e14–20. doi: 10.2500/aap.2013.34.3641. [DOI] [PubMed] [Google Scholar]
- 15.Williamson PA, Clearie K, Menzies D, Vaid-yanathan S, Lipworth BJ. Assessment of small-airways disease using alveolar nitric oxide and impulse oscillometry in asthma and COPD. Lung. 2011;189:121–129. doi: 10.1007/s00408-010-9275-y. [DOI] [PubMed] [Google Scholar]
- 16.Miedinger D, Stohr S, Pletscher C. [Asthma and COPD in the workplace] . Ther Umsch. 2014;71:275–281. doi: 10.1024/0040-5930/a000513. [DOI] [PubMed] [Google Scholar]
- 17.Magureanu IL, Furtunescu F. [The importance of determining the prevalence of COPD] . Pneumologia. 2013;62:239–246. [PubMed] [Google Scholar]
- 18.Gibson PG, Simpson JL. The overlap syndrome of asthma and COPD: what are its features and how important is it? Thorax. 2009;64:728–735. doi: 10.1136/thx.2008.108027. [DOI] [PubMed] [Google Scholar]
- 19.Fu JJ, McDonald VM, Gibson PG, Simpson JL. Systemic Inflammation in Older Adults With Asthma-COPD Overlap Syndrome. Allergy Asthma Immunol Res. 2014;6:316–324. doi: 10.4168/aair.2014.6.4.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee HY, Kang JY, Yoon HK, Lee SY, Kwon SS, Kim YK, Rhee CK. Clinical characteristics of asthma combined with COPD feature. Yonsei Med J. 2014;55:980–986. doi: 10.3349/ymj.2014.55.4.980. [DOI] [PMC free article] [PubMed] [Google Scholar]


