Abstract
Colorectal cancer (CRC) is a major cause of cancer morbidity and mortality worldwide. Bevacizumab plays an important role in the treatment of metastatic CRC (mCRC). The aim of this study was to evaluate the efficacy and safety of chemotherapy plus bevacizumab as first-line treatment in patients with mCRC. Randomized-controlled clinical trials comparing the efficacy of chemotherapy plus bevacizumab or chemotherapy alone in patients with mCRC were searched using the following electronic database of PubMed, Medline, Embase and CNKI. Total 9 trials, containing 1843 patients in chemotherapy plus bevacizumab group and 1741 patients in chemotherapy alone group, were included. Our results showed that chemotherapy plus bevacizumab statistically increased the Overall response rate (ORR) in patients with mCRC (OR = 1.57, 95% CI = 1.17-2.11, P = 0.003) in a random-effects model. The complete response rate and partial response rate were statistically increased as well (P ≤ 0.05). Subgroup analysis by bevacizumab dosage found that bevacizumab 5 mg/kg statistically increased the ORR. Significant differences were found in PFS (HR = 0.56, 95% CI = 0.46-0.69, P < 0.00001) and OS (HR = 0.83, 95% CI = 0.76-0.91, P < 0.0001) as well. No significant difference was found in adverse events. Overall, the combination of chemotherapy and bevacizumab as first-line treatment is an effective and well-tolerated regimen for patients with mCRC.
Keywords: Metastatic colorectal cancer, bevacizumab, chemotherapy, meta-analysis
Introduction
Colorectal cancer (CRC), arising from the lining of the large intestine (colon and rectum), is a malignant neoplasm [1]. In the late 1940s and early 1950s, CRC is a major cause of cancer morbidity and mortality in the United States, and today it is the third most common cancer and the third leading cause of cancer death in men and women [2]. According to Colorectal Cancer Statistics, an estimated 71,830 men and 65,000 women were diagnosed with CRC and 26,270 men and 24,040 women died of this disease in 2014 [3]. The etiological factors and pathogenetic mechanisms underlying CRC development appear to be complex and heterogeneous. Moreover, there is substantial variation in tumor location by age, and the average age at diagnosis is 66 years [4].
Approximately, 20% of CRC cases have been metastasized at the time of diagnosis [5]. The most common sites of metastatic disease for CRC are the liver followed by the lungs [6]. The presentation of metastatic disease can present treatment dilemmas for physicians. Since the late 1950s, fluorouracil has been the only drug approved for treating metastatic colorectal cancer (mCRC) [7]. Subsequently, irinotecan, oxaliplatin and leucovorin combined with fluorouracil-based chemotherapy regimens have been proved to be well-tolerated and increased response rate, time to progression and survival in patients with mCRC, and are considered as a reference first-line treatment for mCRC [8-10].
Although there are improvements in the treatment of mCRC, there is a strong medical need for more effective and safe therapies. During the last decade, novel targeted therapies, as a single agent or in combination, were used for the treatment of mCRC, especially bevacizumab (Avastin). Bevacizumab, an antibody against vascular endothelial growth factor (VEGF), was first approved as a treatment for mCRC in 2004. It is well suited for use in combination with first- or second-line chemotherapy in the treatment of mCRC because its side effects are predictable and appear not to add to the incidence or severity of the side effects of chemotherapy [11,12]. Clinical trials of bevacizumab in combination with oxaliplatin-containing and 5-fluorouracil-based regimens have shown that combination therapy is well tolerated and its toxicity is not substantially greater than that of the chemotherapy alone [13,14]. Preliminary evidences from observational studies show that the incidence and severity of adverse events with combinations of bevacizumab and newer chemotherapy regimens are similar to those in the pivotal phase III trial with irinotecan, 5-fluorouracil, and leucovorin plus bevacizumab.
Though bevacizumab is used in clinical practice, it is toxicities, and the results remain inconclusive. Thus, we conducted this meta-analysis to systematically evaluate the safety and efficacy of chemotherapy plus bevacizumab as first-line treatment in patients with mCRC.
Materials and methods
Study selection
We conducted a thorough investigation for relevant articles published between January 2000 and July 2014 using the following electronic database of PubMed, Medline, Embase and CNKI (China National Knowledge Infrastructure). The related trials were retrieved by using the following keywords “metastatic colorectal cancer”, “bevacizumab”, “chemotherapy” and “first-line therapy or treatment” as well as their combinations. The corresponding Chinese terms were used in the Chinese library. References of retrieved articles were searched without language restrictions. The search was focused on studies that had been conducted in humans.
Criteria for inclusion
The inclusion criteria were as follows: 1) the paper should be randomized-controlled trials (RCT) or cohort studies evaluating the efficacy and safety of chemotherapy plus bevacizumab as first-line therapy in patients with mCRC; 2) patients should be in prospective phase II and III with previously untreated mCRC; 3) patients in experiment group treated with chemotherapy plus bevacizumab, patients in control group treated with chemotherapy alone; and 4) data including therapeutic effects and adverse events were available to extract.
Data extraction
Two investigators independently assessed the quality of the included trials according to the descriptions provided by the authors of the included studies. Any disagreement was subsequently resolved by discussion with a third author. The following information was extracted from each article: first author, year of publication, country, mean age, total numbers, phase and bevacizumab schedule.
Statistical analysis
The overall effect was measured by odds ratios (ORs), risk ratios (RRs) and hazard ratios (HRs) with their 95% confidence interval (CI). The Z test was employed to determine the significance of the pooled ratios, and a P-value less than 0.05 was considered statistically significant. The I2 test was used to assess the proportion of statistical heterogeneity and the Q-statistic test was used to define the degree of heterogeneity. A P-value less than 0.10 for the Q-test and I2 more than 50% was considered significant among the studies. Data were combined using both a fixed-effects model and a random-effects model. The fixed-effects model is used when the effects are assumed to be homogenous, while the random-effects model is employed when they are heterogenous. The evidence of publication bias was assessed by visual funnel plot inspection. Egger’s regression test was also conducted to identify study effects (P-value less than 0.10 was considered significant). Statistical analyses were conducted by the Review Manager (RevMan version 5.2, the Cochrane Collaboration, Oxford, England). All the tests were two-sided.
Results
Study selection and characteristics
The electronic database search identified 121 references. After applying the inclusion criteria, 9 articles were ultimately included in the systematic review and meta-analysis. The study selection process is shown in Figure 1. The 9 reports, one in Chinese [15] and eight in English [16-23], included 3584 mCRC cases, with 1843 patients in the chemotherapy plus bevacizumab group and 1741 patients in the chemotherapy alone group, and conducted in 5 countries. The detailed characteristics of the studies included were shown in Table 1.
Figure 1.
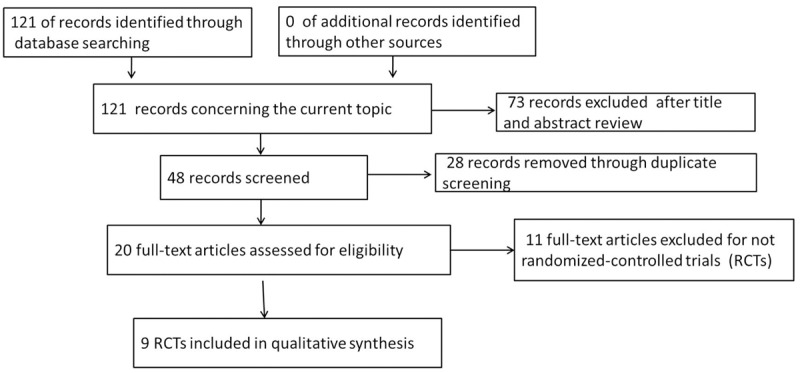
Flow chart of the search process.
Table 1.
Main characteristic of included trials
| First author | Year | Country | Phase | Regimen | Bevacizumab schedule | Mean age (B/C) | Total number | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| B | C | |||||||
| Kabbinavar BF | 2003 | USA | II | C: FU/LV | 5 or 10 mg/kg every two weeks | - | 35/33 | 36 |
| B: FU/LV plus Bevacizumab | ||||||||
| Hurwitz H | 2004 | UK | II | C: IFL plus Placebo | 5 mg/kg every two weeks | 59.5/59.2 | 402 | 411 |
| B: IFL plus Bevacizumab | ||||||||
| Kabbinavar FF | 2005 | USA | II | C: FU/LV plus Placebo | 5 mg/kg every two weeks | 71.3/70.7 | 104 | 105 |
| B: FU/LV plus Bevacizumab | ||||||||
| Saltz LB | 2008 | UK | III | C: FOLFOX-4 or XELOX plus placebo | 7.5 mg/kg every 3 weeks | 60.0 (18-86)/60 (18-83) | 699 | 701 |
| B: FOLFOX-4 or XELOX plus Bevacizumab | ||||||||
| Stathopoulos GP | 2010 | Greece | III | C: IFL | 7.5 mg/kg every 3 weeks | 67 (45-82)/62 (30-87) | 114 | 108 |
| B: IFL plus Bevacizumab | ||||||||
| Tebbutt NC | 2010 | Australia | III | C: Capecitabine | 7.5 mg/kg every 3 weeks | 67 (32-85)/69 (37-86) | 157 | 156 |
| B: Capecitabine plus Bevacizumab | ||||||||
| Guan ZZ | 2011 | China | III | C: mIFL | 5 mg/kg every two weeks | 53 (23-77)/50 (22-72) | 139 | 64 |
| B: mIFL plus Bevacizumab | ||||||||
| Zhang HM | 2012 | China | II | C: FOLFIRI | 5 mg/kg every two weeks | 52 (20-77) | 20 | 20 |
| B: FOLFIRI plus Bevacizumab | ||||||||
| Cunningham D | 2013 | UK | III | C: Capecitabine | 7.5 mg/kg every 3 weeks | 76 (70-87)/77 (70-87) | 140 | 140 |
| B: Capecitabine plus Bevacizumab | ||||||||
B, Bevacizumab-based group; C, control group FU/LV, fluorouracil and leucovorin. mIFL, modified irinotecan, leucovorin bolus, and 5-fluorouracil intravenous infusion; FOLFOX, xaliplatin plus infusional 5-FU.
Overall response rate (ORR)
All the nine trials reported ORR. The ORR was higher in the chemotherapy plus bevacizumab group (experimental group) than that in the chemotherapy alone group (control group) (36.6% versus 26.9%). As shown in Figure 2A, we found that chemotherapy plus bevacizumab statistically increased the ORR in patients with mCRC (OR = 1.57, 95% CI = 1.17-2.11, P = 0.003) in a random-effects model.
Figure 2.
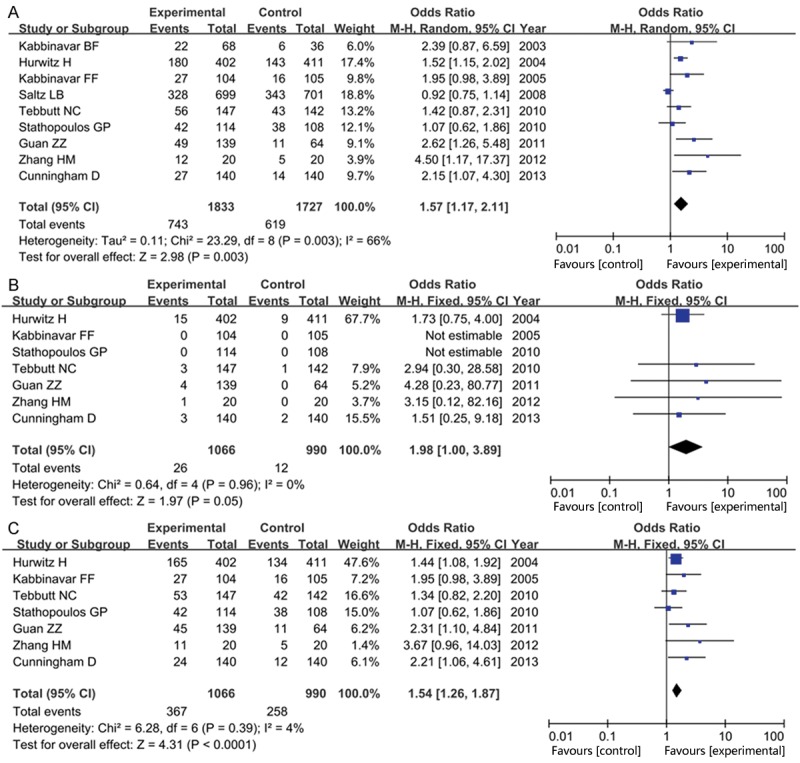
Forest plot of response rate in experiment group (chemotherapy plus bevacizumab) versus control group (chemotherapy alone): A: overall response rate; B: complete response rate; C: partial response rate.
Seven articles reported the complete response rate (CR) and partial response rate (PR). Meta-analysis of these trials showed that there was a statistically significant difference in CR (OR = 1.98, 95% CI = 1.00-3.89, P = 0.05, Figure 2B) and PR (OR = 1.54, 95% CI = 1.26-1.87, P < 0.0001, Figure 2C ) between both groups, indicating that patients in the experimental group improved nearly twice as much the response rate as that in the control group.
When evaluating the efficacy by subgroup analysis of bevacizumab dosage, we found that bevacizumab 5 mg/kg group statistically increased the ORR in patients with mCRC than that in the control group (OR = 2.00, 95% CI = 1.41-2.82, P < 0.001), while no significant advantage was found in bevacizumab 7.5 mg/kg group (OR = 1.21, 95% CI = 0.86-1.71, P = 0.28) as shown in Figure 3.
Figure 3.
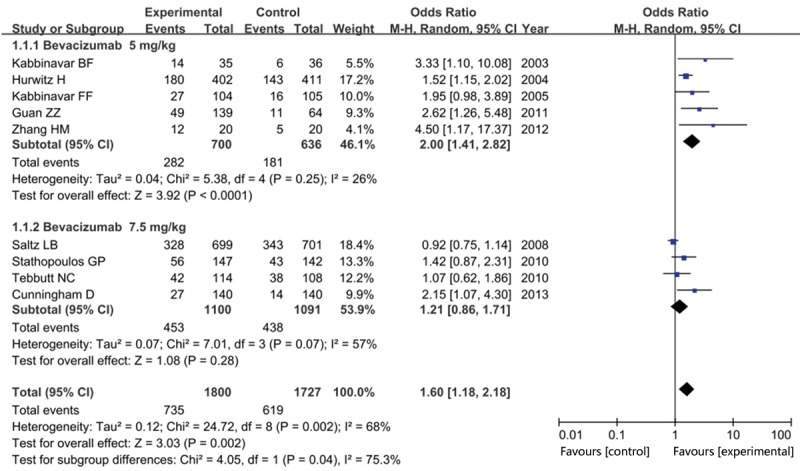
Forest plot of overall response rate in experiment group versus control group in subgroup analysis by bevacizumab dosage.
Progression-free survival (PFS, months)
Eight trials reported the related PFS data, containing 3362 patients with mCRC. Table 2 listed the PFS data in each trial. High heterogeneity between trials was observed (I2 = 78%), so the random-effects model was employed. As shown in Figure 4, we found that patients treated with chemotherapy plus bevacizumab in the experimental group resulted in a statistically significant improvement in PFS compared with first-line chemotherapy alone in control group (HR = 0.56, 95% CI = 0.46-0.69, P < 0.00001).
Table 2.
Therapeutic efficacy of chemotherapy plus bevacizumab (experimental group) versus chemotherapy alone (control group)
| First author | PFS (median months) | OS (median months) | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| B | C | P | HR (95% CI) | B | C | P | HR (95% CI) | |
| Kabbinavar BF | 7.4 | 5.2 | 0.013 | 0.54 (0.31-0.94) | 21.5 | 13.8 | < 0.001 | 0.86 (0.44-1.68) |
| Hurwitz H | 10.6 | 6.2 | < 0.001 | 0.54 (0.45-0.65) | 20.3 | 15.6 | < 0.001 | 0.66 (0.54-0.81) |
| Kabbinavar FF | 9.2 | 5.5 | 0.0002 | 0.50 (0.34-0.73) | 16.6 | 12.9 | 0.016 | 0.79 (0.56-1.10) |
| Saltz LB | 10.4 | 7.9 | <0.0001 | 0.83 (0.74-0.93) | 21.3 | 19.9 | 0.0769 | 0.89 (0.76-1.03) |
| Stathopoulos GP | - | - | - | - | 22 | 25 | 0.1391 | 1.05 (0.81-1.36) |
| Tebbutt NC | 8.5 | 5.7 | < 0.001 | 0.63 (0.50-0.80) | 18.9 | 18.9 | 0.314 | 0.88 (0.68-1.14) |
| Guan ZZ | 8.3 | 4.2 | < 0.001 | 0.44 (0.31-0.63) | 18.7 | 13.4 | 0.014 | 0.62 (0.41-0.95) |
| Zhang HM | 10.1 | 5.1 | 0.002 | 0.65 (0.60-0.71) | 18 | 11 | 0.016 | 0.83 (0.41-1.69) |
| Cunningham D | 9.1 | 5.1 | < 0.0001 | 0.53 (0.41-0.69) | 20.7 | 16.8 | 0.18 | 0.79 (0.57-1.09) |
PFS: Progression-free survival; OS, overall Survival; HR: Hazard ratio; 95% CI, 95% confidence interval; B: Bevacizumab-based group/Experimental group; C: Control group; P: P-value.
Figure 4.
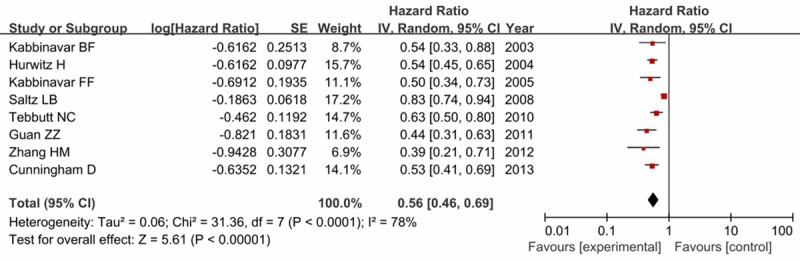
Forest plot of progression-free survival in experiment group versus control group.
Overall survival (OS, months)
All the trials reported the OS data which presented in Table 2. No significant heterogeneity between trials was observed (I2 = 29%, P = 0.18), and the fixed-effects model was used. Our results indicated that the addition of bevacizumab to first-line chemotherapy improved the OS compared with chemotherapy alone (HR = 0.83, 95% CI = 0.76-0.91, P < 0.0001) as shown in Figure 5.
Figure 5.
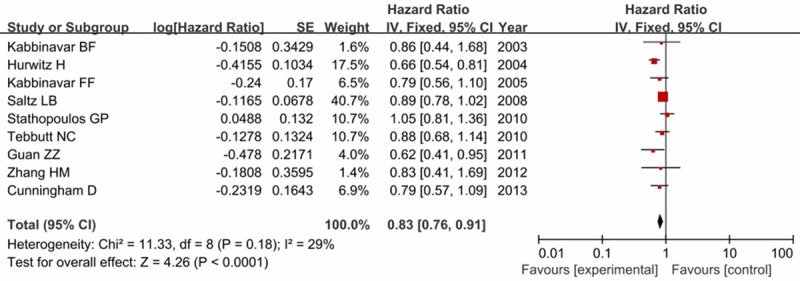
Forest plot of overall survival in experiment group versus control group.
Adverse effect
Treatment-related toxicity included all-cause death within 60 days, diarrhea, leukopenia, bleeding, fever and so on. Each trial reported some of these adverse events. Our results found that the incidence of any Grade 3/4 adverse event was higher among patients in the experiment group than that in the control group (78.1% versus 69.2%), with a statistically significant difference (OR = 1.70, 95% CI = 1.43-0.02, P < 0.0001) as shown in Figure 6. No significant difference was found between these two groups when related to diarrhea (OR = 1.18, 95% CI = 0.95-1.45, P = 0.13), leukopenia (OR = 1.19, 95% CI = 0.93-1.54, P = 0.17), treatment related mortality (RR = 0.97, 95% CI = 0.59-1.73, P = 0.87) and bleeding (OR = 0.51, 95% CI = 0.08-4.37, P = 0.68).
Figure 6.
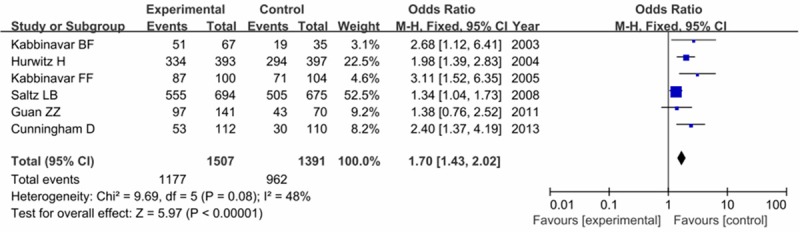
Forest plot of comparison of any grade 3/4 adverse events in experiment group versus control group.
Publication bias
The funnel plots of ORR and PFS revealed an apparent asymmetry that suggested the presence of a potential publication bias. Funnel plots of OS detected no obvious publication bias. Figure 7 showed the results.
Figure 7.
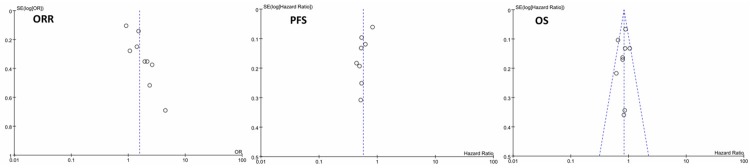
Funnel plot of ORR, PFS and OS in this meta-analysis.
Discussion
CRC is the third most common cancer worldwide. Approximately 20-25% of patients with CRC have synchronous metastases at the time of diagnosis, and 50-60% of the remainder will develop metachronous metastases [24,25]. For most patients with mCRC, treatment remains incurable rather than curative. There are five active agents available for the treatment of advanced disease: 5-fluorouracil (5-FU), oxaliplatin, leucovorin, capecitabine and irinotecan. Saltz et al. have shown that weekly treatment with irinotecan plus fluorouracil and leucovorin is superior to a widely used regimen of fluorouracil and leucovorin for mCRC in terms of PFS and OS [26]. Goldberg et al. have found that when oxaliplatin plus infusional 5-FU (FOLFOX) was compared to irinotecan plus bolus 5-FU (IFL), both the response rate and survival were improved in the oxaliplatin-containing arm [10]. These regimens should be considered as a standard therapy for patients with advanced CRC. However, a randomized study has shown that when 5-FU is administered by the same schedule in each arm, survival is similar between the irinotecan- and oxaliplatin-containing arms [27]. Moreover, serious adverse events and treatment-related toxicity were found among a population-based cohort of patients receiving first-line chemotherapy for mCRC. Thus, new therapies are needed to further treat patients with mCRC.
Recently, the treatment of mCRC has evolved significantly. Bevacizumab, a monoclonal antibody against vascular endothelial growth factor (VEGF), is one of the recent additions to the list of systemic drugs for the treatment of mCRC. It was first approved as a treatment for mCRC in 2004, followed by cetuximab (also in 2004) and panitumumab (2006). Cetuximab and panitumumab both target the epidermal growth factor receptor (EGFR) and are effective only in patients with wild-type KRAS mCRC [28,29]. Panitumumab is the only approved fully human anti-EGFR monoclonal antibody, while cetuximab is a chimeric antibody and bevacizumab is a humanized monoclonal antibody. The National Institute for Health and Clinical Excellence (NICE) invited the manufacturer of bevacizumab (Roche Products) to submit evidence for the clinical and cost effectiveness of this drug for the treatment of patients with mCRC, as part of the Institute’s Single Technology Appraisal (STA) process [30]. The introduction of bevacizumab therapy has improved patient treatment outcomes. A meta-analysis conducted by Dai et al. have found that bevacizumab has efficacy in all treatment regimens for advanced CRC, while the use of bevacizumab among patients with mCRC increased the risk of serious adverse events [31]. Furthermore, bevacizumab in combination with fluoropyrimidine-based chemotherapy is the standard treatment for mCRC in the first-line (1 L) and bevacizumab-naïve second-line (2 L) settings. As well, severe adverse events can occur with these treatment options, and their management can be challenging for patients and clinicians. Therefore, assessing the efficacy and safety of chemotherapy plus bevacizumab as first-line treatment in patients with mCRC is crucial.
In the present meta-analysis, we totally selected 9 RCT trials. Our results showed that the addition of bevacizumab to chemotherapy as first-line therapy for mCRC resulted in a clinically statistically significant improvement in ORR, CR, PR, PFS and OS compared with chemotherapy alone. No significant adverse events were found between two groups. Our study indicated that bevacizumab plus standard chemotherapy has clinical benefits in patients with mCRC, which is consistent with previous meta-analysis conducted by Cao et al. [32], Macedo et al. [33] and Wang et al. [34].
Nowadays, bevacizumab is indicated for the first- and second-line treatment of mCRC in combination with fluoropyrimidine-based chemotherapy. Saltz et al. have shown that the addition of bevacizumab to oxaliplatin-based chemotherapy significantly improved PFS in the first-line trial in patients with mCRC [17]. Kabbinavar et al. have found that addition of bevacizumab to FU/LV as first-line therapy in CRC patients who were not considered optimal candidates for first-line irinotecan treatment provided clinically significant patient benefit, including statistically significant improvement in PFS [16]. Hurwitz et al. have proved that the FU/LV/BV regimen seems as effective as IFL and has an acceptable safety profile, indicating that FU/LV/BV is an active alternative treatment regimen for patients with previously untreated mCRC [35]. The Bevacizumab Expanded Access Trial (BEAT) study shows that the efficacy and safety profile of bevacizumab in routine clinical practice is consistent with results observed in prospective randomized clinical trials and another large observational study in the United States (BRiTE study) [36]. Hochster et al. have suggested that the addition of bevacizumab to oxaliplatin and fluoropyrimidine regimens is well tolerated as first-line treatment of mCRC and does not markedly change overall toxicity, resulting in a median OS of approximately 2 years [12]. Furthermore, for patients with previously treated mCRC, the addition of bevacizumab to oxaliplatin, fluorouracil, and leucovorin improves survival duration [37]. Maintenance of VEGF inhibition with bevacizumab plus standard second-line chemotherapy beyond disease progression has clinical benefits in patients with mCRC [38]. The first randomized study to prospectively investigate the impact of continuing bevacizumab treatment in 2 L mCRC for patients who progressed after receiving a bevacizumab-containing regimen in 1 L has demonstrated that bevacizumab plus chemotherapy (crossed over from 1 L regimen) continued beyond progression significantly prolongs OS and PFS in 2 L mCRC [39].
Conclusions
In conclusion, the addition of bevacizumab to chemotherapy as first-line treatment for patients with mCRC has a statistically significant improvement in ORR, PFS, and OS than that in chemotherapy alone. Its side effects are predictable and manageable. Though bevacizumab can produce a significant treatment benefit, more trials are needed to further evaluate these therapeutic effects and adverse events.
Acknowledgements
We thank the colleagues in department of colorectal surgery, the First Affiliated Hospital, College of Medicine, Zhejiang University, for discussion in this study.
Disclosure of conflict of interest
None.
References
- 1.Cooper K, Squires H, Carroll C, Papaioannou D, Booth A, Logan R, Maguire C, Hind D, Tappenden P. Chemoprevention of colorectal cancer: systematic review and economic evaluation. Heath Technol Assess. 2010;14:1–206. doi: 10.3310/hta14320. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R, DeSantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104–117. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- 4.Hawk ET, Levin B. Colorectal cancer prevention. J. Clin. Oncol. 2005;23:378–391. doi: 10.1200/JCO.2005.08.097. [DOI] [PubMed] [Google Scholar]
- 5.Mills S, Stamos MJ. Complexities in Colorectal Surgery. Springer; 2014. Metastatic Colorectal Cancer; pp. 91–104. [Google Scholar]
- 6.Kanas GP, Taylor A, Primrose JN, Langeberg WJ, Kelsh MA, Mowat FS, Alexander DD, Choti MA, Poston G. Survival after liver resection in metastatic colorectal cancer: review and meta-analysis of prognostic factors. Clin Epidemiol. 2012;4:283. doi: 10.2147/CLEP.S34285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petrelli N, Douglass H, Herrera L, Russell D, Stablein D, Bruckner H, Mayer R, Schinella R, Green M, Muggia F. The modulation of fluorouracil with leucovorin in metastatic colorectal carcinoma: a prospective randomized phase III trial. Gastrointestinal Tumor Study Group. J. Clin. Oncol. 1989;7:1419–1426. doi: 10.1200/JCO.1989.7.10.1419. [DOI] [PubMed] [Google Scholar]
- 8.Douillard J, Cunningham D, Roth A, Navarro M, James R, Karasek P, Jandik P, Iveson T, Carmichael J, Alakl M. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355:1041–1047. doi: 10.1016/s0140-6736(00)02034-1. [DOI] [PubMed] [Google Scholar]
- 9.de Gramont Ad, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J. Clin. Oncol. 2000;18:2938–2947. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 10.Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, Findlay BP, Pitot HC, Alberts SR. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J. Clin. Oncol. 2004;22:23–30. doi: 10.1200/JCO.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 11.Brufsky AM, Hurvitz S, Perez E, Swamy R, Valero V, O’Neill V, Rugo HS. RIBBON-2: A randomized, double-blind, placebo-controlled, phase III trial evaluating the efficacy and safety of bevacizumab in combination with chemotherapy for second-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J. Clin. Oncol. 2011;29:4286–93. doi: 10.1200/JCO.2010.34.1255. [DOI] [PubMed] [Google Scholar]
- 12.Hochster HS, Hart LL, Ramanathan RK, Childs BH, Hainsworth JD, Cohn AL, Wong L, Fehrenbacher L, Abubakr Y, Saif MW. Safety and efficacy of oxaliplatin and fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer: results of the TREE Study. J. Clin. Oncol. 2008;26:3523–3529. doi: 10.1200/JCO.2007.15.4138. [DOI] [PubMed] [Google Scholar]
- 13.Ducreux M, Adenis A, Pignon JP, François E, Chauffert B, Ichanté J, Boucher E, Ychou M, Pierga JY, Montoto-Grillot C. Efficacy and safety of bevacizumab-based combination regimens in patients with previously untreated metastatic colorectal cancer: final results from a randomised phase II study of bevacizumab plus 5-fluorouracil, leucovorin plus irinotecan versus bevacizumab plus capecitabine plus irinotecan (FNCLCC ACCORD 13/0503 study) Eur J Cancer. 2013;49:1236–1245. doi: 10.1016/j.ejca.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 14.Tol J, Koopman M, Rodenburg CJ, Cats A, Creemers GJ, Schrama JG, Erdkamp FL, Vos AH, Mol L, Antonini NF, Punt CJ. A randomised phase III study on capecitabine, oxaliplatin and bevacizumab with or without cetuximab in first-line advanced colorectal cancer, the CAIRO2 study of the Dutch Colorectal Cancer Group (DCCG). An interim analysis of toxicity. Ann Oncol. 2008;19:734–8. doi: 10.1093/annonc/mdm607. [DOI] [PubMed] [Google Scholar]
- 15.Zhang H, Xu L, An G. Evaluation of bevadzumab combined with FOLFIRI as first-line treatment for patients with metastatic colorectal cancer. Cancer Res Pre Treat. 2012;39:1001–1004. [Google Scholar]
- 16.Kabbinavar FF, Schulz J, McCleod M, Patel T, Hamm JT, Hecht JR, Mass R, Perrou B, Nelson B, Novotny WF. Addition of bevacizumab to bolus fluorouracil and leucovorin in first-line metastatic colorectal cancer: results of a randomized phase II trial. J. Clin. Oncol. 2005;23:3697–3705. doi: 10.1200/JCO.2005.05.112. [DOI] [PubMed] [Google Scholar]
- 17.Saltz LB, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang T-S, Rivera F. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J. Clin. Oncol. 2008;26:2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 18.Kabbinavar F, Hurwitz HI, Fehrenbacher L, Meropol NJ, Novotny WF, Lieberman G, Griffing S, Bergsland E. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J. Clin. Oncol. 2003;21:60–65. doi: 10.1200/JCO.2003.10.066. [DOI] [PubMed] [Google Scholar]
- 19.Cunningham D, Lang I, Marcuello E, Lorusso V, Ocvirk J, Shin DB, Jonker D, Osborne S, Andre N, Waterkamp D. Bevacizumab plus capecitabine versus capecitabine alone in elderly patients with previously untreated metastatic colorectal cancer (AVEX): an open-label, randomised phase 3 trial. Lancet Oncol. 2013;14:1077–1085. doi: 10.1016/S1470-2045(13)70154-2. [DOI] [PubMed] [Google Scholar]
- 20.Tebbutt NC, Wilson K, Gebski VJ, Cummins MM, Zannino D, Van Hazel GA, Robinson B, Broad A, Ganju V, Ackland SP. Capecitabine, bevacizumab, and mitomycin in first-line treatment of metastatic colorectal cancer: results of the Australasian Gastrointestinal Trials Group Randomized Phase III MAX Study. J. Clin. Oncol. 2010;28:3191–3198. doi: 10.1200/JCO.2009.27.7723. [DOI] [PubMed] [Google Scholar]
- 21.Guan ZZ, Xu JM, Luo RC, Feng FY, Wang LW, Shen L, Yu SY, Ba Y, Liang J, Wang D. Efficacy and safety of bevacizumab plus chemotherapy in Chinese patients with metastatic colorectal cancer: a randomized phase III ARTIST trial. Chin JCancer. 2011;30:682–689. doi: 10.5732/cjc.011.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 23.Stathopoulos GP, Batziou C, Trafalis D, Koutantos J, Batzios S, Stathopoulos J, Legakis J, Armakolas A. Treatment of colorectal cancer with and without bevacizumab: a phase III study. Oncol. 2010;78:376–381. doi: 10.1159/000320520. [DOI] [PubMed] [Google Scholar]
- 24.Van Cutsem E, Oliveira J. Advanced colorectal cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;20:iv61–iv63. doi: 10.1093/annonc/mdp130. [DOI] [PubMed] [Google Scholar]
- 25.Yoo PS, Lopez-Soler RI, Longo WE, Cha CH. Liver resection for metastatic colorectal cancer in the age of neoadjuvant chemotherapy and bevacizumab. Clin Colorectal Cancer. 2006;6:202–207. doi: 10.3816/CCC.2006.n.036. [DOI] [PubMed] [Google Scholar]
- 26.Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, Maroun JA, Ackland SP, Locker PK, Pirotta N. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. N Engl J Med. 2000;343:905–914. doi: 10.1056/NEJM200009283431302. [DOI] [PubMed] [Google Scholar]
- 27.Tournigand C, André T, Achille E, Lledo G, Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J. Clin. Oncol. 2004;22:229–237. doi: 10.1200/JCO.2004.05.113. [DOI] [PubMed] [Google Scholar]
- 28.Van Cutsem E, Köhne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D’Haens G, Pintér T, Lim R, Bodoky G. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 29.Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, Freeman DJ, Juan T, Sikorski R, Suggs S, Radinsky R. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J. Clin. Oncol. 2008;26:1626–1634. doi: 10.1200/JCO.2007.14.7116. [DOI] [PubMed] [Google Scholar]
- 30.Whyte S, Pandor A, Stevenson M. Bevacizumab for metastatic colorectal cancer. Pharmacoeconomics. 2012;30:1119–1132. doi: 10.2165/11597210-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 31.Dai F, Shu L, Bian Y, Wang Z, Yang Z, Chu W, Gao S. Safety of bevacizumab in treating metastatic colorectal cancer: a systematic review and meta-analysis of all randomized clinical trials. Clin Drug Inves. 2013;33:779–788. doi: 10.1007/s40261-013-0125-6. [DOI] [PubMed] [Google Scholar]
- 32.Cao Y, Tan A, Gao F, Liu L, Liao C, Mo Z. A meta-analysis of randomized controlled trials comparing chemotherapy plus bevacizumab with chemotherapy alone in metastatic colorectal cancer. Int J Colorectal Dis. 2009;24:677–685. doi: 10.1007/s00384-009-0655-9. [DOI] [PubMed] [Google Scholar]
- 33.Macedo LT, da Costa Lima AB, Sasse AD. Addition of bevacizumab to first-line chemotherapy in advanced colorectal cancer: a systematic review and meta-analysis, with emphasis on chemotherapy subgroups. BMC Cancer. 2012;12:89. doi: 10.1186/1471-2407-12-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang M, Zheng X, Ruan X, Ye B, Cai L, Lin F, Tu J, Jiang F, Li S. Efficacy and safety of first-line chemotherapy plus bevacizumab in patients with metastatic colorectal cancer: a meta-analysis. Chin Med J. 2014;127:538–546. [PubMed] [Google Scholar]
- 35.Hurwitz HI, Fehrenbacher L, Hainsworth JD, Heim W, Berlin J, Holmgren E, Hambleton J, Novotny WF, Kabbinavar F. Bevacizumab in combination with fluorouracil and leucovorin: an active regimen for first-line metastatic colorectal cancer. J. Clin. Oncol. 2005;23:3502–3508. doi: 10.1200/JCO.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 36.Van Cutsem E, Rivera F, Berry S, Kretzschmar A, Michael M, DiBartolomeo M, Mazier M, Canon J, Georgoulias V, Peeters M, Bridgewater J, Cunningham D. Safety and efficacy of first-line bevacizumab with FOLFOX, XELOX, FOLFIRI and fluoropyrimidines in metastatic colorectal cancer: the BEAT study. Ann Oncol. 2009;20:1842–1847. doi: 10.1093/annonc/mdp233. [DOI] [PubMed] [Google Scholar]
- 37.Giantonio BJ, Catalano PJ, Meropol NJ, O’Dwyer PJ, Mitchell EP, Alberts SR, Schwartz MA, Benson AB. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J. Clin. Oncol. 2007;25:1539–1544. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 38.Bennouna J, Sastre J, Arnold D, Österlund P, Greil R, Van Cutsem E, von Moos R, Viéitez JM, Bouché O, Borg C. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet oncol. 2013;14:29–37. doi: 10.1016/S1470-2045(12)70477-1. [DOI] [PubMed] [Google Scholar]
- 39.Arnold D, Andre T, Bennouna J, Sastre J, Osterlund PJ, Greil R, Van Cutsem E, Von Moos R, Reyes-Rivera I, Bendahmane B. Bevacizumab (BEV) plus chemotherapy (CT) continued beyond first progression in patients with metastatic colorectal cancer (mCRC) previously treated with BEV plus CT: results of a randomized phase III intergroup study (TML study) J Clin Oncol (Meeting Abstracts) 2012;30:CRA3503. [Google Scholar]


